Detection of Embryonic Suspensor Cell Death by Whole-Mount TUNEL Assay in Tobacco
Abstract
:1. Introduction
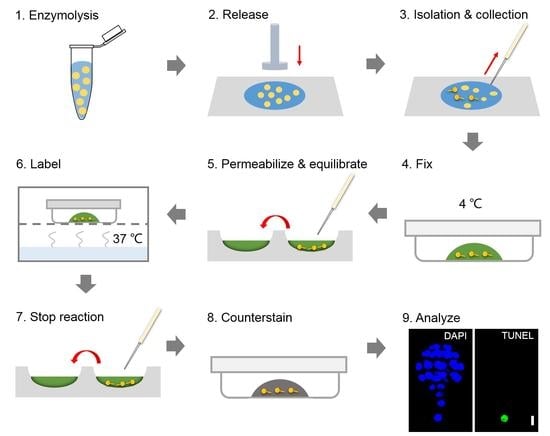
2. Results
2.1. Preparation of Hand-Made Tools
2.2. Collection of Living Embryos
2.3. TUNEL Assay
2.3.1. Fix the Embryos
2.3.2. Permeabilize and Equilibrate the Embryos
2.3.3. Label the Embryos
2.3.4. Analyze the Fluorescence
3. Materials and Equipment
3.1. Plant Materials
3.2. Reagents
3.3. Equipment
3.4. Solutions
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kawashima, T.; Goldberg, R.B. The suspensor: Not just suspending the embryo. Trends Plant Sci. 2010, 15, 23–30. [Google Scholar] [CrossRef]
- Peng, X.; Sun, M.-X. The suspensor as a model system to study the mechanism of cell fate specification during early embryogenesis. Plant Reprod. 2018, 31, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, X.; Zhao, J.; Tang, X.; Tian, S.; Chen, J.; Shi, C.; Wang, W.; Zhang, L.; Feng, X.; et al. Direct evidence that suspensor cells have embryogenic potential that is suppressed by the embryo proper during normal embryogenesis. Proc. Natl. Acad. Sci. USA 2015, 112, 12432. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Zhou, X.-M.; Zhang, L.-Y.; Wang, W.; Ma, L.-G.; Yang, L.-B.; Peng, X.-B.; Bozhkov, P.V.; Sun, M.-X. A bipartite molecular module controls cell death activation in the basal cell lineage of plant embryos. PLoS Biol. 2013, 11, e1001655. [Google Scholar] [CrossRef] [Green Version]
- Bozhkov, P.V.; Filonova, L.H.; Suarez, M.F. Programmed cell death in plant embryogenesis. Curr. Top. Dev. Biol. 2005, 67, 135–179. [Google Scholar] [PubMed]
- Bozhkov, P.V.; Suarez, M.F.; Filonova, L.H.; Daniel, G.; Zamyatnin, A.A.; Rodriguez-Nieto, S.; Zhivotovsky, B.; Smertenko, A. Cysteine protease mcII-Pa executes programmed cell death during plant embryogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 14463–14468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smertenko, A.P.; Bozhkov, P.V.; Filonova, L.H.; von Arnold, S.; Hussey, P.J. Re-organisation of the cytoskeleton during developmental programmed cell death in Picea abies embryos. Plant J. 2003, 33, 813–824. [Google Scholar] [CrossRef]
- Filonova, L.H.; Bozhkov, P.V.; Brukhin, V.B.; Daniel, G.; Arnold, S.V. Two waves of programmed cell death occur during formation and development of somatic embryos in the gymnosperm, Norway spruce. J. Cell Sci. 2000, 113, 4399–4411. [Google Scholar] [PubMed]
- Bozhkov, P.V.; Filonova, L.H.; Suarez, M.F.; Helmersson, A.; Smertenko, A.P.; Zhivotovsky, B.; von Arnold, S. VEIDase is a principal caspase-like activity involved in plant programmed cell death and essential for embryonic pattern formation. Cell Death Differ. 2004, 11, 175–182. [Google Scholar] [CrossRef]
- Wredle, U.; Walles, B.; Hakman, I. DNA fragmentation and nuclear degradation during programmed cell death in the suspensor and endosperm of Vicia faba. Int. J. Plant Sci. 2001, 162, 1053–1063. [Google Scholar] [CrossRef]
- Lombardi, L.; Ceccarelli, N.; Picciarelli, P.; Lorenzi, R. DNA degradation during programmed cell death in Phaseolus coccineus suspensor. Plant Physiol. Biochem. 2007, 45, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, L.; Ceccarelli, N.; Picciarelli, P.; Lorenzi, R. Caspase-like proteases involvement in programmed cell death of Phaseolus coccineus suspensor. Plant Sci. 2007, 172, 573–578. [Google Scholar] [CrossRef]
- Gavrieli, Y.; Sherman, Y.; Ben-Sasson, S.A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 1992, 119, 493–501. [Google Scholar] [CrossRef]
- Rath, N.C.; Huff, W.E.; Bayyari, G.R.; Balog, J.M. Cell death in avian tibial dyschondroplasia. Avian Dis. 1998, 42, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xiong, H.; Lin, R.; Zhao, N.; Zhao, P.; Sun, M.-X. VPE-like protease NtTPE8 exclusively expresses in the integumentary tapetum and is involved in seed development. J. Integr. Plant Biol. 2018, 61, 598–610. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Luo, P.; Du, Y.-T.; Chen, H.; Huang, X.; Cheng, T.-H.; Luo, A.; Li, H.-J.; Yang, W.-C.; Zhao, P.; et al. Maternal control of suspensor programmed cell death via gibberellin signaling. Nat. Commun. 2019, 10, 3484. [Google Scholar] [CrossRef] [Green Version]
- Luo, A.; Zhao, P.; Zhang, L.Y.; Sun, M.-X. Initiation of programmed cell death in the suspensor is predominantly regulated maternally in a tobacco hybrid. Sci. Rep. 2016, 6, 29467. [Google Scholar] [CrossRef]
- Zhao, P.; Zhou, X.-M.; Shi, C.; Sun, M.-X. Manual isolation of living early embryos from tobacco seeds. Methods Mol. Biol. 2020, 2122, 101–111. [Google Scholar]
- Palovaara, J.; Saiga, S.; Weijers, D. Transcriptomics approaches in the early Arabidopsis embryo. Trends Plant Sci. 2013, 18, 514–521. [Google Scholar] [CrossRef]
- Zhou, X.-M.; Shi, C.; Zhao, P.; Sun, M.-X. Isolation of living apical and basal cell lineages of early proembryos for transcriptome analysis. Plant Reprod. 2019, 32, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, A.K.; Pareek, A.; Singla-Pareek, S.L. A NAP-Family Histone chaperone functions in abiotic stress response and adaptation. Plant Physiol. 2016, 171, 2854–2868. [Google Scholar] [PubMed] [Green Version]
- Biswas, M.S.; Mano, J.I. Lipid peroxide-derived short-chain carbonyls mediate hydrogen peroxide-induced and salt-induced programmed cell death in plants. Plant Physiol. 2015, 168, 885–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fendrych, M.; Van Hautegem, T.; Van Durme, M.; Olvera-Carrillo, Y.; Huysmans, M.; Karimi, M.; Lippens, S.; Guérin, C.J.; Krebs, M.; Schumacher, K.; et al. Programmed cell death controlled by ANAC033/SOMBRERO determines root cap organ size in Arabidopsis. Curr. Biol. 2014, 24, 931–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, C.; Luo, P.; Zhao, P.; Sun, M.-X. Detection of Embryonic Suspensor Cell Death by Whole-Mount TUNEL Assay in Tobacco. Plants 2020, 9, 1196. https://doi.org/10.3390/plants9091196
Shi C, Luo P, Zhao P, Sun M-X. Detection of Embryonic Suspensor Cell Death by Whole-Mount TUNEL Assay in Tobacco. Plants. 2020; 9(9):1196. https://doi.org/10.3390/plants9091196
Chicago/Turabian StyleShi, Ce, Pan Luo, Peng Zhao, and Meng-Xiang Sun. 2020. "Detection of Embryonic Suspensor Cell Death by Whole-Mount TUNEL Assay in Tobacco" Plants 9, no. 9: 1196. https://doi.org/10.3390/plants9091196