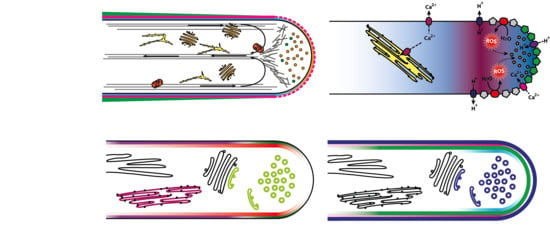
Signalling Pinpointed to the Tip: The Complex Regulatory Network That Allows Pollen Tube Growth
Abstract
:1. Introduction
2. Pectin Is an Important Component of the Pollen Tube Cell Wall
3. Pectin Secretion during Pollen Tube Growth
4. Pollen Tube Growth Is Regulated by Several Interwoven Signalling Networks
5. Reactive Oxygen Species in Pollen Tube Growth
6. Ion Gradients in Growing Pollen Tubes
7. Small GTPases Define the Pollen Tube Tip
8. Phosphoinositides and Derived Lipids form a Signalling Network
9. All Phosphoinositides Derive from Phosphatidylinositol
10. PI4P Has Regulatory Roles in the Trans-Golgi Network
11. PI4P 5-Kinases Catalyse the Formation of PI(4,5)P2 at the Pollen Tube Tip
12. PI(4,5)P2 Interacts with Components of the Exocyst Complex
13. Dephosphorylation Reactions on PI4P and PI(4,5)P2
14. Activity of Phospholipase C Is the Main Degradation Route of Phosphoinositides
15. Inositol(poly)phosphates Are Receptors’ Cofactors
16. Diacylgylcerol Kinases Have Distinct Functions in Growing Pollen Tubes
17. Phospholipase D-Produced Phosphatidic Acid Regulates Pollen Tube Growth
18. Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dehors, J.; Mareck, A.; Kiefer-Meyer, M.-C.; Menu-Bouaouiche, L.; Lehner, A.; Mollet, J.-C. Evolution of Cell Wall Polymers in Tip-Growing Land Plant Gametophytes: Composition, Distribution, Functional Aspects and Their Remodeling. Front. Plant. Sci. 2019, 10, 441. [Google Scholar] [CrossRef]
- Johnson, M.A.; Harper, J.F.; Palanivelu, R. A Fruitful Journey: Pollen Tube Navigation from Germination to Fertilization. Ann. Rev. Plant. Biol. 2019, 70, 809–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amien, S.; Kliwer, I.; Márton, M.L.; Debener, T.; Geiger, D.; Becker, D.; Dresselhaus, T. Defensin-like ZmES4 mediates pollen tube burst in maize via opening of the potassium channel KZM1. PLoS Biol. 2010, 8, e1000388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Z.; Bergonci, T.; Zhao, Y.; Zou, Y.; Du, S.; Liu, M.-C.; Luo, X.; Ruan, H.; García-Valencia, L.E.; Zhong, S.; et al. Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling. Science 2017, 358, 1596–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamamura, Y.; Saito, C.; Awai, C.; Kurihara, D.; Miyawaki, A.; Nakagawa, T.; Kanaoka, M.M.; Sasaki, N.; Nakano, A.; Berger, F.; et al. Live-cell imaging reveals the dynamics of two sperm cells during double fertilization in Arabidopsis thaliana. Curr. Biol. 2011, 21, 497–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grebnev, G.; Ntefidou, M.; Kost, B. Secretion and Endocytosis in Pollen Tubes: Models of Tip Growth in the Spot Light. Front. Plant. Sci. 2017, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, I.; Ischebeck, T. Male functions and malfunctions: The impact of phosphoinositides on pollen development and pollen tube growth. Plant. Reprod. 2016, 29, 3–20. [Google Scholar] [CrossRef]
- Stephan, O.O.H. Actin fringes of polar cell growth. J. Exp. Bot. 2017, 68, 3303–3320. [Google Scholar] [CrossRef]
- Williams, J.H. Novelties of the flowering plant pollen tube underlie diversification of a key life history stage. Proc. Natl. Acad. Sci. USA 2008, 105, 11259–11263. [Google Scholar] [CrossRef] [Green Version]
- Bidhendi, A.J.; Geitmann, A. Finite Element Modeling of Shape Changes in Plant Cells. Plant. Physiol. 2018, 176, 41–56. [Google Scholar] [CrossRef] [Green Version]
- Fayant, P.; Girlanda, O.; Chebli, Y.; Aubin, C.-E.; Villemure, I.; Geitmann, A. Finite Element Model of Polar Growth in Pollen Tubes. Plant. Cell 2010, 22, 2579–2593. [Google Scholar] [CrossRef] [Green Version]
- Luo, N.; Yan, A.; Liu, G.; Guo, J.; Rong, D.; Kanaoka, M.M.; Xiao, Z.; Xu, G.; Higashiyama, T.; Cui, X.; et al. Exocytosis-coordinated mechanisms for tip growth underlie pollen tube growth guidance. Nat. Commun. 2017, 8, 1687. [Google Scholar] [CrossRef]
- Zerzour, R.; Kroeger, J.; Geitmann, A. Polar growth in pollen tubes is associated with spatially confined dynamic changes in cell mechanical properties. Dev. Biol. 2009, 334, 437–446. [Google Scholar] [CrossRef]
- Chebli, Y.; Kaneda, M.; Zerzour, R.; Geitmann, A. The Cell Wall of the Arabidopsis Pollen Tube--Spatial Distribution, Recycling, and Network Formation of Polysaccharides. Plant Physiol. 2012, 160, 1940–1955. [Google Scholar] [CrossRef] [Green Version]
- Parre, E.; Geitmann, A. Pectin and the role of the physical properties of the cell wall in pollen tube growth of Solanum chacoense. Planta 2005, 220, 582–592. [Google Scholar] [CrossRef]
- Dardelle, F.; Lehner, A.; Ramdani, Y.; Bardor, M.; Lerouge, P.; Driouich, A.; Mollet, J.-C. Biochemical and immunocytological characterizations of Arabidopsis pollen tube cell wall. Plant. Physiol. 2010, 153, 1563–1576. [Google Scholar] [CrossRef] [Green Version]
- Driouich, A.; Follet-Gueye, M.-L.; Bernard, S.; Kousar, S.; Chevalier, L.; Vicré-Gibouin, M.; Lerouxel, O. Golgi-mediated synthesis and secretion of matrix polysaccharides of the primary cell wall of higher plants. Front. Plant. Sci. 2012, 3, 79. [Google Scholar] [CrossRef] [Green Version]
- Lenartowska, M.; Rodríguez-García, M.I.; Bednarska, E. Immunocytochemical localization of esterified and unesterified pectins in unpollinated and pollinated styles of Petunia hybrida Hort. Planta 2001, 213, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Chen, F.; Linskens, H.F.; Cresti, M. Distribution of unesterified and esterified pectins in cell walls of pollen tubes of flowering plants. Sex. Plant. Reprod. 1994, 7. [Google Scholar] [CrossRef]
- Mollet, J.-C.; Leroux, C.; Dardelle, F.; Lehner, A. Cell Wall Composition, Biosynthesis and Remodeling during Pollen Tube Growth. Plants 2013, 2, 107–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mravec, J.; Kračun, S.K.; Rydahl, M.G.; Westereng, B.; Pontiggia, D.; De Lorenzo, G.; Domozych, D.S.; Willats, W.G.T. An oligogalacturonide-derived molecular probe demonstrates the dynamics of calcium-mediated pectin complexation in cell walls of tip-growing structures. Plant. J. 2017, 91, 534–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bidhendi, A.J.; Chebli, Y.; Geitmann, A. Fluorescence visualization of cellulose and pectin in the primary plant cell wall. J. Microsc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.; Teeri, T.T.; Siika-aho, M.; Read, S.M.; Bacic, A. Location of cellulose and callose in pollen tubes and grains of Nicotiana tabacum. Planta 1998, 206, 452–460. [Google Scholar] [CrossRef]
- Bosch, M.; Cheung, A.Y.; Hepler, P.K. Pectin methylesterase, a regulator of pollen tube growth. Plant. Physiol. 2005, 138, 1334–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.; Yang, S.-L.; Xie, L.-F.; Puah, C.S.; Zhang, X.-Q.; Yang, W.-C.; Sundaresan, V.; Ye, D. VANGUARD1 Encodes a Pectin Methylesterase That Enhances Pollen Tube Growth in the Arabidopsis Style and Transmitting Tract. Plant. Cell 2005, 17, 584–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, C.; Zhu, X.; Qiao, X.; Gao, H.; Li, Q.; Wang, P.; Wu, J.; Zhang, S. Characterization of the pectin methyl-esterase gene family and its function in controlling pollen tube growth in pear (Pyrus bretschneideri). Genomics 2020, 112, 2467–2477. [Google Scholar] [CrossRef]
- Tian, G.-W.; Chen, M.-H.; Zaltsman, A.; Citovsky, V. Pollen-specific pectin methylesterase involved in pollen tube growth. Dev. Biol. 2006, 294, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Pelloux, J.; Rusterucci, C.; Mellerowicz, E. New insights into pectin methylesterase structure and function. Trends Plant. Sci. 2007, 12, 267–277. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, D.; Gao, W.; Li, Y.; Tan, J.; Zhang, X. A comparative genome analysis of PME and PMEI families reveals the evolution of pectin metabolism in plant cell walls. PLoS ONE 2013, 8, e72082. [Google Scholar] [CrossRef] [Green Version]
- Wormit, A.; Usadel, B. The Multifaceted Role of Pectin Methylesterase Inhibitors (PMEIs). Int. J. Mol. Sci. 2018, 19, 2878. [Google Scholar] [CrossRef] [Green Version]
- Micheli, F. Pectin methylesterases: Cell wall enzymes with important roles in plant physiology. Trends Plant. Sci. 2001, 6, 414–419. [Google Scholar] [CrossRef]
- Röckel, N.; Wolf, S.; Kost, B.; Rausch, T.; Greiner, S. Elaborate spatial patterning of cell-wall PME and PMEI at the pollen tube tip involves PMEI endocytosis, and reflects the distribution of esterified and de-esterified pectins. Plant. J. 2008, 53, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Derksen, J.; Rutten, T.; Lichtscheidl, I.K.; de Win, A.H.N.; Pierson, E.S.; Rongen, G. Quantitative analysis of the distribution of organelles in tobacco pollen tubes: Implications for exocytosis and endocytosis. Protoplasma 1995, 188, 267–276. [Google Scholar] [CrossRef]
- Guan, Y.; Guo, J.; Li, H.; Yang, Z. Signaling in Pollen Tube Growth: Crosstalk, Feedback, and Missing Links. Mol. Plant. 2013, 6, 1053–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mecchia, M.A.; Santos-Fernandez, G.; Duss, N.N.; Somoza, S.C.; Boisson-Dernier, A.; Gagliardini, V.; Martínez-Bernardini, A.; Fabrice, T.N.; Ringli, C.; Muschietti, J.P.; et al. RALF4/19 peptides interact with LRX proteins to control pollen tube growth in Arabidopsis. Science 2017, 358, 1600–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derksen, J.; Rutten, T.; Van Amstel, T.; De Win, A.; Doris, F.; Steer, M. Regulation of pollen tube growth. Acta Bot. Neerl. 1995, 44, 93–119. [Google Scholar] [CrossRef] [Green Version]
- Picton, J.M.; Steer, M.W. Membrane recycling and the control of secretory activity in pollen tubes. J. Cell. Sci. 1983, 63, 303–310. [Google Scholar]
- Steer, M.W. Plasma Membrane Turnover in Plant Cells. J. Exp. Bot. 1988, 39, 987–996. [Google Scholar] [CrossRef]
- Guo, J.; Yang, Z. Exocytosis and endocytosis: Coordinating and fine-tuning the polar tip growth domain in pollen tubes. J. Exp. Bot. 2020. [Google Scholar] [CrossRef]
- Cole, R.A.; Fowler, J.E. Polarized growth: Maintaining focus on the tip. Curr. Opin. Plant. Biol. 2006, 9, 579–588. [Google Scholar] [CrossRef]
- Cai, G.; Parrotta, L.; Cresti, M. Organelle trafficking, the cytoskeleton, and pollen tube growth: Organelle trafficking in pollen tubes. J. Integr. Plant. Biol. 2015, 57, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Hepler, P.K.; Winship, L.J. The pollen tube clear zone: Clues to the mechanism of polarized growth: Differential organelle movement creates a clear zone. J. Integr. Plant. Biol. 2015, 57, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Hepler, P.K.; Vidali, L.; Cheung, A.Y. Polarized Cell Growth in Higher Plants. Ann. Rev. Cell Dev. Biol. 2001, 17, 159–187. [Google Scholar] [CrossRef]
- Chebli, Y.; Kroeger, J.; Geitmann, A. Transport Logistics in Pollen Tubes. Mol. Plant. 2013, 6, 1037–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhikari, P.B.; Liu, X.; Wu, X.; Zhu, S.; Kasahara, R.D. Fertilization in flowering plants: An odyssey of sperm cell delivery. Plant. Mol. Biol. 2020. [Google Scholar] [CrossRef]
- Ischebeck, T.; Seiler, S.; Heilmann, I. At the poles across kingdoms: Phosphoinositides and polar tip growth. Protoplasma 2010, 240, 13–31. [Google Scholar] [CrossRef] [Green Version]
- Michard, E.; Simon, A.A.; Tavares, B.; Wudick, M.M.; Feijó, J.A. Signaling with Ions: The Keystone for Apical Cell Growth and Morphogenesis in Pollen Tubes. Plant. Physiol. 2017, 173, 91–111. [Google Scholar] [CrossRef] [Green Version]
- Scheible, N.; McCubbin, A. Signaling in Pollen Tube Growth: Beyond the Tip of the Polarity Iceberg. Plants (Basel) 2019, 8, 156. [Google Scholar] [CrossRef] [Green Version]
- Steinhorst, L.; Kudla, J. Calcium—A central regulator of pollen germination and tube growth. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2013, 1833, 1573–1581. [Google Scholar] [CrossRef] [Green Version]
- Vogler, H.; Santos-Fernandez, G.; Mecchia, M.A.; Grossniklaus, U. To preserve or to destroy, that is the question: The role of the cell wall integrity pathway in pollen tube growth. Curr. Opin. Plant. Biol. 2019, 52, 131–139. [Google Scholar] [CrossRef]
- Zhang, M.J.; Zhang, X.S.; Gao, X.-Q. ROS in the Male-Female Interactions During Pollination: Function and Regulation. Front. Plant. Sci. 2020, 11, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Y.; Fu, Y.; Dowd, P.; Li, S.; Vernoud, V.; Gilroy, S.; Yang, Z. A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J. Cell Biol. 2005, 169, 127–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ischebeck, T.; Stenzel, I.; Hempel, F.; Jin, X.; Mosblech, A.; Heilmann, I. Phosphatidylinositol-4,5-bisphosphate influences Nt-Rac5-mediated cell expansion in pollen tubes of Nicotiana tabacum: PtdIns(4,5)P2 and polar tip growth. Plant. J. 2011, 65, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Kost, B.; Lemichez, E.; Spielhofer, P.; Hong, Y.; Tolias, K.; Carpenter, C.; Chua, N.-H. Rac Homologues and Compartmentalized Phosphatidylinositol 4, 5-Bisphosphate Act in a Common Pathway to Regulate Polar Pollen Tube Growth. J. Cell Biol. 1999, 145, 317–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haduch-Sendecka, A.; Pietruszka, M.; Zajdel, P. Power spectrum, growth velocities and cross-correlations of longitudinal and transverse oscillations of individual Nicotiana tabacum pollen tube. Planta 2014, 240, 263–276. [Google Scholar] [CrossRef] [Green Version]
- Hemelryck, M.V.; Bernal, R.; Ispolatov, Y.; Dumais, J. Lily Pollen Tubes Pulse According to a Simple Spatial Oscillator. Sci. Rep. 2018, 8, 12135. [Google Scholar] [CrossRef]
- Hwang, J.-U.; Gu, Y.; Lee, Y.-J.; Yang, Z. Oscillatory ROP GTPase activation leads the oscillatory polarized growth of pollen tubes. Mol. Biol. Cell 2005, 16, 5385–5399. [Google Scholar] [CrossRef]
- Qin, Y.; Dong, J. Focusing on the Focus: What Else beyond the Master Switches for Polar Cell Growth? Mol. Plant. 2015, 8, 582–594. [Google Scholar] [CrossRef] [Green Version]
- Yan, A.; Xu, G.; Yang, Z.-B. Calcium participates in feedback regulation of the oscillating ROP1 Rho GTPase in pollen tubes. Proc. Natl. Acad. Sci. USA 2009, 106, 22002–22007. [Google Scholar] [CrossRef] [Green Version]
- Duan, Q.; Kita, D.; Johnson, E.A.; Aggarwal, M.; Gates, L.; Wu, H.-M.; Cheung, A.Y. Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat. Commun. 2014, 5, 3129. [Google Scholar] [CrossRef]
- Potocký, M.; Jones, M.A.; Bezvoda, R.; Smirnoff, N.; Žárský, V. Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol. 2007, 174, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Potocký, M.; Pejchar, P.; Gutkowska, M.; Jiménez-Quesada, M.J.; Potocká, A.; de Dios Alché, J.; Kost, B.; Žárský, V. NADPH oxidase activity in pollen tubes is affected by calcium ions, signaling phospholipids and Rac/Rop GTPases. J. Plant. Physiol. 2012, 169, 1654–1663. [Google Scholar] [CrossRef]
- Cárdenas, L.; McKenna, S.T.; Kunkel, J.G.; Hepler, P.K. NAD(P)H oscillates in pollen tubes and is correlated with tip growth. Plant. Physiol. 2006, 142, 1460–1468. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.-B.; Wang, C.-L.; Wu, J.-Y.; Zhou, H.-S.; Jiang, X.-T.; Wu, J.; Zhang, S.-L. Low temperature inhibits pollen tube growth by disruption of both tip-localized reactive oxygen species and endocytosis in Pyrus bretschneideri Rehd. Plant. Physiol. Biochem. 2014, 74, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Quesada, M.J.; Traverso, J.A.; Potocký, M.; Žárský, V.; Alché, J.D.D. Generation of Superoxide by OeRbohH, a NADPH Oxidase Activity During Olive (Olea europaea L.) Pollen Development and Germination. Front. Plant. Sci. 2019, 10, 1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Li, R.-L.; Zhang, L.; Wang, Q.-L.; Niehaus, K.; Baluska, F.; Samaj, J.; Lin, J.-X. Lipid microdomain polarization is required for NADPH oxidase-dependent ROS signaling in Picea meyeri pollen tube tip growth. Plant. J. 2009, 60, 303–313. [Google Scholar] [CrossRef]
- Speranza, A.; Crinelli, R.; Scoccianti, V.; Geitmann, A. Reactive oxygen species are involved in pollen tube initiation in kiwifruit. Plant. Biol. (Stuttg) 2012, 14, 64–76. [Google Scholar] [CrossRef]
- Kaya, H.; Nakajima, R.; Iwano, M.; Kanaoka, M.M.; Kimura, S.; Takeda, S.; Kawarazaki, T.; Senzaki, E.; Hamamura, Y.; Higashiyama, T.; et al. Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant. Cell 2014, 26, 1069–1080. [Google Scholar] [CrossRef] [Green Version]
- Lassig, R.; Gutermuth, T.; Bey, T.D.; Konrad, K.R.; Romeis, T. Pollen tube NAD(P)H oxidases act as a speed control to dampen growth rate oscillations during polarized cell growth. Plant. J. 2014, 78, 94–106. [Google Scholar] [CrossRef]
- Boisson-Dernier, A.; Lituiev, D.S.; Nestorova, A.; Franck, C.M.; Thirugnanarajah, S.; Grossniklaus, U. ANXUR Receptor-Like Kinases Coordinate Cell Wall Integrity with Growth at the Pollen Tube Tip Via NADPH Oxidases. PLoS Biol. 2013, 11, e1001719. [Google Scholar] [CrossRef] [Green Version]
- Feng, H.; Liu, C.; Fu, R.; Zhang, M.; Li, H.; Shen, L.; Wei, Q.; Sun, X.; Xu, L.; Ni, B.; et al. LORELEI-LIKE GPI-ANCHORED PROTEINS 2/3 Regulate Pollen Tube Growth as Chaperones and Coreceptors for ANXUR/BUPS Receptor Kinases in Arabidopsis. Mol. Plant. 2019, 12, 1612–1623. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chu, L.-C.; Liang, Y.; Zhang, X.-Q.; Chen, L.-Q.; Ye, D. The Arabidopsis CrRLK1L protein kinases BUPS1 and BUPS2 are required for normal growth of pollen tubes in the pistil. Plant. J. 2018, 95, 474–486. [Google Scholar] [CrossRef] [Green Version]
- Ge, Z.; Cheung, A.Y.; Qu, L. Pollen tube integrity regulation in flowering plants: Insights from molecular assemblies on the pollen tube surface. New Phytol. 2019, 222, 687–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benkő, P.; Jee, S.; Kaszler, N.; Fehér, A.; Gémes, K. Polyamines treatment during pollen germination and pollen tube elongation in tobacco modulate reactive oxygen species and nitric oxide homeostasis. J. Plant. Physiol. 2020, 244, 153085. [Google Scholar] [CrossRef]
- Wu, J.; Shang, Z.; Wu, J.; Jiang, X.; Moschou, P.N.; Sun, W.; Roubelakis-Angelakis, K.A.; Zhang, S. Spermidine oxidase-derived H2O2 regulates pollen plasma membrane hyperpolarization-activated Ca(2+) -permeable channels and pollen tube growth. Plant. J. 2010, 63, 1042–1053. [Google Scholar] [CrossRef] [PubMed]
- Do, T.H.T.; Choi, H.; Palmgren, M.; Martinoia, E.; Hwang, J.-U.; Lee, Y. Arabidopsis ABCG28 is required for the apical accumulation of reactive oxygen species in growing pollen tubes. Proc. Natl. Acad. Sci. USA 2019, 116, 12540–12549. [Google Scholar] [CrossRef] [Green Version]
- Mangano, S.; Juárez, S.P.D.; Estevez, J.M. ROS Regulation of Polar Growth in Plant Cells. Plant. Physiol. 2016, 171, 1593–1605. [Google Scholar] [CrossRef] [Green Version]
- Podolyan, A.; Maksimov, N.; Breygina, M. Redox-regulation of ion homeostasis in growing lily pollen tubes. J. Plant. Physiol. 2019, 243, 153050. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, H.; Zhang, Q.; Li, M.; Yan, M.; Wang, R.; Wang, L.; Welti, R.; Zhang, W.; Wang, X. Phospholipase Dα1 and Phosphatidic Acid Regulate NADPH Oxidase Activity and Production of Reactive Oxygen Species in ABA-Mediated Stomatal Closure in Arabidopsis. Plant. Cell 2009, 21, 2357–2377. [Google Scholar] [CrossRef] [Green Version]
- Wudick, M.M.; Feijó, J.A. At the intersection: Merging Ca2+ and ROS signaling pathways in pollen. Mol. Plant. 2014, 7, 1595–1597. [Google Scholar] [CrossRef] [Green Version]
- Feijó, J.A.; Sainhas, J.; Hackett, G.R.; Kunkel, J.G.; Hepler, P.K. Growing pollen tubes possess a constitutive alkaline band in the clear zone and a growth-dependent acidic tip. J. Cell Biol. 1999, 144, 483–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Certal, A.C.; Almeida, R.B.; Carvalho, L.M.; Wong, E.; Moreno, N.; Michard, E.; Carneiro, J.; Rodriguéz-Léon, J.; Wu, H.-M.; Cheung, A.Y.; et al. Exclusion of a Proton ATPase from the Apical Membrane Is Associated with Cell Polarity and Tip Growth in Nicotiana tabacum Pollen Tubes. Plant. Cell 2008, 20, 614–634. [Google Scholar] [CrossRef] [Green Version]
- Michard, E.; Dias, P.; Feijó, J.A. Tobacco pollen tubes as cellular models for ion dynamics: Improved spatial and temporal resolution of extracellular flux and free cytosolic concentration of calcium and protons using pHluorin and YC3.1 CaMeleon. Sex. Plant. Reprod. 2008, 21, 169–181. [Google Scholar] [CrossRef]
- Fricker, M.D.; White, N.S.; Obermeyer, G. pH gradients are not associated with tip growth in pollen tubes of Lilium longiflorum. J. Cell. Sci. 1997, 110 Pt 15, 1729–1740. [Google Scholar]
- Lovy-Wheeler, A.; Kunkel, J.G.; Allwood, E.G.; Hussey, P.J.; Hepler, P.K. Oscillatory increases in alkalinity anticipate growth and may regulate actin dynamics in pollen tubes of lily. Plant. Cell 2006, 18, 2182–2193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winship, L.J.; Rounds, C.; Hepler, P.K. Perturbation Analysis of Calcium, Alkalinity and Secretion during Growth of Lily Pollen Tubes. Plants (Basel) 2016, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Domingos, P.; Dias, P.N.; Tavares, B.; Portes, M.T.; Wudick, M.M.; Konrad, K.R.; Gilliham, M.; Bicho, A.; Feijó, J.A. Molecular and electrophysiological characterization of anion transport in Arabidopsis thaliana pollen reveals regulatory roles for pH, Ca2+ and GABA. New Phytol. 2019, 223, 1353–1371. [Google Scholar] [CrossRef]
- Hoffmann, R.D.; Portes, M.T.; Olsen, L.I.; Damineli, D.S.C.; Hayashi, M.; Nunes, C.O.; Pedersen, J.T.; Lima, P.T.; Campos, C.; Feijó, J.A.; et al. Plasma membrane H+-ATPases sustain pollen tube growth and fertilization. Nat. Commun. 2020, 11, 2395. [Google Scholar] [CrossRef]
- Brewbaker, J.L.; Kwack, B.H. THE ESSENTIAL ROLE OF CALCIUM ION IN POLLEN GERMINATION AND POLLEN TUBE GROWTH. Am. J. Bot. 1963, 50, 859–865. [Google Scholar] [CrossRef]
- Denninger, P.; Bleckmann, A.; Lausser, A.; Vogler, F.; Ott, T.; Ehrhardt, D.W.; Frommer, W.B.; Sprunck, S.; Dresselhaus, T.; Grossmann, G. Male–female communication triggers calcium signatures during fertilization in Arabidopsis. Nat. Commun. 2014, 5, 4645. [Google Scholar] [CrossRef] [Green Version]
- Hamamura, Y.; Nishimaki, M.; Takeuchi, H.; Geitmann, A.; Kurihara, D.; Higashiyama, T. Live imaging of calcium spikes during double fertilization in Arabidopsis. Nat. Commun. 2014, 5, 4722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holdaway-Clarke, T.L.; Feijo, J.A.; Hackett, G.R.; Kunkel, J.G.; Hepler, P.K. Pollen Tube Growth and the Intracellular Cytosolic Calcium Gradient Oscillate in Phase while Extracellular Calcium Influx Is Delayed. Plant. Cell 1997, 9, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Pierson, E.S.; Miller, D.D.; Callaham, D.A.; van Aken, J.; Hackett, G.; Hepler, P.K. Tip-localized calcium entry fluctuates during pollen tube growth. Dev. Biol. 1996, 174, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Malho, R.; Trewavas, A.J. Localized Apical Increases of Cytosolic Free Calcium Control Pollen Tube Orientation. Plant. Cell 1996, 8, 1935–1949. [Google Scholar] [CrossRef]
- Zheng, R.; Su, S.; Xiao, H.; Tian, H. Calcium: A Critical Factor in Pollen Germination and Tube Elongation. Int. J. Mol. Sci. 2019, 20, 420. [Google Scholar] [CrossRef] [Green Version]
- Iwano, M.; Entani, T.; Shiba, H.; Kakita, M.; Nagai, T.; Mizuno, H.; Miyawaki, A.; Shoji, T.; Kubo, K.; Isogai, A.; et al. Fine-tuning of the cytoplasmic Ca2+ concentration is essential for pollen tube growth. Plant. Physiol. 2009, 150, 1322–1334. [Google Scholar] [CrossRef] [Green Version]
- Obermeyer, G.; Weisenseel, M.H. Calcium channel blocker and calmodulin antagonists affect the gradient of free calcium ions in lily pollen tubes. Eur. J. Cell Biol. 1991, 56, 319–327. [Google Scholar] [PubMed]
- Pierson, E.S.; Miller, D.D.; Callaham, D.A.; Shipley, A.M.; Rivers, B.A.; Cresti, M.; Hepler, P.K. Pollen tube growth is coupled to the extracellular calcium ion flux and the intracellular calcium gradient: Effect of BAPTA-type buffers and hypertonic media. Plant. Cell 1994, 6, 1815–1828. [Google Scholar] [CrossRef]
- Dutta, R.; Robinson, K.R. Identification and characterization of stretch-activated ion channels in pollen protoplasts. Plant. Physiol. 2004, 135, 1398–1406. [Google Scholar] [CrossRef] [Green Version]
- Frietsch, S.; Wang, Y.-F.; Sladek, C.; Poulsen, L.R.; Romanowsky, S.M.; Schroeder, J.I.; Harper, J.F. A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc. Natl. Acad. Sci. USA 2007, 104, 14531–14536. [Google Scholar] [CrossRef] [Green Version]
- Michard, E.; Lima, P.T.; Borges, F.; Silva, A.C.; Portes, M.T.; Carvalho, J.E.; Gilliham, M.; Liu, L.-H.; Obermeyer, G.; Feijó, J.A. Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science 2011, 332, 434–437. [Google Scholar] [CrossRef] [Green Version]
- Wudick, M.M.; Portes, M.T.; Michard, E.; Rosas-Santiago, P.; Lizzio, M.A.; Nunes, C.O.; Campos, C.; Santa Cruz Damineli, D.; Carvalho, J.C.; Lima, P.T.; et al. CORNICHON sorting and regulation of GLR channels underlie pollen tube Ca2+ homeostasis. Science 2018, 360, 533–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamport, D.T.A.; Tan, L.; Held, M.A.; Kieliszewski, M.J. Pollen tube growth and guidance: Occam’s razor sharpened on a molecular arabinogalactan glycoprotein Rosetta Stone. New Phytol. 2018, 217, 491–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Guo, J.; Yang, Z.; Yang, D.-L. Plasma Membrane-Localized Calcium Pumps and Copines Coordinately Regulate Pollen Germination and Fertility in Arabidopsis. Int. J. Mol. Sci. 2018, 19, 1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiøtt, M.; Romanowsky, S.M.; Baekgaard, L.; Jakobsen, M.K.; Palmgren, M.G.; Harper, J.F. A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc. Natl. Acad. Sci. USA 2004, 101, 9502–9507. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, S.; Bera, M.; Coleman, J.; Rothman, J.E.; Krishnakumar, S.S. Synergistic roles of Synaptotagmin-1 and complexin in calcium-regulated neuronal exocytosis. Elife 2020, 9. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhou, P.; Wang, A.L.; Wu, D.; Zhao, M.; Südhof, T.C.; Brunger, A.T. The primed SNARE-complexin-synaptotagmin complex for neuronal exocytosis. Nature 2017, 548, 420–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pozzi, D.; Corradini, I.; Matteoli, M. The Control of Neuronal Calcium Homeostasis by SNAP-25 and its Impact on Neurotransmitter Release. Neuroscience 2019, 420, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Harmon, A.C.; Gribskov, M.; Gubrium, E.; Harper, J.F. The CDPK superfamily of protein kinases: Research review. New Phytol. 2001, 151, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, Y.; Deng, Y.; Chen, P.; Feng, F.; Chen, W.; Zhou, X.; Wang, Y. A calcium-dependent protein kinase, ZmCPK32, specifically expressed in maize pollen to regulate pollen tube growth. PLoS ONE 2018, 13, e0195787. [Google Scholar] [CrossRef]
- Myers, C.; Romanowsky, S.M.; Barron, Y.D.; Garg, S.; Azuse, C.L.; Curran, A.; Davis, R.M.; Hatton, J.; Harmon, A.C.; Harper, J.F. Calcium-dependent protein kinases regulate polarized tip growth in pollen tubes. Plant. J. 2009, 59, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Yoon, G.M.; Dowd, P.E.; Gilroy, S.; McCubbin, A.G. Calcium-dependent protein kinase isoforms in Petunia have distinct functions in pollen tube growth, including regulating polarity. Plant. Cell 2006, 18, 867–878. [Google Scholar] [CrossRef] [Green Version]
- Gutermuth, T.; Lassig, R.; Portes, M.-T.; Maierhofer, T.; Romeis, T.; Borst, J.-W.; Hedrich, R.; Feijo, J.A.; Konrad, K.R. Pollen Tube Growth Regulation by Free Anions Depends on the Interaction between the Anion Channel SLAH3 and Calcium-Dependent Protein Kinases CPK2 and CPK20. Plant. Cell 2013, 25, 4525–4543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.-N.; Shen, L.-K.; Zhang, W.-Z.; Zhang, W.; Wang, Y.; Wu, W.-H. Ca2+-dependent protein kinase11 and 24 modulate the activity of the inward rectifying K+ channels in Arabidopsis pollen tubes. Plant. Cell 2013, 25, 649–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutermuth, T.; Herbell, S.; Lassig, R.; Brosché, M.; Romeis, T.; Feijó, J.A.; Hedrich, R.; Konrad, K.R. Tip-localized Ca2+ -permeable channels control pollen tube growth via kinase-dependent R- and S-type anion channel regulation. New Phytol. 2018, 218, 1089–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbell, S.; Gutermuth, T.; Konrad, K.R. An interconnection between tip-focused Ca2+ and anion homeostasis controls pollen tube growth. Plant. Signal. Behav. 2018, 13, e1529521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mähs, A.; Steinhorst, L.; Han, J.-P.; Shen, L.-K.; Wang, Y.; Kudla, J. The calcineurin B-like Ca2+ sensors CBL1 and CBL9 function in pollen germination and pollen tube growth in Arabidopsis. Mol. Plant. 2013, 6, 1149–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinhorst, L.; Mähs, A.; Ischebeck, T.; Zhang, C.; Zhang, X.; Arendt, S.; Schültke, S.; Heilmann, I.; Kudla, J. Vacuolar CBL-CIPK12 Ca2+-Sensor-Kinase Complexes Are Required for Polarized Pollen Tube Growth. Curr. Biol. 2015, 25, 1475–1482. [Google Scholar] [CrossRef] [Green Version]
- Suwińska, A.; Wasąg, P.; Zakrzewski, P.; Lenartowska, M.; Lenartowski, R. Calreticulin is required for calcium homeostasis and proper pollen tube tip growth in Petunia. Planta 2017, 245, 909–926. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.-S.; Diao, W.-Z.; Yang, X.; Qiao, Z.; Wang, M.; Acharya, B.R.; Zhang, W. Arabidopsis thaliana CML25 mediates the Ca(2+) regulation of K(+) transmembrane trafficking during pollen germination and tube elongation. Plant. Cell Environ. 2015, 38, 2372–2386. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.-S.; Wang, M.; Qiao, Z.; Bao, C.-C.; Zhang, W. Arabidopsis thaliana calmodulin-like protein CML24 regulates pollen tube growth by modulating the actin cytoskeleton and controlling the cytosolic Ca(2+) concentration. Plant. Mol. Biol. 2014, 86, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Xiang, Y. Actin Cytoskeleton as Actor in Upstream and Downstream of Calcium Signaling in Plant Cells. Int. J. Mol. Sci. 2019, 20, 1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, L.; Moore, I.; Kirchhelle, C. Spatio-temporal control of post-Golgi exocytic trafficking in plants. J. Cell. Sci. 2020, 133. [Google Scholar] [CrossRef] [PubMed]
- Feiguelman, G.; Fu, Y.; Yalovsky, S. ROP GTPases Structure-Function and Signaling Pathways. Plant. Physiol. 2018, 176, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Bourne, H.R.; Sanders, D.A.; McCormick, F. The GTPase superfamily: Conserved structure and molecular mechanism. Nature 1991, 349, 117–127. [Google Scholar] [CrossRef]
- Berken, A.; Thomas, C.; Wittinghofer, A. A new family of RhoGEFs activates the Rop molecular switch in plants. Nature 2005, 436, 1176–1180. [Google Scholar] [CrossRef]
- Wu, G.; Li, H.; Yang, Z. Arabidopsis RopGAPs are a novel family of rho GTPase-activating proteins that require the Cdc42/Rac-interactive binding motif for rop-specific GTPase stimulation. Plant. Physiol. 2000, 124, 1625–1636. [Google Scholar] [CrossRef] [Green Version]
- DerMardirossian, C.; Bokoch, G.M. GDIs: Central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005, 15, 356–363. [Google Scholar] [CrossRef]
- Bischoff, F.; Molendijk, A.; Rajendrakumar, C.S.V.; Palme, K. GTP-binding proteins in plants. Cell. Mol. Life Sci. CMLS 1999, 55, 233–256. [Google Scholar] [CrossRef]
- Cheung, A.Y.; Chen, C.Y.-H.; Glaven, R.H.; de Graaf, B.H.J.; Vidali, L.; Hepler, P.K.; Wu, H. Rab2 GTPase regulates vesicle trafficking between the endoplasmic reticulum and the Golgi bodies and is important to pollen tube growth. Plant. Cell 2002, 14, 945–962. [Google Scholar] [CrossRef] [Green Version]
- de Graaf, B.H.J.; Cheung, A.Y.; Andreyeva, T.; Levasseur, K.; Kieliszewski, M.; Wu, H. Rab11 GTPase-regulated membrane trafficking is crucial for tip-focused pollen tube growth in tobacco. Plant. Cell 2005, 17, 2564–2579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szumlanski, A.L.; Nielsen, E. The Rab GTPase RabA4d regulates pollen tube tip growth in Arabidopsis thaliana. Plant. Cell 2009, 21, 526–544. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Ilarslan, H.; Wurtele, E.S.; Bassham, D.C. AtRabD2b and AtRabD2c have overlapping functions in pollen development and pollen tube growth. BMC Plant. Biol. 2011, 11, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutkowska, M.; Wnuk, M.; Nowakowska, J.; Lichocka, M.; Stronkowski, M.M.; Swiezewska, E. Rab geranylgeranyl transferase β subunit is essential for male fertility and tip growth in Arabidopsis. J. Exp. Bot. 2015, 66, 213–224. [Google Scholar] [CrossRef]
- Klahre, U.; Kost, B. Tobacco RhoGTPase ACTIVATING PROTEIN1 spatially restricts signaling of RAC/Rop to the apex of pollen tubes. Plant. Cell 2006, 18, 3033–3046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kost, B. Spatial control of Rho (Rac-Rop) signaling in tip-growing plant cells. Trends Cell Biol. 2008, 18, 119–127. [Google Scholar] [CrossRef]
- Bloch, D.; Pleskot, R.; Pejchar, P.; Potocký, M.; Trpkošová, P.; Cwiklik, L.; Vukašinović, N.; Sternberg, H.; Yalovsky, S.; Žárský, V. Exocyst SEC3 and phosphoinositides define sites of exocytosis in pollen tube initiation and growth. Plant. Physiol. 2016, 172, 980–1002. [Google Scholar] [CrossRef] [Green Version]
- Lavy, M.; Bloch, D.; Hazak, O.; Gutman, I.; Poraty, L.; Sorek, N.; Sternberg, H.; Yalovsky, S. A Novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Curr. Biol. 2007, 17, 947–952. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Gu, Y.; Yan, A.; Lord, E.; Yang, Z.-B. RIP1 (ROP Interactive Partner 1)/ICR1 marks pollen germination sites and may act in the ROP1 pathway in the control of polarized pollen growth. Mol. Plant. 2008, 1, 1021–1035. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.-H.; Cheung, A.Y.; Wu, H. Actin-depolymerizing factor mediates Rac/Rop GTPase-regulated pollen tube growth. Plant. Cell 2003, 15, 237–249. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.J.; Szumlanski, A.; Nielsen, E.; Yang, Z. Rho-GTPase-dependent filamentous actin dynamics coordinate vesicle targeting and exocytosis during tip growth. J. Cell Biol. 2008, 181, 1155–1168. [Google Scholar] [CrossRef] [Green Version]
- Stephan, O.; Cottier, S.; Fahlén, S.; Montes-Rodriguez, A.; Sun, J.; Eklund, D.M.; Klahre, U.; Kost, B. RISAP is a TGN-associated RAC5 effector regulating membrane traffic during polar cell growth in tobacco. Plant. Cell 2014, 26, 4426–4447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, H.; Higashiyama, T. Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis. Nature 2016, 531, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, Y.; Nagashima, Y.; Wakazaki, M.; Sato, M.; Toyooka, K.; Fukuda, H.; Oda, Y. A Rho-actin signaling pathway shapes cell wall boundaries in Arabidopsis xylem vessels. Nat. Commun. 2019, 10, 468. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lin, Y.; Heath, R.M.; Zhu, M.X.; Yang, Z. Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. Plant. Cell 1999, 11, 1731–1742. [Google Scholar]
- Winge, P.; Brembu, T.; Kristensen, R.; Bones, A.M. Genetic structure and evolution of RAC-GTPases in Arabidopsis thaliana. Genetics 2000, 156, 1959–1971. [Google Scholar]
- Klahre, U.; Becker, C.; Schmitt, A.C.; Kost, B. Nt-RhoGDI2 regulates Rac/Rop signaling and polar cell growth in tobacco pollen tubes. Plant. J. 2006, 46, 1018–1031. [Google Scholar] [CrossRef]
- Sun, J.; Eklund, D.M.; Montes-Rodriguez, A.; Kost, B. In vivo Rac/Rop localization as well as interaction with RhoGAP and RhoGDI in tobacco pollen tubes: Analysis by low-level expression of fluorescent fusion proteins and bimolecular fluorescence complementation. Plant J. 2015, 84, 83–98. [Google Scholar] [CrossRef]
- Feng, Q.-N.; Kang, H.; Song, S.-J.; Ge, F.-R.; Zhang, Y.-L.; Li, E.; Li, S.; Zhang, Y. Arabidopsis RhoGDIs Are Critical for Cellular Homeostasis of Pollen Tubes. Plant. Physiol. 2016, 170, 841–856. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.-J.; Park, S.-W.; Hong, W.-J.; Silva, J.; Liang, W.; Zhang, D.; Jung, K.-H.; Kim, Y.-J. Genome-wide analysis of RopGEF gene family to identify genes contributing to pollen tube growth in rice (Oryza sativa). BMC Plant. Biol. 2020, 20, 95. [Google Scholar] [CrossRef] [Green Version]
- Chang, F.; Gu, Y.; Ma, H.; Yang, Z. AtPRK2 promotes ROP1 activation via RopGEFs in the control of polarized pollen tube growth. Mol. Plant. 2013, 6, 1187–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, E.; Cui, Y.; Ge, F.-R.; Chai, S.; Zhang, W.-T.; Feng, Q.-N.; Jiang, L.; Li, S.; Zhang, Y. AGC1.5 Kinase Phosphorylates RopGEFs to Control Pollen Tube Growth. Mol. Plant. 2018, 11, 1198–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashiyama, T.; Yang, W.-C. Gametophytic Pollen Tube Guidance: Attractant Peptides, Gametic Controls, and Receptors. Plant. Physiol. 2017, 173, 112–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, H.; Higashiyama, T. A Species-Specific Cluster of Defensin-Like Genes Encodes Diffusible Pollen Tube Attractants in Arabidopsis. PLoS Biol. 2012, 10, e1001449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Liang, L.; Xue, Y.; Jia, P.-F.; Chen, W.; Zhang, M.-X.; Wang, Y.-C.; Li, H.-J.; Yang, W.-C. A receptor heteromer mediates the male perception of female attractants in plants. Nature 2016, 531, 241–244. [Google Scholar] [CrossRef]
- Gu, Y.; Li, S.; Lord, E.M.; Yang, Z. Members of a novel class of Arabidopsis Rho guanine nucleotide exchange factors control Rho GTPase-dependent polar growth. Plant. Cell 2006, 18, 366–381. [Google Scholar] [CrossRef] [Green Version]
- Meijer, H.J.G.; Munnik, T. PHOSPHOLIPID-BASED SIGNALING IN PLANTS. Ann. Rev. Plant. Biol. 2003, 54, 265–306. [Google Scholar] [CrossRef]
- Noack, L.C.; Pejchar, P.; Sekereš, J.; Jaillais, Y.; Potocký, M. Transient Gene Expression as a Tool to Monitor and Manipulate the Levels of Acidic Phospholipids in Plant Cells. Methods Mol. Biol. 2019, 1992, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Collin, S.; Justin, A.M.; Cantrel, C.; Arondel, V.; Kader, J.C. Identification of AtPIS, a phosphatidylinositol synthase from Arabidopsis. Eur. J. Biochem. 1999, 262, 652–658. [Google Scholar] [CrossRef]
- Löfke, C.; Ischebeck, T.; König, S.; Freitag, S.; Heilmann, I. Alternative metabolic fates of phosphatidylinositol produced by phosphatidylinositol synthase isoforms in Arabidopsis thaliana. Biochem. J. 2008, 413, 115–124. [Google Scholar] [CrossRef]
- Ischebeck, T.; Vu, L.H.; Jin, X.; Stenzel, I.; Löfke, C.; Heilmann, I. Functional Cooperativity of Enzymes of Phosphoinositide Conversion According to Synergistic Effects on Pectin Secretion in Tobacco Pollen Tubes. Mol. Plant. 2010, 3, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Gerth, K.; Lin, F.; Menzel, W.; Krishnamoorthy, P.; Stenzel, I.; Heilmann, M.; Heilmann, I. Guilt by Association: A Phenotype-Based View of the Plant Phosphoinositide Network. Ann. Rev. Plant. Biol. 2017, 68, 349–374. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, I. Phosphoinositide signaling in plant development. Development 2016, 143, 2044–2055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meijer, H.J.; Berrie, C.P.; Iurisci, C.; Divecha, N.; Musgrave, A.; Munnik, T. Identification of a new polyphosphoinositide in plants, phosphatidylinositol 5-monophosphate (PtdIns5P), and its accumulation upon osmotic stress. Biochem. J. 2001, 360, 491–498. [Google Scholar] [CrossRef]
- Krinke, O.; Ruelland, E.; Valentová, O.; Vergnolle, C.; Renou, J.-P.; Taconnat, L.; Flemr, M.; Burketová, L.; Zachowski, A. Phosphatidylinositol 4-Kinase Activation Is an Early Response to Salicylic Acid in Arabidopsis Suspension Cells. Plant. Physiol. 2007, 144, 1347–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, M.L.A.; Platre, M.P.; Assil, S.; van Wijk, R.; Chen, W.Y.; Chory, J.; Dreux, M.; Munnik, T.; Jaillais, Y. A multi-colour/multi-affinity marker set to visualize phosphoinositide dynamics in Arabidopsis. Plant. J. 2014, 77, 322–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thole, J.M.; Vermeer, J.E.M.; Zhang, Y.; Gadella, T.W.J.; Nielsen, E. ROOT HAIR DEFECTIVE4 Encodes a Phosphatidylinositol-4-Phosphate Phosphatase Required for Proper Root Hair Development in Arabidopsis thaliana. Plant. Cell 2008, 20, 381–395. [Google Scholar] [CrossRef] [Green Version]
- Vermeer, J.E.M.; Thole, J.M.; Goedhart, J.; Nielsen, E.; Munnik, T.; Gadella, T.W.J. Imaging phosphatidylinositol 4-phosphate dynamics in living plant cells. Plant. J. 2009, 57, 356–372. [Google Scholar] [CrossRef] [Green Version]
- Kang, B.-H.; Nielsen, E.; Preuss, M.L.; Mastronarde, D.; Staehelin, L.A. Electron tomography of RabA4b- and PI-4Kβ1-labeled trans Golgi network compartments in Arabidopsis. Traffic 2011, 12, 313–329. [Google Scholar] [CrossRef]
- Preuss, M.L.; Schmitz, A.J.; Thole, J.M.; Bonner, H.K.S.; Otegui, M.S.; Nielsen, E. A role for the RabA4b effector protein PI-4Kbeta1 in polarized expansion of root hair cells in Arabidopsis thaliana. J. Cell Biol. 2006, 172, 991–998. [Google Scholar] [CrossRef]
- Jia, P.-F.; Xue, Y.; Li, H.-J.; Yang, W.-C. Golgi-localized LOT regulates trans-Golgi network biogenesis and pollen tube growth. Proc. Natl. Acad. Sci. USA 2018, 115, 12307–12312. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.; Krishnamoorthy, P.; Schubert, V.; Hause, G.; Heilmann, M.; Heilmann, I. A dual role for cell plate-associated PI4Kβ in endocytosis and phragmoplast dynamics during plant somatic cytokinesis. Embo J. 2019, 38. [Google Scholar] [CrossRef] [PubMed]
- Rubilar-Hernández, C.; Osorio-Navarro, C.; Cabello, F.; Norambuena, L. PI4KIIIβ Activity Regulates Lateral Root Formation Driven by Endocytic Trafficking to the Vacuole. Plant. Physiol. 2019, 181, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Roeber, B.; Pical, C. Inositol phospholipid metabolism in Arabidopsis. Characterized and putative isoforms of inositol phospholipid kinase and phosphoinositide-specific phospholipase C. Plant. Physiol. 2002, 130, 22–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strahl, T.; Hama, H.; DeWald, D.B.; Thorner, J. Yeast phosphatidylinositol 4-kinase, Pik1, has essential roles at the Golgi and in the nucleus. J. Cell Biol. 2005, 171, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Ohya, Y.; Goebl, M.; Nakano, A.; Anraku, Y. A novel gene, STT4, encodes a phosphatidylinositol 4-kinase in the PKC1 protein kinase pathway of Saccharomyces cerevisiae. J. Biol. Chem. 1994, 269, 1166–1172. [Google Scholar] [PubMed]
- Im, Y.J.; Davis, A.J.; Perera, I.Y.; Johannes, E.; Allen, N.S.; Boss, W.F. The N-terminal Membrane Occupation and Recognition Nexus Domain of Arabidopsis Phosphatidylinositol Phosphate Kinase 1 Regulates Enzyme Activity. J. Biol. Chem. 2007, 282, 5443–5452. [Google Scholar] [CrossRef] [Green Version]
- Stenzel, I.; Ischebeck, T.; Quint, M.; Heilmann, I. Variable Regions of PI4P 5-Kinases Direct PtdIns(4,5)P2 Toward Alternative Regulatory Functions in Tobacco Pollen Tubes. Front. Plant. Sci. 2012, 2. [Google Scholar] [CrossRef] [Green Version]
- Honys, D.; Twell, D. Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biol. 2004, 5, R85. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, W.-Z.; Song, L.-F.; Zou, J.-J.; Su, Z.; Wu, W.-H. Transcriptome Analyses Show Changes in Gene Expression to Accompany Pollen Germination and Tube Growth in Arabidopsis. Plant. Physiol. 2008, 148, 1201–1211. [Google Scholar] [CrossRef] [Green Version]
- Ischebeck, T.; Stenzel, I.; Heilmann, I. Type B Phosphatidylinositol-4-Phosphate 5-Kinases Mediate Arabidopsis and Nicotiana tabacum Pollen Tube Growth by Regulating Apical Pectin Secretion. Plant Cell Online 2008, 20, 3312–3330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grobei, M.A.; Qeli, E.; Brunner, E.; Rehrauer, H.; Zhang, R.; Roschitzki, B.; Basler, K.; Ahrens, C.H.; Grossniklaus, U. Deterministic protein inference for shotgun proteomics data provides new insights into Arabidopsis pollen development and function. Genome Res. 2009, 19, 1786–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, E.; Kost, B.; Malho, R. Arabidopsis Phosphatidylinositol-4-Monophosphate 5-Kinase 4 Regulates Pollen Tube Growth and Polarity by Modulating Membrane Recycling. Plant Cell Online 2008, 20, 3050–3064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Yan, A.; Feijó, J.A.; Furutani, M.; Takenawa, T.; Hwang, I.; Fu, Y.; Yang, Z. Phosphoinositides regulate clathrin-dependent endocytosis at the tip of pollen tubes in Arabidopsis and tobacco. Plant. Cell 2010, 22, 4031–4044. [Google Scholar] [CrossRef] [Green Version]
- Dowd, P.E.; Coursol, S.; Skirpan, A.L.; Kao, T.; Gilroy, S. Petunia phospholipase c1 is involved in pollen tube growth. Plant. Cell 2006, 18, 1438–1453. [Google Scholar] [CrossRef] [Green Version]
- Hempel, F.; Stenzel, I.; Heilmann, M.; Krishnamoorthy, P.; Menzel, W.; Golbik, R.; Helm, S.; Dobritzsch, D.; Baginsky, S.; Lee, J.; et al. MAPKs Influence Pollen Tube Growth by Controlling the Formation of Phosphatidylinositol 4,5-Bisphosphate in an Apical Plasma Membrane Domain. Plant. Cell 2017, 29, 3030–3050. [Google Scholar] [CrossRef] [Green Version]
- Kaneda, M.; van Oostende-Triplet, C.; Chebli, Y.; Testerink, C.; Bednarek, S.Y.; Geitmann, A. Plant AP180 N-Terminal Homolog Proteins Are Involved in Clathrin-Dependent Endocytosis during Pollen Tube Growth in Arabidopsis thaliana. Plant. Cell Physiol. 2019, 60, 1316–1330. [Google Scholar] [CrossRef]
- Kolay, S.; Basu, U.; Raghu, P. Control of diverse subcellular processes by a single multi-functional lipid phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2]. Biochem. J. 2016, 473, 1681–1692. [Google Scholar] [CrossRef]
- Mähs, A.; Ischebeck, T.; Heilig, Y.; Stenzel, I.; Hempel, F.; Seiler, S.; Heilmann, I. The essential phosphoinositide kinase MSS-4 is required for polar hyphal morphogenesis, localizing to sites of growth and cell fusion in Neurospora crassa. PLoS ONE 2012, 7, e51454. [Google Scholar] [CrossRef]
- Raghu, P.; Joseph, A.; Krishnan, H.; Singh, P.; Saha, S. Phosphoinositides: Regulators of Nervous System Function in Health and Disease. Front. Mol. NeuroSci. 2019, 12, 208. [Google Scholar] [CrossRef] [Green Version]
- Hille, B.; Dickson, E.J.; Kruse, M.; Vivas, O.; Suh, B.-C. Phosphoinositides regulate ion channels. Biochim. Biophys. Acta 2015, 1851, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.F.J. Role of PI(4,5)P(2) in vesicle exocytosis and membrane fusion. Subcell. Biochem. 2012, 59, 111–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, B.; Guo, W. The Exocyst at a Glance. J. Cell. Sci. 2015, 128, 2957–2964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Žárský, V.; Sekereš, J.; Kubátová, Z.; Pečenková, T.; Cvrčková, F. Three subfamilies of exocyst EXO70 family subunits in land plants: Early divergence and ongoing functional specialization. J. Exp. Bot. 2020, 71, 49–62. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Xi, F.; Zhang, X.; Zhang, J.; Guo, W. Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. Embo J. 2007, 26, 4053–4065. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Tamanoi, F.; Novick, P. Spatial regulation of the exocyst complex by Rho1 GTPase. Nat. Cell Biol. 2001, 3, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Orlando, K.; He, B.; Xi, F.; Zhang, J.; Zajac, A.; Guo, W. Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J. Cell Biol. 2008, 180, 145–158. [Google Scholar] [CrossRef] [Green Version]
- Elias, M.; Drdova, E.; Ziak, D.; Bavlnka, B.; Hala, M.; Cvrckova, F.; Soukupova, H.; Zarsky, V. The exocyst complex in plants. Cell Biol. Int. 2003, 27, 199–201. [Google Scholar] [CrossRef]
- Hála, M.; Cole, R.; Synek, L.; Drdová, E.; Pecenková, T.; Nordheim, A.; Lamkemeyer, T.; Madlung, J.; Hochholdinger, F.; Fowler, J.E.; et al. An exocyst complex functions in plant cell growth in Arabidopsis and tobacco. Plant. Cell 2008, 20, 1330–1345. [Google Scholar] [CrossRef] [Green Version]
- TerBush, D.R.; Maurice, T.; Roth, D.; Novick, P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. Embo J. 1996, 15, 6483–6494. [Google Scholar] [CrossRef]
- Dubuke, M.L.; Maniatis, S.; Shaffer, S.A.; Munson, M. The Exocyst Subunit Sec6 Interacts with Assembled Exocytic SNARE Complexes. J. Biol. Chem. 2015, 290, 28245–28256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, W.; Roth, D.; Walch-Solimena, C.; Novick, P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. Embo J. 1999, 18, 1071–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pleskot, R.; Cwiklik, L.; Jungwirth, P.; Žárský, V.; Potocký, M. Membrane targeting of the yeast exocyst complex. Biochim. Biophys. Acta 2015, 1848, 1481–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Synek, L.; Schlager, N.; Eliás, M.; Quentin, M.; Hauser, M.-T.; Zárský, V. AtEXO70A1, a member of a family of putative exocyst subunits specifically expanded in land plants, is important for polar growth and plant development. Plant. J. 2006, 48, 54–72. [Google Scholar] [CrossRef] [Green Version]
- Yue, P.; Zhang, Y.; Mei, K.; Wang, S.; Lesigang, J.; Zhu, Y.; Dong, G.; Guo, W. Sec3 promotes the initial binary t-SNARE complex assembly and membrane fusion. Nat. Commun. 2017, 8, 14236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, R.A.; Synek, L.; Zarsky, V.; Fowler, J.E. SEC8, a subunit of the putative Arabidopsis exocyst complex, facilitates pollen germination and competitive pollen tube growth. Plant. Physiol. 2005, 138, 2005–2018. [Google Scholar] [CrossRef] [Green Version]
- Synek, L.; Vukašinović, N.; Kulich, I.; Hála, M.; Aldorfová, K.; Fendrych, M.; Žárský, V. EXO70C2 Is a Key Regulatory Factor for Optimal Tip Growth of Pollen. Plant. Physiol. 2017, 174, 223–240. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Tan, X.; Wang, M.; Li, B.; Zhao, Y.; Wu, C.; Rui, Q.; Wang, J.; Liu, Z.; Bao, Y. Exocyst subunit SEC3A marks the germination site and is essential for pollen germination in Arabidopsis thaliana. Sci. Rep. 2017, 7, 40279. [Google Scholar] [CrossRef]
- Zárský, V.; Kulich, I.; Fendrych, M.; Pečenková, T. Exocyst complexes multiple functions in plant cells secretory pathways. Curr. Opin. Plant. Biol. 2013, 16, 726–733. [Google Scholar] [CrossRef]
- Fendrych, M.; Synek, L.; Pecenková, T.; Toupalová, H.; Cole, R.; Drdová, E.; Nebesárová, J.; Sedinová, M.; Hála, M.; Fowler, J.E.; et al. The Arabidopsis exocyst complex is involved in cytokinesis and cell plate maturation. Plant. Cell 2010, 22, 3053–3065. [Google Scholar] [CrossRef] [Green Version]
- Pecenková, T.; Markovic, V.; Sabol, P.; Kulich, I.; Žárský, V. Exocyst and autophagy-related membrane trafficking in plants. J. Exp. Bot. 2017, 69, 47–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabol, P.; Kulich, I.; Žárský, V. RIN4 recruits the exocyst subunit EXO70B1 to the plasma membrane. J. Exp. Bot. 2017, 68, 3253–3265. [Google Scholar] [CrossRef] [PubMed]
- Stegmann, M.; Anderson, R.G.; Ichimura, K.; Pecenkova, T.; Reuter, P.; Žársky, V.; McDowell, J.M.; Shirasu, K.; Trujillo, M. The ubiquitin ligase PUB22 targets a subunit of the exocyst complex required for PAMP-triggered responses in Arabidopsis. Plant. Cell 2012, 24, 4703–4716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubátová, Z.; Pejchar, P.; Potocký, M.; Sekereš, J.; Žárský, V.; Kulich, I. Arabidopsis Trichome Contains Two Plasma Membrane Domains with Different Lipid Compositions Which Attract Distinct EXO70 Subunits. Int. J. Mol. Sci. 2019, 20, 3803. [Google Scholar] [CrossRef] [Green Version]
- Sekereš, J.; Pejchar, P.; Šantrůček, J.; Vukašinović, N.; Žárský, V.; Potocký, M. Analysis of Exocyst Subunit EXO70 Family Reveals Distinct Membrane Polar Domains in Tobacco Pollen Tubes. Plant. Physiol. 2017, 173, 1659–1675. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; van Os, G.M.A.; Ren, S.; Yu, D.; Ketelaar, T.; Emons, A.M.C.; Liu, C.-M. Expression and functional analyses of EXO70 genes in Arabidopsis implicate their roles in regulating cell type-specific exocytosis. Plant. Physiol. 2010, 154, 1819–1830. [Google Scholar] [CrossRef] [Green Version]
- Pecenková, T.; Hála, M.; Kulich, I.; Kocourková, D.; Drdová, E.; Fendrych, M.; Toupalová, H.; Zársky, V. The role for the exocyst complex subunits Exo70B2 and Exo70H1 in the plant-pathogen interaction. J. Exp. Bot. 2011, 62, 2107–2116. [Google Scholar] [CrossRef] [Green Version]
- Boyd, C.; Hughes, T.; Pypaert, M.; Novick, P. Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J. Cell Biol. 2004, 167, 889–901. [Google Scholar] [CrossRef] [Green Version]
- Fendrych, M.; Synek, L.; Pecenková, T.; Drdová, E.J.; Sekeres, J.; de Rycke, R.; Nowack, M.K.; Zársky, V. Visualization of the exocyst complex dynamics at the plasma membrane of Arabidopsis thaliana. Mol. Biol. Cell 2013, 24, 510–520. [Google Scholar] [CrossRef] [Green Version]
- Zhong, R.; Ye, Z.-H. The SAC Domain-Containing Protein Gene Family in Arabidopsis. Plant. Physiol. 2003, 132, 544–555. [Google Scholar] [CrossRef] [Green Version]
- Despres, B.; Bouissonnie, F.; Wu, H.-J.; Gomord, V.; Guilleminot, J.; Grellet, F.; Berger, F.; Delseny, M.; Devic, M. Three SAC1-like genes show overlapping patterns of expression in Arabidopsis but are remarkably silent during embryo development. Plant. J. 2003, 34, 293–306. [Google Scholar] [CrossRef]
- Ton, J.; Jakab, G.; Toquin, V.; Flors, V.; Iavicoli, A.; Maeder, M.N.; Métraux, J.-P.; Mauch-Mani, B. Dissecting the beta-aminobutyric acid-induced priming phenomenon in Arabidopsis. Plant. Cell 2005, 17, 987–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, T.; Chen, H.-C.; Lu, Z.-Q.; Diao, M.; Chen, K.; Dong, N.-Q.; Shan, J.-X.; Ye, W.-W.; Huang, S.; Lin, H.-X. A SAC Phosphoinositide Phosphatase Controls Rice Development via Hydrolyzing PI4P and PI(4,5)P2. Plant. Physiol. 2020, 182, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.E.; Torabinejad, J.; Cohick, E.; Parker, K.; Drake, E.J.; Thompson, J.E.; Hortter, M.; Dewald, D.B. Mutations in the Arabidopsis phosphoinositide phosphatase gene SAC9 lead to overaccumulation of PtdIns(4,5)P2 and constitutive expression of the stress-response pathway. Plant. Physiol. 2005, 138, 686–700. [Google Scholar] [CrossRef] [Green Version]
- Vollmer, A.H.; Youssef, N.N.; DeWald, D.B. Unique cell wall abnormalities in the putative phosphoinositide phosphatase mutant AtSAC9. Planta 2011, 234, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Luo, Z.; Kong, S.; Zhao, Y.; Zhang, C.; Zhang, W.; Yuan, H.; Cheng, L. Expansion and Functional Divergence of Inositol Polyphosphate 5-Phosphatases in Angiosperms. Genes (Basel) 2019, 10, 393. [Google Scholar] [CrossRef] [Green Version]
- Berdy, S.E.; Kudla, J.; Gruissem, W.; Gillaspy, G.E. Molecular characterization of At5PTase1, an inositol phosphatase capable of terminating inositol trisphosphate signaling. Plant. Physiol. 2001, 126, 801–810. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.A.; Raymond, M.J.; Smirnoff, N. Analysis of the root-hair morphogenesis transcriptome reveals the molecular identity of six genes with roles in root-hair development in Arabidopsis. Plant. J. 2006, 45, 83–100. [Google Scholar] [CrossRef]
- Ringli, C.; Baumberger, N.; Keller, B. The Arabidopsis root hair mutants der2-der9 are affected at different stages of root hair development. Plant. Cell Physiol. 2005, 46, 1046–1053. [Google Scholar] [CrossRef] [Green Version]
- Zhong, R.; Ye, Z.-H. Molecular and biochemical characterization of three WD-repeat-domain-containing inositol polyphosphate 5-phosphatases in Arabidopsis thaliana. Plant. Cell Physiol. 2004, 45, 1720–1728. [Google Scholar] [CrossRef]
- Hanahan, D.J.; Chaikoff, I.L. A new phospholipide-splitting enzyme specific for the ester linkage between the nitrogenous base and the phosphoric acid grouping. J. Biol. Chem. 1947, 169, 699–705. [Google Scholar] [PubMed]
- Irvine, R.F.; Letcher, A.J.; Dawson, R.M. Phosphatidylinositol phosphodiesterase in higher plants. Biochem. J. 1980, 192, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ryu, S.; Wang, X. Plant Phospholipases: An Overview. In Lipases and Phospholipases; Sandoval, G., Ed.; Humana Press: Totowa, NJ, USA, 2012; Volume 861, pp. 123–137. ISBN 978-1-61779-599-2. [Google Scholar]
- Pokotylo, I.; Pejchar, P.; Potocký, M.; Kocourková, D.; Krčková, Z.; Ruelland, E.; Kravets, V.; Martinec, J. The plant non-specific phospholipase C gene family. Novel competitors in lipid signalling. Prog. Lipid Res. 2013, 52, 62–79. [Google Scholar] [CrossRef]
- Pokotylo, I.; Kolesnikov, Y.; Kravets, V.; Zachowski, A.; Ruelland, E. Plant phosphoinositide-dependent phospholipases C: Variations around a canonical theme. Biochimie 2014, 96, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Ngo, A.H.; Lin, Y.-C.; Liu, Y.-C.; Gutbrod, K.; Peisker, H.; Dörmann, P.; Nakamura, Y. A pair of nonspecific phospholipases C, NPC2 and NPC6, are involved in gametophyte development and glycerolipid metabolism in Arabidopsis. New Phytol. 2018, 219, 163–175. [Google Scholar] [CrossRef] [Green Version]
- Ngo, A.H.; Kanehara, K.; Nakamura, Y. Non-specific phospholipases C, NPC2 and NPC6, are required for root growth in Arabidopsis. Plant. J. 2019, 100, 825–835. [Google Scholar] [CrossRef]
- Wimalasekera, R.; Pejchar, P.; Holk, A.; Martinec, J.; Scherer, G.F.E. Plant phosphatidylcholine-hydrolyzing phospholipases C NPC3 and NPC4 with roles in root development and brassinolide signaling in Arabidopsis thaliana. Mol. Plant. 2010, 3, 610–625. [Google Scholar] [CrossRef]
- Helling, D.; Possart, A.; Cottier, S.; Klahre, U.; Kost, B. Pollen tube tip growth depends on plasma membrane polarization mediated by tobacco PLC3 activity and endocytic membrane recycling. Plant. Cell 2006, 18, 3519–3534. [Google Scholar] [CrossRef] [Green Version]
- Stenzel, I.; Ischebeck, T.; Vu-Becker, L.H.; Riechmann, M.; Krishnamoorthy, P.; Fratini, M.; Heilmann, I. Coordinated Localization and Antagonistic Function of NtPLC3 and PI4P 5-Kinases in the Subapical Plasma Membrane of Tobacco Pollen Tubes. Plants 2020, 9, 452. [Google Scholar] [CrossRef] [Green Version]
- Hokin, L.E. Receptors and phosphoinositide-generated second messengers. Ann. Rev. Biochem. 1985, 54, 205–235. [Google Scholar] [CrossRef]
- Munnik, T.; Testerink, C. Plant phospholipid signaling: “in a nutshell”. J. Lipid Res. 2009, 50, S260–S265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Y.; Zhao, J.; Guo, L.; Kim, S.-C.; Deng, X.; Wang, G.; Zhang, G.; Li, M.; Wang, X. Plant phospholipases D and C and their diverse functions in stress responses. Prog. Lipid Res. 2016, 62, 55–74. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo-Orts, L.; Couto, D.; Hothorn, M. Identity and functions of inorganic and inositol polyphosphates in plants. New Phytol. 2020, 225, 637–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsui, M.M.; York, J.D. Roles of inositol phosphates and inositol pyrophosphates in development, cell signaling and nuclear processes. Adv. Enzym. Regul. 2010, 50, 324–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderón Villalobos, L.I.A.; Lee, S.; De Oliveira, C.; Ivetac, A.; Brandt, W.; Armitage, L.; Sheard, L.B.; Tan, X.; Parry, G.; Mao, H.; et al. A combinatorial TIR1/AFB-Aux/IAA co-receptor system for differential sensing of auxin. Nat. Chem. Biol. 2012, 8, 477–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laha, D.; Parvin, N.; Dynowski, M.; Johnen, P.; Mao, H.; Bitters, S.T.; Zheng, N.; Schaaf, G. Inositol Polyphosphate Binding Specificity of the Jasmonate Receptor Complex. Plant. Physiol. 2016, 171, 2364–2370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosblech, A.; Thurow, C.; Gatz, C.; Feussner, I.; Heilmann, I. Jasmonic acid perception by COI1 involves inositol polyphosphates in Arabidopsis thaliana. Plant. J. 2011, 65, 949–957. [Google Scholar] [CrossRef]
- Sheard, L.B.; Tan, X.; Mao, H.; Withers, J.; Ben-Nissan, G.; Hinds, T.R.; Kobayashi, Y.; Hsu, F.-F.; Sharon, M.; Browse, J.; et al. Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 2010, 468, 400–405. [Google Scholar] [CrossRef]
- Tan, X.; Calderon-Villalobos, L.I.A.; Sharon, M.; Zheng, C.; Robinson, C.V.; Estelle, M.; Zheng, N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 2007, 446, 640–645. [Google Scholar] [CrossRef]
- Muradoğlu, F.; Yıldız, K.; Balta, F. Methyl jasmonate influences of pollen germination and pollen tube growth of apricot (Prunus armeniaca L.). Yüzüncü Yıl Univ. J. Agr. Sci. (Turk.) 2010, 183–188. [Google Scholar]
- Gao, C.; Wang, Y.; Qu, H. Study of auxin regulation of pollen tube growth through calcium channels in Pyrus pyrifolia. Plant. Growth Regul. 2019, 89, 99–108. [Google Scholar] [CrossRef]
- Zhan, H.; Zhong, Y.; Yang, Z.; Xia, H. Enzyme activities of Arabidopsis inositol polyphosphate kinases AtIPK2α and AtIPK2β are involved in pollen development, pollen tube guidance and embryogenesis. Plant. J. 2015, 82, 758–771. [Google Scholar] [CrossRef] [Green Version]
- Nishizuka, Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature 1988, 334, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Valverde, A.M.; Sinnett-Smith, J.; Van Lint, J.; Rozengurt, E. Molecular cloning and characterization of protein kinase D: A target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proc. Natl. Acad. Sci. USA 1994, 91, 8572–8576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islas-Flores, T.; Rahman, A.; Ullah, H.; Villanueva, M.A. The Receptor for Activated C Kinase in Plant Signaling: Tale of a Promiscuous Little Molecule. Front. Plant. Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arisz, S.A.; Testerink, C.; Munnik, T. Plant PA signaling via diacylglycerol kinase. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2009, 1791, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Lv, H.; Xia, G.; Wang, M. Does diacylglycerol serve as a signaling molecule in plants? Plant. Signal. Behav. 2012, 7, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Kim, S.-C.; Devaiah, S.; Li, M.; Wang, X. Non-specific phospholipase C5 and diacylglycerol promote lateral root development under mild salt stress in Arabidopsis. Plant. Cell Environ. 2014, 37, 2002–2013. [Google Scholar] [CrossRef]
- Potocký, M.; Pleskot, R.; Pejchar, P.; Vitale, N.; Kost, B.; Žárský, V. Live-cell imaging of phosphatidic acid dynamics in pollen tubes visualized by Spo20p-derived biosensor. New Phytol. 2014, 203, 483–494. [Google Scholar] [CrossRef]
- Wissing, J.; Heim, S.; Wagner, K.G. Diacylglycerol kinase from suspension cultured plant cells: Purification and properties. Plant. Physiol. 1989, 90, 1546–1551. [Google Scholar] [CrossRef] [Green Version]
- Pleskot, R.; Pejchar, P.; Bezvoda, R.; Lichtscheidl, I.; Wolters-Arts, M.; Marc, J.; Žárský, V.; Potocký, M. Turnover of Phosphatidic Acid through Distinct Signaling Pathways Affects Multiple Aspects of Pollen Tube Growth in Tobacco. Front. Plant. Sci. 2012, 3, 54. [Google Scholar] [CrossRef] [Green Version]
- Ischebeck, T.; Valledor, L.; Lyon, D.; Gingl, S.; Nagler, M.; Meijón, M.; Egelhofer, V.; Weckwerth, W. Comprehensive cell-specific protein analysis in early and late pollen development from diploid microsporocytes to pollen tube growth. Mol. Cell Proteom. 2014, 13, 295–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaz Dias, F.; Serrazina, S.; Vitorino, M.; Marchese, D.; Heilmann, I.; Godinho, M.; Rodrigues, M.; Malhó, R. A role for diacylglycerol kinase 4 in signalling crosstalk during Arabidopsis pollen tube growth. New Phytol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Donaldson, L.; Portes, M.T.; Eppinger, J.; Feijó, J.; Gehring, C. The Arabidopsis Diacylglycerol Kinase 4 is involved in nitric oxide-dependent pollen tube guidance and fertilization. Development 2020. [Google Scholar] [CrossRef]
- Angkawijaya, A.E.; Nguyen, V.C.; Gunawan, F.; Nakamura, Y. A Pair of Arabidopsis Diacylglycerol Kinases Essential for Gametogenesis and ER Phospholipid Metabolism in Leaves and Flowers. Plant. Cell 2020. [Google Scholar] [CrossRef] [PubMed]
- Eliáš, M.; Potocký, M.; Cvrčková, F.; Žárský, V. Molecular diversity of phospholipase D in angiosperms. BMC Genom. 2002, 3, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wang, X. Phospholipase D and phosphatidic acid in plant immunity. Plant. Sci. 2019, 279, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Testerink, C.; Munnik, T. Phosphatidic acid: A multifunctional stress signaling lipid in plants. Trends Plant. Sci. 2005, 10, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Potocký, M.; Eliás, M.; Profotová, B.; Novotná, Z.; Valentová, O.; Zárský, V. Phosphatidic acid produced by phospholipase D is required for tobacco pollen tube growth. Planta 2003, 217, 122–130. [Google Scholar] [CrossRef]
- Baneux, C.; Tanguy, E.; Thahouly, T.; Vitale, A.; Chasserot-Golaz, S.; Bader, M.-F.; Gasman, S.; Vitale, N. Phosphatidic acid metabolism regulates neuroendocrine secretion but is not under the direct control of lipins. Iubmb Life 2020, 72, 533–543. [Google Scholar] [CrossRef]
- Tanguy, E.; Wang, Q.; Vitale, N. Role of Phospholipase D-Derived Phosphatidic Acid in Regulated Exocytosis and Neurological Disease. Handb Exp Pharm. 2020, 259, 115–130. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; Xiao, H. Mechanism of membrane fusion: Protein-protein interaction and beyond. Int. J. Physiol. Pathophysiol. Pharm. 2019, 11, 250–257. [Google Scholar]
- Noack, L.C.; Jaillais, Y. Functions of Anionic Lipids in Plants. Ann. Rev. Plant. Biol. 2020, 71, 71–102. [Google Scholar] [CrossRef] [PubMed]
- Pleskot, R.; Potocký, M.; Pejchar, P.; Linek, J.; Bezvoda, R.; Martinec, J.; Valentová, O.; Novotná, Z.; Žárský, V. Mutual regulation of plant phospholipase D and the actin cytoskeleton: Reciprocal regulation of plant PLD and actin. Plant. J. 2010, 62, 494–507. [Google Scholar] [CrossRef]
- Pejchar, P.; Sekereš, J.; Novotný, O.; Žárský, V.; Potocký, M. Functional analysis of phospholipase Dδ family in tobacco pollen tubes. Plant. J. 2020. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scholz, P.; Anstatt, J.; Krawczyk, H.E.; Ischebeck, T. Signalling Pinpointed to the Tip: The Complex Regulatory Network That Allows Pollen Tube Growth. Plants 2020, 9, 1098. https://doi.org/10.3390/plants9091098
Scholz P, Anstatt J, Krawczyk HE, Ischebeck T. Signalling Pinpointed to the Tip: The Complex Regulatory Network That Allows Pollen Tube Growth. Plants. 2020; 9(9):1098. https://doi.org/10.3390/plants9091098
Chicago/Turabian StyleScholz, Patricia, Jannis Anstatt, Hannah Elisa Krawczyk, and Till Ischebeck. 2020. "Signalling Pinpointed to the Tip: The Complex Regulatory Network That Allows Pollen Tube Growth" Plants 9, no. 9: 1098. https://doi.org/10.3390/plants9091098