Assessment of the Nutritional and Medicinal Potential of Tubers from Hairy Stork’s-Bill (Erodium crassifolium L ’Hér), a Wild Plant Species Inhabiting Arid Southeast Mediterranean Regions
Abstract
:1. Introduction
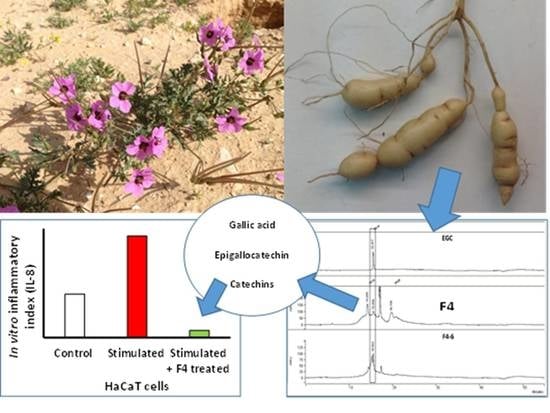
2. Results and Discussion
3. Materials and Methods
3.1. Ecosystem and Plant Material
3.2. Field Experiments
3.2.1. Simulated Precipitation Regimes
3.2.2. Agricultural Practice
3.3. Evaluation of Tubers Nutrition Facts
3.4. Ethanolic Extract (EE)
3.5. High-Performance Liquid Chromatography (HPLC) Analysis
3.6. GC–MS Analysis
3.7. Human Cell Culture
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zohary, D.; Hopf, M.; Weiss, E. Domestication of Plants in the Old World, 4th ed.; Clarendon Press: Oxford, UK, 2012. [Google Scholar]
- Doebley, J.F.; Gaut, B.S.; Smith, B.D. The molecular genetics of crop domestication. Cell 2006, 127, 1309–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardin, G. Extensions of “the tragedy of the commons”. Science 1998, 280, 682–683. [Google Scholar] [CrossRef] [Green Version]
- Massawe, F.; Mayes, S.; Cheng, A. Crop diversity: An unexploited treasure trove for food security. Trends Plant Sci. 2016, 21, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.Z.; Alberti, K.G.M. Introduction: Globalization and the non-communicable disease epidemic. Obesity 2006, 14, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E. Benefits of the Mediterranean diet: Insights from the PREDIMED study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Dogan, Y. Wild edible plants: From the past to the future. Austin Food Sci. 2016, 1, 1006. [Google Scholar]
- Shelef, O.; Guy, O.; Solowey, E.; Kam, M.; Degen, A.A.; Rachmilevitch, S. Domestication of plants for sustainable agriculture in drylands: Experience from the Negev Desert. Arid Land Res. Manag. 2016, 30, 209–228. [Google Scholar] [CrossRef]
- Leonti, M.; Casu, L. Traditional medicines and globalization: Current and future perspectives in ethnopharmacology. Front. Pharmacol. 2013, 4, 92. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, M.; Jäger, A. Ethnopharmacolgy; John Wiley & Sons Ltd.: Chichester, West Sussex, UK, 2015. [Google Scholar]
- De-Cortes Sánchez-Mata, M.; Tardío, J. (Eds.) Mediterranean Wild Edible Plants: Ethnobotany and Food Composition Tables; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Benvenuti, S.; Maggini, R.; Pardossi, A. Agronomic, nutraceutical, and organoleptic performances of wild herbs of ethnobotanical tradition. Int. J. Veg. Sci. 2017, 23, 270–281. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Barata, A.M. The consumption of wild edible plants. In Wild Plants, Mushrooms and Nuts: Functional Food Properties and Applications; Ferreira, I.C.F.R.P., Morales, P., Barros, L., Eds.; Wiley-Blackwell: Chichester, UK, 2017; pp. 159–198. [Google Scholar]
- Dwivedi, S.L.; Van Bueren, E.T.L.; Ceccarelli, S.; Grando, S.; Upadhyaya, H.D.; Ortiz, R. Diversifying food systems in the pursuit of sustainable food production and healthy diets. Trends Plant Sci. 2017, 22, 842–856. [Google Scholar] [CrossRef] [Green Version]
- Ceccanti, C.; Landi, M.; Benvenuti, S.; Pardossi, A.; Guidi, L. Mediterranean wild edible plants: Weeds or “new functional crops”? Molecules 2018, 23, 2299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renna, M. Wild edible plants as a source of mineral elements in the daily diet. Progr. Nutr. 2017, 19, 219–222. [Google Scholar]
- Barros, L.; Morales, P.; Carvalho, A.M.; Ferreira, I.C. Antioxidant potential of wild food plants. In Mediterranean Wild Edible Plants: Ethnobotany and Food Composition Tables; De-Cortez Sánchez-Mata, M., Tardio, J., Eds.; Springer: New York, NY, USA, 2015; pp. 209–232. [Google Scholar]
- Zhu, F.; Du, B.; Xu, B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Küpeli, E.; Tatli, I.I.; Akdemir, Z.S.; Yesilade, E. Estimation of antinociceptive and antiinflammatory activity of Geranium pretense subsp finitum and its phenolic compounds. J. Ethnopharmacol. 2007, 114, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Li, Y.Y.; Li, L.J.; Liang, L.Y.; Shen, Y.M. Anti-inflammatory activities of fractions from Geranium nepalense and related polyphenols. Drug Discov. Ther. 2012, 6, 194–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velázquez-González, C.; Cariño-Cortes, R.; De Lucio, J.; Ortiz, M.I.; Arciniega, M.D.O.; Altamirano-Baez, D.A.; Jimenez-Angeles, L.; Bautista-Avila, M. Anti-nociceptive and anti-inflammatory activities of Geranium bellumandits isolated compounds. BMC Complement. Altern. Med. 2014, 14, 506. [Google Scholar] [CrossRef]
- Bilić, V.L.; Rodríguez, J.V.; Grubešić, R.J.; Kremer, D.; Juričić, Ž. Quantitative analysis of polyphenols and antioxidant activity of Croatian populations of Erodium cicutarium (L.) L’Herit (Geraniaceae). Planta Med. 2016, 82, P498. [Google Scholar]
- Shim, J.U.; Oh, P.S.; Lim, K.T. Anti-inflammatory activity of ethanol extract from Geranium sibiricum Linne. J. Ethnopharmacol. 2009, 126, 90–95. [Google Scholar] [CrossRef]
- Fecka, I.; Kowalczyk, A.; Cisowski, W. Phenolic acids and depsides from some species of the Erodium genera. Z. Naturforsch C 2001, 56, 943–950. [Google Scholar] [CrossRef]
- Lakhdari, W.; Dehliz, A.; Acheuk, F.; Mlik, R.; Hammi, H.; Doumandji-Mitiche, B.; Gheriani, S.; Berrekbia, M.; Guermit, K.; Chergui, S. Ethnobotanical study of some plants used in traditional medicine in the region of Oued Righ (Algerian Sahara). J. Med. Plants Stud. 2016, 4, 204–211. [Google Scholar]
- Busso, C.A.; Lobartini, J.C. Mineral composition of Medicago minima and Erodium cicutarium under various water regimes. Commun. Soil Sci. Plant Anal. 2004, 35, 2243–2267. [Google Scholar] [CrossRef]
- Lisbalchin, M.; Guittonneau, G.G. Preliminary investigations on the presence of alkaloids in the genus Erodium Lher (Geraniaceae). Acta Bot. Gall. 1995, 142, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Helmstadter, A. Ethnopharmacology in the work of Melville William Hilton-Simpson (1881–1938)—Historical analysis and current research opportunities. Pharmazie 2016, 71, 352–360. [Google Scholar] [PubMed]
- Brullo, S.; Giusso del Galdo, G.; Guarino, R. Phytosociological notes on Lygeum Spartum grasslands from Crete. Lazaroa 2002, 23, 65–72. [Google Scholar]
- Bergmeier, E.; Kypriotakis, Z.; Jahn, R.; Böhling, N.; Dimopoulos, P.; Raus, T.; Tzanoudakis, D. Flora and phytogeographical significance of the islands Chrisi, Koufonisi and nearby islets (S Aegean, Greece). Willdenowia 2001, 31, 329–356. [Google Scholar] [CrossRef] [Green Version]
- El-Mokasabi, F.M. Floristic composition and traditional uses of plant species at Wadi Alkuf, Al-Jabal Al-Akhder, Libya. Am.-Eurasian J. Agric. Environ. Sci. 2014, 14, 685–697. [Google Scholar]
- Sharawy, S.M.; Badr, A. Systematic revision of Erodium species in Egypt as reflected by variation in morphological characters and seed protein electrophoretic profiles. Int. J. Bot. 2008, 4, 225–230. [Google Scholar]
- Bidak, L.M.; Kamal, S.A.; Halmy, M.W.; Heneidy, S.Z. Goods and services provided by native plants in desert ecosystems: Examples from the northwestern coastal desert of Egypt. Glob. Ecol. Conserv. 2015, 3, 433–447. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Wahab, R.H. Condition assessment of plant diversity of Gebel Maghara, North Sinai, Egypt. Catrina 2008, 3, 21–40. [Google Scholar]
- Hand, R. Supplementary notes to the flora of Cyprus VIII. Willdenowia 2015, 245–259. [Google Scholar] [CrossRef] [Green Version]
- Danin, A. Flora and vegetation of Israel and adjacent areas. In The Zoogeography of Israel; Springer: Drodrecht, The Netherlands, 1988; pp. 18–42. [Google Scholar]
- Osman, A.K.; Al-Ghamdi, F.; Bawadekji, A. Floristic diversity and vegetation analysis of Wadi Arar: A typical desert Wadi of the Northern Border region of Saudi Arabia. Saudi J. Biol. Sci. 2014, 21, 554–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbo, S.; Gopher, A.; Lev-Yadun, S. The domestication of crop plants. In Encyclopedia of Applied Plant Sciences; Thomas, B., Murray, B.G., Murphy, D.J., Eds.; Academic Press: Waltham, MA, USA, 2017; Volume 3, pp. 50–54. [Google Scholar]
- USDA. Carrot. 2020. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/787522/nutrients (accessed on 20 July 2020).
- Yang, C.S.; Wang, H. Cancer preventive activities of tea catechins. Molecules 2016, 21, 1679. [Google Scholar] [CrossRef] [PubMed]
- Mangels, D.R.; Mohler, E.R., III. Catechins as potential mediators of cardiovascular health. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Kopustinskiene, D.M. The role of catechins in cellular responses to oxidative stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choubey, S.; Goyal, S.; Varughese, L.R.; Kumar, V.; Sharma, A.K.; Beniwal, V. Probing gallic acid for its broad-spectrum applications. Mini Rev. Med. Chem. 2018, 18, 1283–1293. [Google Scholar] [CrossRef]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Mazibuko-Mbeje, S.E. Inflammation and oxidative stress in an obese state and the protective effects of gallic acid. Nutrients 2019, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Liu, A.; Xiong, W.; Lin, H.; Xiao, W.; Huang, J.; Liu, Z. Catechins enhance skeletal muscle performance. Crit. Rev. Food Sci. Nutr. 2020, 60, 515–528. [Google Scholar] [CrossRef]
- Koltai, H.; Kapulnik, Y.; Fridlender, M.; Gati, E.M.; Ahmed, N.; Shemer, B.E.N. U.S. Patent Application No. 15/563,595, 2018. Erodium crassifolium L’Her plant extracts and uses thereof. Filing date: 31/03/2016.
- Yadav, R.P.; Tarun, G. Versatility of turmeric: A review the golden spice of life. J. Pharmacogn. Phytochem. 2017, 6, 41–46. [Google Scholar]
- Ladizinsky, G. Plant Evolution under Domestication; Springer: Drodrecht, The Netherlands, 1998. [Google Scholar]
- Purugganan, M.D.; Fuller, D.Q. The nature of selection during plant domestication. Nature 2009, 457, 843–848. [Google Scholar] [CrossRef]
- Aronson, J.; Shmida, A. Plant species diversity along a Mediterranean-desert gradient and its correlation with interannual rainfall fluctuations. J. Arid Environ. 1992, 23, 235–247. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoop, V.M.; Fusenig, N.E.; Mirancea, N. Epidermal organization and differentiation of HaCaT keratinocytes in organotypic coculture with human dermal fibroblasts. J. Investig. Dermatol. 1999, 112, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Stieger, M.; Yawalkar, N.; Kakeda, M. Cytokines and chemokines in irritant contact dermatitis. Mediators Inflamm. 2013, 916497. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yang, X.; Wang, H.; Zhao, B.; Wu, X.; Su, L.; Xie, S.; Wang, Y.; Li, J.; Liu, J.; et al. PKCζ as a promising therapeutic target for TNFα-induced inflammatory disorders in chronic cutaneous wounds. Int. J. Mol. Med. 2017, 40, 1335–1346. [Google Scholar] [CrossRef]
- Mizuno, K.; Morizane, S.; Takiguchi, T.; Iwatsuki, K. Dexamethasone but not tacrolimus suppresses TNF-α-induced thymic stromal lymphopoietin expression in lesional keratinocytes of atopic dermatitis model. J. Dermatol. Sci. 2015, 80, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Wufuer, M.; Lee, G.; Hur, W.; Jeon, B.; Kim, B.J.; Choi, T.H.; Lee, S. Skin-on-a-chip model simulating inflammation, edema and drug-based treatment. Sci. Rep. 2016, 6, 37471. [Google Scholar] [CrossRef] [Green Version]






| Nutritional Profile | Measure Units /100 g | HSB Tubers | Carrot |
|---|---|---|---|
| Water | g | 90.6 | 88.3 |
| Ash | 0.8 | 0.9 | |
| Caloric value | Kcal | 23 | 41 |
| Carbohydrates | g | 7.9 | 9.6 |
| Sugars | 4.3 | 6 | |
| Polysaccharides | 3.0 | 2.8 | |
| Lipids | 0.1 | 0.24 | |
| Protein | 0.6 | 0.93 | |
| Potassium | mg | 223.4 | 320 |
| Calcium | 74.3 | 33 | |
| Phosphorus | 28.0 | 35 | |
| Sulfur | 27.4 | ||
| Magnesium | 20.0 | 12 | |
| Sodium | 18.9 | 69 | |
| Iron | 2.2 | 0.3 | |
| Zinc | 0.4 | 0.24 | |
| Vitamin A | μg | <200 | 835 |
| Vitamin C (ascorbic acid) | mg | 2.03 | 5.9 |
| A-tocopherol (vitamin E) | IU | <1 |
| Compound | RT (min) | Percentage (%) from Total Amount |
|---|---|---|
| Mannofuranose | 28.024 | 17.2 |
| α-D-xylopyranose | 29.047 | 2.7 |
| Gallic acid | 29.766 | 5.7 |
| Palmitic acid | 30.853 | 6.4 |
| Stearic acid | 33.831 | 2.0 |
| Trans-catechin | 42.166 | 11.7 |
| Cis-catechin | 42.435 | 12.8 |
| Epigallocatechin | 43.007 | 41.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen, S.; Koltai, H.; Selvaraj, G.; Mazuz, M.; Segoli, M.; Bustan, A.; Guy, O. Assessment of the Nutritional and Medicinal Potential of Tubers from Hairy Stork’s-Bill (Erodium crassifolium L ’Hér), a Wild Plant Species Inhabiting Arid Southeast Mediterranean Regions. Plants 2020, 9, 1069. https://doi.org/10.3390/plants9091069
Cohen S, Koltai H, Selvaraj G, Mazuz M, Segoli M, Bustan A, Guy O. Assessment of the Nutritional and Medicinal Potential of Tubers from Hairy Stork’s-Bill (Erodium crassifolium L ’Hér), a Wild Plant Species Inhabiting Arid Southeast Mediterranean Regions. Plants. 2020; 9(9):1069. https://doi.org/10.3390/plants9091069
Chicago/Turabian StyleCohen, Shabtai, Hinanit Koltai, Gopinath Selvaraj, Moran Mazuz, Moran Segoli, Amnon Bustan, and Ofer Guy. 2020. "Assessment of the Nutritional and Medicinal Potential of Tubers from Hairy Stork’s-Bill (Erodium crassifolium L ’Hér), a Wild Plant Species Inhabiting Arid Southeast Mediterranean Regions" Plants 9, no. 9: 1069. https://doi.org/10.3390/plants9091069