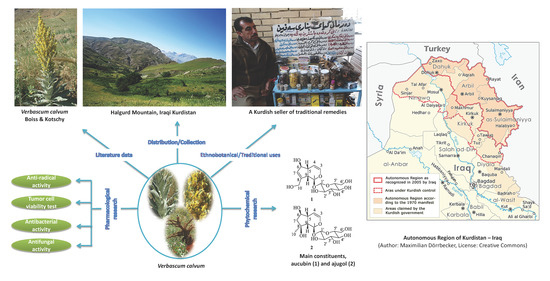
Phytochemistry of Verbascum Species Growing in Iraqi Kurdistan and Bioactive Iridoids from the Flowers of Verbascum calvum
Abstract
:1. Introduction
2. Literature Data about the Verbascum Species Growing in Kurdistan
3. Phytochemical Studies on Vebascum Calvum
3.1. General Experimental Techniques and Procedures
3.2. Plant Material
3.3. Extraction of V. Calvum Flowers
3.4. Preliminary Phytochemical Analysis of Residues B and C
3.5. Preliminary Chromatographic Purification of Residues B and C
3.6. Folin–Ciocalteu Assay
3.7. Anti-radical Activity Test
3.8. Antibacterial Activity Test
3.9. Antifungal Activity Test
3.10. Tumor Cell Viability Test (MTS Assay)
3.11. Chromatographic Separation of Residue B’
3.12. Spectroscopic Data of Compounds 43 (Aucubin) and 42 (Ajugol)
3.13. Acidic Hydrolysis of Compounds 42 and 43
4. Results and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Solecki, R.S. Shanidar IV, a Neanderthal flower burial in Northern Iraq. Science 1975, 190, 880–881. [Google Scholar] [CrossRef]
- Amin, H.I.M.; Ibrahim, M.F.; Hussain, F.H.S.; Sardar, A.S.; Vidari, G. Phytochemistry and ethnopharmacology of some medicinal plants used in the Kurdistan region of Iraq. Nat. Prod. Commun. 2016, 11, 291–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdullah, F.O.; Hussain, F.H.S.; Sardar, A.S.; Vita-Finzi, P.; Vidari, G. Phytochemistry and ethnopharmacology of medicinal plants used on Safeen Mountain in the Kurdistan region of Iraq. Nat. Prod. Commun. 2016, 11, 1923–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mati, E.; de Boer, H. Ethnobotany and trade of medicinal plants in the Qaysari Market, Kurdish autonomous region, Iraq. J. Ethnopharmacol. 2011, 133, 490–510. [Google Scholar] [CrossRef]
- Naqishbandi, A. Plants used in Iraqi traditional medicine in Erbil-Kurdistan region. Zanco J. Med. Sci. 2018, 18, 811–815. [Google Scholar] [CrossRef]
- Ahmed, H.M. Ethnopharmacobotanical study on the medicinal plants used by herbalists in Sulaymaniyah Province, Kurdistan, Iraq. J. Ethnobiol. Ethnomed. 2016, 12, 8. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, S.A.; Al-Habib, O.A.M.; Bugonl, S.; Clericuzio, M.; Vidari, G. A new ursane-type triterpenoid and other constituents from the leaves of Crataegus azarolus var. aronia. Nat. Prod. Commun. 2016, 11, 1637–1639. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.F.; Hussain, F.H.S.; Zanoni, G.; Vidari, G. The main constituents of Tulipa systola Stapf. roots and flowers; their antioxidant activities. Nat. Prod. Res. 2017, 31, 2001–2007. [Google Scholar] [CrossRef]
- Abdullah, F.O.; Hussain, F.H.; Clericuzio, M.; Porta, A.; Vidari, G. A new iridoid dimer and other constituents from the traditional Kurdish plant Pterocephalus nestorianus Nábělek. Chem. Biodivers. 2017, 14, e1600281. [Google Scholar] [CrossRef]
- Amin, H.I.M.; Amin, A.A.; Tosi, S.; Mellerio, G.G.; Hussain, F.H.S.; Picco, A.M.; Vidari, G. Chemical composition and antifungal activity of essential oils from flowers, leaves, rhizomes, and bulbs of the wild Iraqi Kurdish plant Iris persica. Nat. Prod. Commun. 2017, 12, 441–444. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, F.O.; Hussain, F.H.S.; Mannucci, B.; Lappano, R.; Tosi, S.; Maggiolini, M.; Vidari, G. Composition, antifungal and antiproliferative activities of the hydrodistilled oils from leaves and flower heads of Pterocephalus nestorianus Nábělek. Chem. Biodivers. 2017, 14, e1700009. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.I.M.; Hussain, F.H.S.; Maggiolini, M.; Vidari, G. Bioactive constituents from the traditional Kurdish plant Iris persica. Nat. Prod. Commun. 2018, 13, 1127–1128. [Google Scholar] [CrossRef] [Green Version]
- Kheder, D.A.; Al-Habib, O.A.M.; Gilardoni, G.; Vidari, G. Components of volatile fractions from Eucalyptus camaldulensis leaves from Iraqi–Kurdistan and their potent spasmolytic effects. Molecules 2020, 25, 804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Plant List. A Working List of All Known Plant Species. Version 1.1. Available online: http://www.theplantlist.org/ (accessed on 1 January 2013).
- Tatli, I.I.; Akadimir, Z.S. Traditional uses and biological activities of Verbascum species. FABAD J. Pharm. Sci. 2006, 31, 85–96. [Google Scholar]
- Al-Rawi, A. Wild Plants of Iraq with Their Distribution; Ministry of Agriculture & Irrigation, State Board for Agricultural & Water Resources Research, National Herbarium of Iraq: Baghdad, Iraq, 1964. [Google Scholar]
- Al-Bermani, A.K. Systematic Study of the Genus Verbascum (Scrophulareaceae) as it Occurs in Iraq. Master’s. Thesis, University of Baghdad, Baghdad, Iraq, 1981; pp. 139–141. [Google Scholar]
- Dalar, A.; Konczak, I. Botanicals from Eastern Anatolia region of Turkey: Antioxidant capacity and phenolic constituents of endemic herbal medicines. J. Herb. Med. 2012, 2, 126–135. [Google Scholar] [CrossRef]
- Dalar, A.; Guo, Y.; Konczak, I. Phenolic composition and potential anti-inflammatory properties of Verbascum cheiranthifolium var. cheiranthifolium leaf. J. Herb. Med. 2014, 4, 195–200. [Google Scholar] [CrossRef]
- Gürbüz, I.; Ozkan, A.M.; Yesilada, E.; Kutsal, O. Anti-ulcerogenic activity of some plants used in folk medicine of Pinarbasi (Kayseri, Turkey). J. Ethnopharmacol. 2005, 101, 313–318. [Google Scholar] [CrossRef]
- Küçük, S.; Özdemir, F.; İşcan, G.; İncesu, Z. Determination of cytotoxic and anticandidal activities of three Verbascum L. species from Turkey: V. cheiranthifolium Boiss. var. asperulum (Boiss.) Murb. Monorg., V. pycnostachyum Boiss. & Heldr and V. orgyale Boiss. & Heldr. Turk. J. Pharm. Sci. 2016, 13, 318–322. [Google Scholar]
- Khoshnoud, H.; Nemati, N.; Amirnia, R.; Ghiyasi, M.; Ghourttapeh, A.H.; Tajbakhsh, M.; Talati, F.; Salehzadeh, H. Insecticidal properties of Verbascum cheiranthifolium against R. dominica on wheat and barley. Pak. J. Biol. Sci. 2008, 11, 783–787. [Google Scholar] [CrossRef] [Green Version]
- Khoshnoud, H.; Ghiyasi, M.; Amirnia, R.; Fard, S.S.; Tajbakhsh, M.; Salehzadeh, H.; Alahyary, P. The potential of using insecticidal properties of medicinal plants against insect pests. Pak. J. Biol. Sci. 2008, 11, 1380–1384. [Google Scholar] [CrossRef]
- Kunduhoglu, B.; Pilatin, S.; Caliskan, F. Antimicrobial screening of some medicinal plants collected from Eskisehir, Turkey. Fresenius Environ. Bull. 2011, 20, 945–952. [Google Scholar]
- Eribekyan, M.I.; Arutyunyan, L.S.; Mnatsakanyan, V.A. Iridoid glycosides of Verbascum cheiranthifolium. Chem. Nat. Compd. 1989, 25, 622. [Google Scholar] [CrossRef]
- Nadiroğlu, M.; Behçet, L.; Çakılcıoğlu, U. An ethnobotanical survey of medicinal plants in Karlıova (Bingöl-Turkey). Indian J. Tradit. Knowl. 2019, 18, 76–87. [Google Scholar]
- Makhatova, B.G.; Datkhayev, U.M.; Makhatov, Z.B.; Orazbekov, Y.K. Antibacterial activity of Verbascum songaricum various extracts against Staphylococcus. New Armen. Med. J. 2017, 11, 67–69. [Google Scholar]
- Yanar, Y.; Kadioğlu, I.; Gökçe, A.; Demirtas, I.; Gören, N.; Çam, H.; Whalon, M. In vitro antifungal activities of 26 plant extracts on mycelial growth of Phytophthora infestans (Mont.) de Bary. Afr. J. Biotechnol. 2011, 10, 2625–2629. [Google Scholar]
- Seifert, K.; Preiss, A.; Johne, S.; Schmidt, J.; Lien, N.T.; Lavaud, C.; Massiot, G. Triterpene saponins from Verbascum songaricum. Phytochemistry 1991, 30, 3395–3400. [Google Scholar] [CrossRef]
- Hartleb, I.; Seifert, K. Songarosaponin D—A triterpenoid saponins from Verbascum songaricum. Phytochemistry 1994, 35, 1009–1011. [Google Scholar] [CrossRef]
- Hartleb, I.; Seifert, K. Triterpenoid saponins from Verbascum songaricum. Phytochemistry 1995, 38, 221–224. [Google Scholar] [CrossRef]
- Iida, A.; Kiuchi, F.; Ito, M.; Honda, G.; Mizushina, Y.; Yoshida, H.; Sarsenbaev, K. Phenylethanoid glycosides targeting mammalian DNA polymerases. Nat. Med. 2003, 54, 146–149. [Google Scholar]
- Bahmani, M.; Saki, K.; Shahsavari, S.; Rafieian-Kopaei, M.; Sepahvand, R.; Adineh, A. Identification of medicinal plants effective in infectious diseases in Urmia, Northwest of Iran. Asian Pac. J. Trop. Biomed. 2015, 5, 858–864. [Google Scholar] [CrossRef] [Green Version]
- Nofouzi, K.; Mahmudi, R.; Tahapour, K.; Amini, E.; Yousefi, K. Verbascum speciosum methanolic extract: Phytochemical components and antibacterial properties. J. Essent. Oil Bear. Plants 2016, 19, 499–505. [Google Scholar] [CrossRef]
- Asgarpanah, J.; Hashemi, S.J.; Hashemi, E.; Askari, K. In vitro antifungal activity of some traditional Persian medicinal plants on pathogenic fungi. Chin. J. Integr. Med. 2017, 23, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Kayır, S.; Demirci, Y.; Demirci, S.; Ertürk, E.; Ayaz, E.; Doğan, A.; Şahin, F.; Demirci, S. The in vivo effects of Verbascum speciosum on wound healing. S. Afr. J. Bot. 2018, 119, 226–229. [Google Scholar] [CrossRef]
- Vahedi, H.; Lari, J.; Halimi, M.; Nasrabadi, M.; Vahedi, A. Chemical composition of the n-hexane extract of Verbascum speciosum growing wild in Iran. J. Essent. Oil Bear. Plants 2012, 15, 895–899. [Google Scholar] [CrossRef]
- CABI. Verbascum thapsus (Common mullein). Available online: https://www.cabi.org/isc/datasheet/56652#:~:text=Native%20to%20Asia%20and%20Europe,as%20′cowboy%20toilet%20paper’ (accessed on 13 July 2019).
- Turker, A.U.; Camper, N.D. Biological activity of common mullein, a medicinal plant. J. Ethnopharmacol. 2002, 82, 117–125. [Google Scholar] [CrossRef]
- Riaz, M.; Zia-Ul-Haq, M.; Jaafar, H.Z.E. Common mullein, pharmacological and chemical aspects. Rev. Bras. Farmacogn. 2013, 23, 948–959. [Google Scholar] [CrossRef] [Green Version]
- Turker, A.U.; Gurel, E. Common mullein (Verbascum thapsus L.): Recent advances in research. Phytother. Res. 2005, 19, 733–739. [Google Scholar] [CrossRef]
- Mohd, A.D.; Mohammad, F.B.; Reyaz, H.; Mubashir, H.M.; Showkat, R.M.; Roohi, M. Extensive phytochemistry, comprehensive traditional uses, and critical pharmacological profile of the great mullein: Verbascum thapsus L. Nat. Prod. J. 2019, 9, 158–171. [Google Scholar] [CrossRef]
- Drugs. com. Mullein. Available online: https://www.drugs.com/npp/mullein.html (accessed on 13 July 2019).
- Benítez, G.; González-Tejero, M.R.; Molero-Mesa, J. Knowledge of ethnoveterinary medicine in the province of Granada, Andalusia, Spain. J. Ethnopharmacol. 2012, 139, 429–439. [Google Scholar] [CrossRef]
- Derakhshanfar, A.; Moayedi, J.; Derakhshanfar, G.; Poostforoosh Fard, A. The role of Iranian medicinal plants in experimental surgical skin wound healing: An integrative review. Iran. J. Basic Med. Sci. 2019, 22, 590–600. [Google Scholar] [CrossRef]
- Ullah, M.; Khan, M.U.; Mahmood, A.; Malik, R.N.; Hussain, M.; Wazir, S.M.; Daud, M.; Shinwari, Z.K. An ethnobotanical survey of indigenous medicinal plants in Wana district South Waziristan agency, Pakistan. J. Ethnopharmacol. 2013, 150, 918–924. [Google Scholar] [CrossRef]
- De Rus Jacquet, A.; Timmers, M.; Ma, S.Y.; Thieme, A.; McCabe, G.P.; Vest, J.H.C.; Lila, M.A.; Rochet, J.-C. Lumbee traditional medicine: Neuroprotective activities of medicinal plants used to treat Parkinson’s disease-related symptoms. J. Ethnopharmacol. 2017, 206, 408–425. [Google Scholar] [CrossRef]
- McCune, L.M.; Johns, T. Symptom-specific antioxidant activity of boreal diabetes treatments. Pharm. Biol. 2003, 41, 362–370. [Google Scholar] [CrossRef]
- McCune, L.M.; Johns, T. Antioxidant activity relates to plant part, life form and growing condition in some diabetes remedies. J. Ethnopharmacol. 2007, 112, 461–469. [Google Scholar] [CrossRef]
- McCune, L.M.; Johns, T. Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus used by the indigenous peoples of the North American Boreal forest. J. Ethnopharmacol. 2002, 82, 197–205. [Google Scholar] [CrossRef]
- Miara, M.D.; Bendif, H.; Rebbas, K.; Rabah, B.; Hammou, M.A.; Maggi, F. Medicinal plants and their traditional uses in the highland region of Bordj Bou Arreridj (Northeast Algeria). J. Herb. Med. 2019, 16, 100262. [Google Scholar] [CrossRef]
- Shah, A.; Bharati, K.A.; Ahmad, J.; Sharma, M.P. New ethnomedicinal claims from Gujjar and Bakerwals tribes of Rajouri and Poonch districts of Jammu and Kashmir, India. J. Ethnopharmacol. 2015, 166, 119–128. [Google Scholar] [CrossRef]
- Sharma, J.; Gairola, S.; Sharma, Y.P.; Gaur, R.D. Ethnomedicinal plants used to treat skin diseases by Tharu community of district Udham Singh Nagar, Uttarakhand, India. J. Ethnopharmacol. 2014, 158, 140–206. [Google Scholar] [CrossRef]
- Murad, W.; Ahmad, A.; Gilani, S.A.; Khan, M.A. Indigenous knowledge and folk use of medicinal plants by the tribal communities of Hazar Nao Forest, Malakand district, North Pakistan. J. Med. Plant Res. 2011, 5, 1072–1086. [Google Scholar]
- Verma, J.; Thakur, K.; Kusum. Ethnobotanically important plants of Mandi and Solan districts of Himachal Pradesh, Northwest Himalaya. Plant Arch. 2012, 12, 185–190. [Google Scholar]
- Mihailović, V.; Kreft, S.; Benković, E.T.; Ivanović, N.; Stanković, M.S. Chemical profile, antioxidant activity and stability in stimulated gastrointestinal tract model system of three Verbascum species. Ind. Crops Prod. 2016, 89, 141–151. [Google Scholar] [CrossRef]
- Dulger, G.; Tutenocakli, T.; Dulger, B. Anti-staphylococcal activity of Verbascum thapsus L. against methicillin-resistant Staphylococcus aureus. Konuralp Med. J. 2017, 9, 53–57. [Google Scholar]
- Ghasemi, F.; Rezaei, F.; Araghi, A.; Abouhosseini Tabari, M. Antimicrobial activity of aqueous-alcoholic extracts and the essential oil of Verbascum thapsus L. Jundishapur J. Nat. Pharm. Prod. 2015, 10, e23004. [Google Scholar] [CrossRef] [Green Version]
- Sepahi, S.; Ghorani-Azam, A.; Sepahi, S.; Asoodeh, A.; Rostami, S. In vitro study to evaluate antibacterial and non-haemolytic activities of four Iranian medicinal plants. West Indian Med. J. 2014, 63, 289–293. [Google Scholar]
- Escobar, F.M.; Sabini, M.C.; Zanon, S.M.; Tonn, C.E.; Sabini, L.I. Antiviral effect and mode of action of methanolic extract of Verbascum thapsus L. on pseudorabies virus (strain RC/79). Nat. Prod. Res. 2012, 26, 1621–1625. [Google Scholar] [CrossRef]
- Amber, R.; Adnan, M.; Tariq, A.; Mussarat, S. A review on antiviral activity of the Himalayan medicinal plants traditionally used to treat bronchitis and related symptoms. J. Pharm. Pharmacol. 2017, 69, 109–122. [Google Scholar] [CrossRef]
- Jassim, S.A.A.; Naji, M.A. Novel antiviral agents: A medicinal plant perspective. J. Appl. Microbiol. 2003, 95, 412–427. [Google Scholar] [CrossRef] [Green Version]
- McCutcheon, A.R.; Roberts, T.E.; Gibbons, E.; Ellis, S.M.; Babiuk, L.A.; Hancock, R.E.W.; Towers, G.H.N. Antiviral screening of British Columbian medicinal plants. J. Ethnopharmacol. 1995, 49, 101–110. [Google Scholar] [CrossRef]
- Ali, N.; Ali Shah, S.W.; Shah, I.; Ahmed, G.; Ghias, M.; Khan, I.; Ali, W. Anthelmintic and relaxant activities of Verbascum thapsus mullein. BMC Complement Altern. Med. 2012, 12, 29. [Google Scholar] [CrossRef] [Green Version]
- Riaz, M.; Rahman, N.; Zia-Ul-Haq, M. Anthelmintic and insecticidal activities of Verbascum thapsus L. Pak. J. Zool. 2013, 45, 1593–1598. [Google Scholar]
- Samani, Z.N.; Kopaei, M.R. Effective medicinal plants in treating hepatitis B. Int. J. Pharm. Sci. Res. 2018, 9, 3589–3596. [Google Scholar] [CrossRef]
- Lin, L.-T.; Liu, L.-T.; Chiang, L.-C.; Lin, C.-C. In vitro anti-hepatoma activity of fifteen natural medicines from Canada. Phytother. Res. 2002, 16, 440–444. [Google Scholar] [CrossRef]
- Kashan, Z.F.; Delavari, M.; Arbabi, M.; Hooshyar, H. Therapeutic effects of Iranian herbal extracts against Trichomonas vaginalis. Iran. Biomed. J. 2017, 21, 285–293. [Google Scholar]
- Mehriardestani, M.; Aliahmadi, A.; Toliat, T.; Rahimi, R. Medicinal plants and their isolated compounds showing anti-Trichomonas vaginalis-activity. Biomed. Pharmacother. 2017, 88, 885–893. [Google Scholar] [CrossRef]
- Kheiri Manjili, H.; Jafari, H.; Ramazani, A.; Davoudi, N. Anti-leishmanial and toxicity activities of some selected Iranian medicinal plants. Parasitol. Res. 2012, 111, 2115–2121. [Google Scholar] [CrossRef]
- Zhao, Y.-L.; Wang, S.-F.; Li, Y.; He, Q.-X.; Liu, K.-C.; Yang, Y.-P.; Li, X.-L. Isolation of chemical constituents from the aerial parts of Verbascum thapsus and their antiangiogenic and antiproliferative activities. Arch. Pharm. Res. 2011, 34, 703–707. [Google Scholar] [CrossRef]
- Mehrotra, R.; Ahmed, B.; Vishwakarma, R.A.; Thakur, R.S. Verbacoside: A new luteolin glycoside from Verbascum thapsus. J. Nat. Prod. 1989, 52, 640–643. [Google Scholar] [CrossRef]
- Hussain, H.; Aziz, S.; Miana, G.A.; Ahmad, V.U.; Anwar, S.; Ahmed, I. Minor chemical constituents of Verbascum thapsus. Biochem. Syst. Ecol. 2009, 37, 124–126. [Google Scholar] [CrossRef]
- Khuroo, M.A.; Qureshi, M.A.; Razdan, T.K.; Nichols, P. Sterones, iridoids and a sesquiterpene from Verbascum thapsus. Phytochemistry 1988, 27, 3541–3544. [Google Scholar] [CrossRef]
- Warashina, T.; Miyase, T.; Ueno, A. Iridoid glycosides from Verbascum thapsus L. Chem. Pharm. Bull. 1991, 39, 3261–3264. [Google Scholar] [CrossRef] [Green Version]
- Pardo, F.; Perich, F.; Torres, R.; Monache, F.D. Phytotoxic iridoid glucosides from the roots of Verbascum thapsus. J. Chem. Ecol. 1998, 24, 645–653. [Google Scholar] [CrossRef]
- Warashina, T.; Miyase, T.; Ueno, A. Phenylethanoid and lignan glycosides from Verbascum thapsus. Phytochemistry 1992, 31, 961–965. [Google Scholar] [CrossRef]
- Frezza, C.; Bianco, A.; Serafini, M.; Foddai, S.; Salustri, M.; Reverberi, M.; Gelardi, L.; Bonina, A.; Bonina, F.P. HPLC and NMR analysis of the phenyl-ethanoid glycosides pattern of Verbascum thapsus L. cultivated in the Etnean area. Nat. Prod. Res. 2019, 33, 1310–1316. [Google Scholar] [CrossRef]
- Speranza, L.; Franceschelli, S.; Pesce, M.; Reale, M.; Menghini, L.; Vinciguerra, I.; De Lutiis, M.A.; Felaco, M.; Grilli, A. Antiinflammatory effects in THP-1 cells treated with verbascoside. Phytother. Res. 2010, 24, 1398–1404. [Google Scholar] [CrossRef]
- Gilardoni, G.; Chiriboga, X.; Vita Finzi, P.; Vidari, G. New 3,4-secocycloartane and 3,4-secodammarane triterpenes from the Ecuadorian plant Coussarea macrophylla. Chem. Biodivers. 2015, 12, 946–954. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substances by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Gaggeri, R.; Rossi, D.; Hajikarimian, N.; Martino, E.; Bracco, F.; Grisoli, P.; Dacarro, C.; Leoni, F.; Mascheroni, G.; Collina, S.; et al. Preliminary study on TNFα-blocker activity of Amygdalus lycioides Spach extracts. Open Nat. Prod. J. 2010, 3, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Cockerill, F. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard, 10th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015; ISBN 978-1562387839. OCLC 932608948. [Google Scholar]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard, 3rd ed.; M27-A3; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2008; Volume 28. [Google Scholar]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous fungi; Approved Standard, 2nd ed.; M38-A2; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2008; Volume 28. [Google Scholar]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assays Guidance Manual [Internet]; Sittampalam, G.S., Grossman, A., Brimacombe, K., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004–2016. [Google Scholar]
- Ersöz, T.; Berkman, M.Z.; Taşdemir, D.; Çaliş, I.; Ireland, C.M. Iridoid and Phenylethanoid Glycosides from Euphrasia pectinata. Turk. J. Chem. 2002, 26, 179–188. [Google Scholar]
- Akdemir, Z.; Çali, H.; Junior, P. Iridoid and phenylpropanoid glycosides from Pedicularis condensata. Phytochemistry 1991, 30, 2401–2402. [Google Scholar] [CrossRef]
- Pianaro, A.; Pereira Pinto, J.; Trevisan Ferreira, D.; Ishikawa, N.K.; Braz-Filho, R. Iridoid glucoside and antifungal phenolic compounds from Spathodea campanulata roots. Semin. Ciências Agrárias Londrina 2007, 28, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Hansen, S.A. Thin-layer chromatographic method for the identification of mono-, di- and trisaccharides. J. Chromatogr. 1975, 107, 224–226. [Google Scholar] [CrossRef]
- Young, C.N.; Koepke, J.I.; Terlecky, L.J.; Borkin, M.S.; Boyd, S.L.; Terlecky, S.R. Reactive oxygen species in tumor necrosis factor-a-activated primary human keratinocytes: Implications for psoriasis and inflammatory skin disease. J. Investig. Dermatol. 2008, 128, 2606–2614. [Google Scholar] [CrossRef] [Green Version]
- Dinda, B.; Chowdhury, D.R.; Mohanta, B.C. Naturally occurring iridoids, secoiridoids and their bioactivity. An updated review, part 3. Chem. Pharm. Bull. 2009, 57, 765–796. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Guo, F.; Ouyang, D. A review of the pharmacology and toxicology of aucubin. Fitoterapia 2020, 140, 104443. [Google Scholar] [CrossRef]
- Lee, S.W.; Kho, H.S.; Lee, S.G. The effects of iridoid compounds on wound healing. J. Korean Acad. Oral Med. 1999, 24, 137–142. [Google Scholar]
- Kahraman, Ç.; Tatli, İ.İ.; Kart, D.; Ekizoğlu, M.; Akdemir, Z.Ş. Structure elucidation and antimicrobial activities of secondary metabolites from the flowery parts of Verbascum mucronatum Lam. Turk. J. Pharm. Sci. 2018, 15, 231–237. [Google Scholar] [CrossRef]











| Species | Traditional Use | Biological Activity | Metabolites |
|---|---|---|---|
| Verbascum cheiranthifolium Boiss. | Rheumatism, eczema, earache, menstrual pains, haemorrhoids, oedema, earache and arthralgia [18] | Antioxidant [18], anti-inflammatory [19], anti-ulcerogenic [20], cytotoxic [21], insecticide [22,23], and antimicrobial effects [24]. | Aucubin (43), catalpol, 6-O-(E)-coumaroylaucubin, and 6-O-[(E)-p-methoxycinnamoyl]aucubin (1) [25] |
| Verbascum songaricum Schrenk | Emmenagogue and to cure infertility [26] | antibacterial [27] and antifungal activities [28] | 3-O-{[α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranosyl-(1→3)]-[β-D-glucopyranosyl-(1→2)]-β-D-fucopyranosyl}-olea-11,13-diene-3β,23,28-triol (songarosaponin A) (2), 3-O-{[α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranosyl-(1→3)]-[β-D-glucopyranosyl-(1→2)]-β-D-fuco-pyranosyl}-olea-11-ene-3β,13,23,28-tetrol (songarosaponin B), 3-O-{[β-D-glucopyranosyl-(1→4)]-[β-D-glucopyranosyl-(1→3)]-[β-D-glucopyranosyl-(1→2)]-β-D-fucopyranosyl}-13β,28-epoxyolea-11-ene-3β,23-diol (songarosaponin C), 3-O-{[β-D-glucopyranosyl-(1→4)]-[β-D-glucopyranosyl-(1→3)]-[β-D-glucopyranosyl-(1→2)]-β-D-fucopyranosyl}-13β,28-epoxyolea-11-ene-3β,16β,23-triol (songarosaponin D), 3-O-{[β-D-glucopyranosyl-(l→4)-β-D-glucopyranosyl-(1→3)]-[β-D-glucopyranosyl-(1→2)]-β-D-fucopyranosyl}-olea-11,13-diene-3β,23,28-triol (songarosaponin E), 3-O-{[β-D-glucopyranosyl-(1→4)-β-D-glucopyranosyl-(1→3)]-[β-D-glucopyranosyl-(1→2)]-β-D-fucopyranosyl}-olea-11,13-diene-3β,16β,23,28-tetrol (songarosaponin F), and 3-O-{[α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranosyl-(1→3)]-[β-D-glucopyranosyl-(1→2)]-β-D-fucopyra- nosyl}-13β,28-epoxyolea-11-ene-3β,16β,23-triol (buddlejasaponin I) [29,30,31]. Poliumoside and verbascoside (3) inhibited mammalian DNA polymerases [32] |
| Verbascum speciosum Schrad. | Skin diseases and wound bacterial infection [33] | insecticidal [33], antibacterial [34], antifungal [35] and wound healing potential [36] | Palmitic and oleic acids, (3β,5α)-stigmasta-7,25-dien-3-ol [37] |
| Verbascum thapsus L. [38] | Although mullein has a long history as a favored herbal remedy for the treatment of many disorders [39,40,41,42], high-quality clinical researches have not been conducted so far, and there is no approved drug from this plant [43]. The traditional uses of V. thapsus have generally focused on effects aimed at wound healing [44,45,46], and treating Parkinson’s disease [47], diabetes [48,49,50], bronchitis [51], stomachache [52], snake bites [52], spasmodic cough [46], skin diseases [53], asthma [54], and joint pains [54]. The plant is also used as astringent [46] and sedative remedy [55]. | antioxidant [49,50,56], antibacterial [39,57,58,59], antiviral [60,61,62,63], anthelmintic [64,65], antihepatitis [66,67], anti-trichomonas [68,69], and anti-leishmanial effects [70]. | Luteolin (4) [71], apigetrin (apigenin 7-O-glucoside) (5) [71], 5-O-α-L-rhamnopyranoyl(1→3)-[β-D-glucuronopyranosyl-1→6)]-β-D-glucopyranosyl luteolin [72], and the dimer amentoflavone (6) [73]. verbathasin A (7) [71], ningpogenin (8) [71], 10-deoxyeucommiol (9) [71], jioglutolide (10) [71], 6β-hydroxy-2-oxabicyclo[4.3.0]Δ8-9-nonen-1-one (11) [71], (+)-genipin (12) [73], α-gardiol (13) and β-gardiol (14) [73]. Other characteristic terpenoids are buddlindeterpene A-C (15–17) [73], and 3α-hydroxy-drimmanyl-8-methanoate [74]. Iridoid glycosides include lateroside (18) [73,75,76], harpagoside (19) [71,73,75,76], compounds 20–41 [75], ajugol (42) [71,73,75,76], aucubin (43) [74,76], 6-O-β-xyloxyl aucubin [74], 8-cinnamoylmyoporoside (44) [71] and picroside IV (45) [73]. Lignan glycosides include compounds 46–50 [77], whereas phenylethanoid glycosides include compounds 51–56 [77], alyssonoside (57) [77,78], leucosceptoside B (58) [77,78], verbacoside (3) [73,78], leucosceptoside A (59) [78], martynoside (60) [78], samioside (61) [78], and isoverbascoside (62) [78]. Sterones and saponins include 24α-methyl-5α-cholestan-3-one, 24-ξ-ethyl-5α-cholestan-22-en-3-one, 24-ξ-ethyl-5β-cholestan-22-en-3-one, 24-ξ-ethyl-5α-cholestan-7-en-3-one, 24α-ethyl-5α-cholestan-Δ7,22-dien-3-one, 24-ξ-ethyl-5α-cholestan-3-one, 24-ξ-ethyl-5β-cholestan-3-one [74], and 3-O-fucopyranosylsaikogenin F (63) [71].Verbascoside (3) exhibited anti-inflammatory properties [79], whereas luteolin (4) and saponin 63 induced apoptosis of A549 lung cancer cells [71]. |
| Sample. | Candida albicans | Aspergillus niger | Microsporum canis | Bipolaris oryzae |
|---|---|---|---|---|
| Extract B’ | >5 mg/mL | >5 mg/mL | 1 mg/mL | >1 mg/mL |
| Extract C’ | >5 mg/mL | >5 mg/mL | - | >1 mg/mL |
| Cell Line | 600 µg/mL | 60 µg/mL | 6 µg/mL | 0.6 µg/mL | 0.06 µg/mL |
|---|---|---|---|---|---|
| A549 | 48.43 | 44.95 | 14.69 | ̶ | ̶ |
| MCF-7 | 19.62 | 18.35 | ̶ | ̶ | ̶ |
| Proton/Carbon | Aucubin (43) | Ajugol (42) | ||
|---|---|---|---|---|
| 1H a | 13C b | 1H a | 13C b | |
| 1 | 1H, 4.96 d (7.0) | 97.7 (CH) | 1H, 5.47 d (2.2) | 93.7 (CH) |
| 3 | 1H, 6.31 dd (6.0 and 1.8) | 141.6 (CH) | 1H, 6.16 dd (6.3 and 1.9) | 140.4 (CH) |
| 4 | 1H, 5.10 dd (6.0 and 4.0) | 105.7 (CH) | 1H, 4.78–4.82 (m) | 105.9 (CH) |
| 5 | 1H, 2.60–2.70 (m) | 46.3 (CH) | 1H, 2.69–2.74 (m) | 41.2 (CH) |
| 6 | 1H, 4.39–4.48 (m) | 82.8 (CH) | 1H, 3.90 ddd (5.6, 5.0, and 3.0) | 78.0 (CH) |
| 7 | 1H, 5.76 br s (t) | 130.3 (CH) | 1H, 1.79 dd (13.5, 5.0);1H, 2.05 dd (13.5 and 5.6) | 50.0 (CH2) |
| 8 | - | 148.0 (C) | - | 79.5 (C) |
| 9 | 1H, 2.89 br t (7.2) | 47.9 (CH) | 1H, 2.53 dd (9.5 and 2.2) | 51.8 (CH) |
| 10 | 2H, ABq centered at 4.25 (15.4) | 61.4 (CH2) | 3H, 1.30 s | 25.2 (CH3) |
| 1′ | 1H, 4.68 d (7.8) | 99.9 (CH) | 1H, 4.63 d (7.9) | 99.4 (CH) |
| 2′ | 1H, 3.21 dd (7.8 and 9.0) | 74.9 (CH) | 1H, 3.21 dd (8.0 and 9.0) | 74.8 (CH) |
| 3′ | 1H, 3.34 t (9.0) | 77.9 (CH) | 1H, 3.30–3.37 m | 77.8 (CH) |
| 4′ | 1H, 3.28 t (9.0) | 71.6 (CH) | 1H, 3.20–3.25 m | 71.7 (CH) |
| 5′ | 1H, 3.28–3.33 m | 78.3 (CH) | 1H, 3.28–3.33 m | 78.2 (CH) |
| 6′ | 1H, 3.65 dd (12.2 and 5.2);1H, 3.87 dd (12.2 and 1.5) | 62.7 (CH2) | 1H, 3.66 dd (12.0 and 5.2);1H, 3.91 dd (12.0 and 1.5) | 62.9 (CH2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. Amin, H.I.; Hussain, F.H.S.; Gilardoni, G.; Thu, Z.M.; Clericuzio, M.; Vidari, G. Phytochemistry of Verbascum Species Growing in Iraqi Kurdistan and Bioactive Iridoids from the Flowers of Verbascum calvum. Plants 2020, 9, 1066. https://doi.org/10.3390/plants9091066
M. Amin HI, Hussain FHS, Gilardoni G, Thu ZM, Clericuzio M, Vidari G. Phytochemistry of Verbascum Species Growing in Iraqi Kurdistan and Bioactive Iridoids from the Flowers of Verbascum calvum. Plants. 2020; 9(9):1066. https://doi.org/10.3390/plants9091066
Chicago/Turabian StyleM. Amin, Hawraz Ibrahim, Faiq H. S. Hussain, Gianluca Gilardoni, Zaw Min Thu, Marco Clericuzio, and Giovanni Vidari. 2020. "Phytochemistry of Verbascum Species Growing in Iraqi Kurdistan and Bioactive Iridoids from the Flowers of Verbascum calvum" Plants 9, no. 9: 1066. https://doi.org/10.3390/plants9091066