In Vitro Antimicrobial and Antiproliferative Activities of the Root Bark Extract and Isolated Chemical Constituents of Zanthoxylum paracanthum Kokwaro (Rutaceae)
Abstract
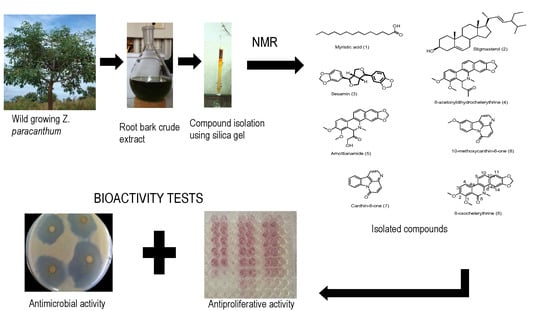
:1. Introduction
2. Results and Discussion
2.1. Identification of the Isolated Compounds
2.2. Antimicrobial Activity
2.3. Antiproliferative Activity
3. Materials and Methods
3.1. General
3.2. Plant Materials
3.3. Extraction, Isolation and Elucidation of Compounds
3.4. In Vitro Antimicrobial Testing
3.5. In Vitro Antiproliferative Activity Testing
3.6. Data Analysis
3.6.1. Calculation of Percentage Cytotoxicity
3.6.2. Selectivity Index Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ang-Lee, M.K.; Moss, J.; Yuan, C.S. Herbal medicines and perioperative care. JAMA 2001, 286, 208–216. [Google Scholar] [CrossRef]
- Ochwang’i, D.O.; Kimwele, C.N.; Oduma, J.A.; Gathumbi, P.K.; Mbaria, J.M.; Kiama, S.G. Medicinal plants used in treatment and management of cancer in Kakamega County, Kenya. J. Ethnopharmacol. 2014, 151, 1040–1055. [Google Scholar] [CrossRef]
- Korir, A.; Okerosi, N.; Ronoh, V.; Mutuma, G.; Parkin, M. Incidence of cancer in Nairobi, Kenya (2004–2008). Int. J. Cancer. 2015, 137, 2053–2059. [Google Scholar] [CrossRef]
- Macharia, L.W.; Mureithi, M.W.; Anzala, O. Cancer in Kenya: Types and infection-attributable. Data from two National referral hospitals. AAS Open Res. 2019, 1, 25. [Google Scholar] [CrossRef]
- Colditz, G.A.; Wolin, K.Y.; Gehlert, S. Applying what we know to accelerate cancer prevention. Sci. Transl. Med. 2012, 4, 127rv4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, A.; Gali-Muhtasib, H.; Ocker, M.; Schneider-Stock, R. Overview of major classes of plant-derived anticancer drugs. Int. J. Biomed. Sci. IJBS 2009, 5, 1–11. [Google Scholar] [PubMed]
- Shah, U.; Shah, R.; Acharya, S.; Acharya, N. Novel anticancer agents from plant sources. Chin. J. Nat. Med. 2013, 11, 16–23. [Google Scholar] [CrossRef]
- Saunders, F.R.; Wallace, H.M. On the natural chemoprevention of cancer. Plant. Physiol. Biochem. 2010, 48, 621–626. [Google Scholar] [CrossRef]
- Kokwaro, J.O. Medicinal Plants of East Africa, 1st ed.; University of Nairobi Press: Nairobi, Kenya, 1976; p. 120. [Google Scholar]
- Beentje, H.; Adamson, J.; Bhanderi, D. Kenya Trees, Shrubs, and Lianas; National Museums of Kenya: Nairobi, Kenya, 1994. [Google Scholar]
- Omosa, L.K.; Mbogo, G.M.; Korir, E.; Omole, R.; Seo, E.J.; Yenesew, A.; Heydenreich, M.; Midiwo, J.O.; Efferth, T. Cytotoxicity of fagaramide derivative and canthin-6-one from Zanthoxylum (Rutaceae) species against multidrug resistant leukemia cells. Nat. Prod. Res. 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lyimo, M.; Temu, R.P.C.; Mugula, J.K. Identification and nutrient composition of indigenous vegetables of Tanzania. Plant Foods Hum. Nutr. 2003, 48, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Samita, F.N.; Sandjo, L.P.; Ndiege, I.O.; Hassanali, A.; Lwande, W. Zanthoxoaporphines A–C: Three new larvicidal dibenzo [de, g] quinolin-7-one alkaloids from Zanthoxylum paracanthum (Rutaceae). Beilstein J. Org. Chem. 2013, 9, 447–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, A.; Sayeed, A.; Bhuiyan, M.S.A.; Mosaddik, M.A.; Islam, M.A.U.; Khan, G.R.M.A.M. Antimicrobial activity and cytotoxicity of Zanthoxylum budrunga. Fitoterapia 2001, 72, 428–430. [Google Scholar] [CrossRef]
- Chrian, M.; Erasto, P.; Otieno, N.J. Antimycobacterial activity and cytotoxicity effect of extracts of Hallea rubrostipulata and Zanthoxylum chalybeum. Spat DD 2011, 3, 147–152. [Google Scholar] [CrossRef]
- Misra, L.N.; Wouatsa, N.A.V.; Kumar, S.; Kumar, R.V.; Tchoumbougnang, F. Antibacterial, cytotoxic activities and chemical composition of fruits of two Cameroonian Zanthoxylum species. J. Ethnopharmacol. 2013, 148, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Yaouba, S. Phytochemical Investigation of Selected Plants in the Families Anacardiaceae and Asteraceae for Bioactive Principles. Ph.D. Thesis, University of Nairobi, Kenya, December 2018. [Google Scholar]
- Ross, S.A.; Krishnaven, K.; Radwan, M.M.; Takamatsu, S.; Burandt, C.L. Constituents of Zanthoxylum flavum and their antioxidant and antimalarial activities. Nat. Prod. Commun. 2008, 3, 791–794. [Google Scholar] [CrossRef] [Green Version]
- Omosa, L.K.; Okemwa, E.K. Antiplasmodial Activities of the Stem bark Extract and Compounds of Zanthoxylum gilletii (De wild) PG Waterman. Pharmacogn. Commun. 2017, 7, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Adesina, S.K.; Reisch, J. Arnottianamide and other constituents of Zanthoxylum gillettii root. J. Nat. Prod. 1988, 51, 601–602. [Google Scholar] [CrossRef]
- Ferreira, M.E.; De Arias, A.R.; De Ortiz, S.T.; Inchausti, A.; Nakayama, H.; Thouvenel, C.; Hocquemiller, R.; Fournet, A. Leishmanicidal activity of two canthin-6-one alkaloids, two major constituents of Zanthoxylum chiloperone var. angustifolium. J. Ethnopharmacol. 2002, 80, 199–202. [Google Scholar] [CrossRef]
- Koul, S.; Razdan, T.K.; Andotra, C.S.; Kalla, A.K.; Koul, S.; Taneja, S.C. Benzophenanthridine alkaloids from Corydalis flabellata. Planta Med. 2002, 68, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Kaigongi, M.M.; Dossaji, S.F.; Nguta, J.M.; Lukhoba, C.W.; Musila, F.M. Antimicrobial activity, toxicity and phytochemical screening of four medicinal plants traditionally used in Msambweni district, Kenya. J. Biol. Agric. Healthc. 2014, 4, 6–12. [Google Scholar]
- Gaya, C.H.; Kawaka, J.F.; Muchugi, A.; Ngeranwa, J.J. Variation of alkaloids in the Kenyan Zanthoxylum gilletii (De Wild Waterman). Afr. J. Plant Sci. 2013, 7, 438–444. [Google Scholar] [CrossRef]
- Buyinza, D. Phytochemical Investigation of Zanthoxylum Holstzianum for Antimicrobial Principles. Ph.D. Thesis, University of Nairobi, Kenya, December 2012. [Google Scholar]
- Kitahara, T.; Koyama, N.; Matsuda, J.; Aoyama, Y.; Hirakata, Y.; Kamihira, S.; Kohno, S.; Nakashima, M.; Sasaki, H. Antimicrobial activity of saturated fatty acids and fatty amines against methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 2004, 27, 1321–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laggoune, S.; Boutaghane, N.; Kabouche, A.; Kabouche, Z.; Ait-Kaki, Z.; Ait-Kaki, B. Components and antimicrobial activity of Lamium amplexicaule from Algeria. Chem. Nat. Compd. 2008, 3, 363–364. [Google Scholar] [CrossRef]
- Chakraborty, S.; Tiedemann, A.V.; Teng, P.S. Climate change: Potential impact on plant diseases. Environ. Pollut. 2000, 108, 317–326. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, M.; Wang, H.; Yan, X.; Yao, M.; Zhu, C.; Wang, L.J.; Zhou, J.C.; Liu, B.L. Studies on Antibacteria and Antioxidant Properies of Sesamin. Food Sci. 2004, 1, 102–105. [Google Scholar]
- Enright, M.C.; Robinson, D.A.; Randle, G.; Feil, E.J.; Grundmann, H.; Spratt, B.G. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc. Natl. Acad. Sci. USA 2002, 11, 7687–7692. [Google Scholar] [CrossRef] [Green Version]
- Tantapakul, C.; Phakhodee, W.; Ritthiwigrom, T.; Yossathera, K.; Deachathai, S.; Laphookhieo, S. Antibacterial compounds from Zanthoxylum rhetsa. Arch. Pharm. Res. 2012, 35, 1139–1142. [Google Scholar] [CrossRef]
- Queiroz, E.F.; Hay, A.E.; Chaaib, F.; van Diemen, D.; Diallo, D.; Hostettmann, K. New and bioactive aromatic compounds from Zanthoxylum zanthoxyloides. Planta Med. 2006, 72, 746–750. [Google Scholar] [CrossRef] [Green Version]
- Cabral, V.; Luo, X.; Junqueira, E.; Costa, S.S.; Mulhovo, S.; Duarte, A.; Couto, I.; Viveiros, M.; Ferreira, M.J. Enhancing activity of antibiotics against Staphylococcus aureus: Zanthoxylum capense constituents and derivatives. Phytomedicine 2015, 22, 469–476. [Google Scholar] [CrossRef]
- Zhao, F.; Dai, J.K.; Liu, D.; Wang, S.J.; Wang, J.R. Synthesis and evaluation of ester derivatives of 10-hydroxycanthin-6-one as potential antimicrobial agents. Molecules 2016, 21, 390. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Liu, D.; Dai, J.K.; Wang, J.Y.; Wang, J.R. Synthesis and In Vitro Antibacterial Activity of Quaternized 10-Methoxycanthin-6-one Derivatives. Molecules 2019, 24, 1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soriano-Agatón, F.; Lagoutte, D.; Poupon, E.; Roblot, F.; Fournet, A.; Gantier, J.C.; Hocquemiller, R. Extraction, hemisynthesis, and synthesis of canthin-6-one analogues. Evaluation of their antifungal activities. J. Nat. Prod. 2005, 68, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Gazoni, V.F.; Balogun, S.O.; Arunachalam, K.; Oliveira, D.M.; Cechinel Filho, V.; Lima, S.R.; Colodel, E.M.; Soares, I.M.; Ascêncio, S.D.; de Oliveira Martins, D.T. Assessment of toxicity and differential antimicrobial activity of methanol extract of rhizome of Simaba ferruginea A. St.-Hil. and its isolate canthin-6-one. J. Ethnopharmacol. 2018, 223, 122–134. [Google Scholar] [CrossRef]
- Cesari, I.; Grisoli, P.; Paolillo, M.; Milanese, C.; Massolini, G.; Brusotti, G. Isolation and characterization of the alkaloid Nitidine responsible for the traditional use of Phyllanthus muellerianus (Kuntze) Excell stem bark against bacterial infections. J. Pharm. Biomed. Anal. 2015, 105, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Tavares L de, C.; Zanon, G.; Weber, A.D.; Neto, A.T.; Mostardeiro, C.; Da Cruz, I.B.M.; Oliveira, R.M.; Ilha, V.; Dalcol, I.I.; Morel, A.F. Structure-activity relationship of benzophenanthridine alkaloids from Zanthoxylum rhoifolium having antimicrobial activity. PLoS ONE 2014, 9, e97000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boik, J. Natural Compounds in Cancer Therapy; Oregon Medical Press: Princeton, MN, USA, 2001; Volume 25. [Google Scholar]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009, 29, 2993–2996. [Google Scholar]
- Menezes, C.; Valério, E.; Dias, E. The kidney Vero-E6 cell line: A suitable model to study the toxicity of microcystins. In New Sights into Toxicity and Drug Testing; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Matskevich, A.A.; Jung, J.S.; Schümann, M.; Cascallo, M.; Moelling, K. Vero cells as a model to study the effects of adenoviral gene delivery vectors on the RNAi system in context of viral infection. J. Innate Immun. 2009, 1, 389–394. [Google Scholar] [CrossRef]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef]
- Talib, W.H. Anticancer and antimicrobial potential of plant-derived natural products. In Phytochemicals: Bioactivities and Impact on Health; IntechOpen: London, UK, 2011; pp. 141–158. [Google Scholar]
- Singh, T.D.; Meitei, H.T.; Sharma, A.L.; Robinson, A.; Singh, L.S.; Singh, T.R. Anticancer properties and enhancement of therapeutic potential of cisplatin by leaf extract of Zanthoxylum armatum DC. Biol. Res. 2015, 48, 46. [Google Scholar] [CrossRef] [Green Version]
- Wongtangtintharn, S.; Oku, H.; Iwasaki, H.; Toda, T. Effect of branched-chain fatty acids on fatty acid biosynthesis of human breast cancer cells. J. Nutr. Sci. Vitaminol (Tokyo) 2004, 50, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Frankfurt, O.S.; Krishan, A. Apoptosis-based drug screening and detection of selective toxicity to cancer cells. Anticancer Drugs 2003, 14, 555–561. [Google Scholar] [CrossRef]
- Ghosh, T.; Maity, T.K.; Singh, J. Evaluation of antitumor activity of stigmasterol, a constituent isolated from Bacopa monnieri Linn aerial parts against Ehrlich Ascites Carcinoma in mice. Orient. Pharm. Exp. Med. 2011, 11, 41–49. [Google Scholar] [CrossRef]
- Fiorentino, A.; DellaGreca, M.; D’Abrosca, B.; Oriano, P.; Golino, A.; Izzo, A.; Zarrelli, A.; Monaco, P. Lignans, neolignans and sesquilignans from Cestrum parqui l’Her. Biochem. Syst. Ecol. 2007, 35, 392–396. [Google Scholar] [CrossRef]
- Cutillo, F.; DellaGreca, M.; Gionti, M.; Previtera, L.; Zarrelli, A. Phenols and lignans from Chenopodium album. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2006, 17, 344–349. [Google Scholar] [CrossRef]
- Hirose, N.; Doi, F.; Ueki, T.; Akazawa, K.; Chijiiwa, K.; Sugano, M.; Akimoto, K.; Shimizu, S.; Yamada, H. Suppressive effect of sesamin against 7, 12-dimethylbenz [a]-anthracene induced rat mammary carcinogenesis. Anticancer Res. 1992, 12, 1259–1265. [Google Scholar]
- Lee, C.C.; Liu, K.J.; Wu, Y.C.; Lin, S.J.; Chang, C.C.; Huang, T.S. Sesamin inhibits macrophage-induced vascular endothelial growth factor and matrix metalloproteinase-9 expression and proangiogenic activity in breast cancer cells. Inflammation 2011, 34, 209–221. [Google Scholar] [CrossRef]
- Yokota, T.; Matsuzaki, Y.; Koyama, M.; Hitomi, T.; Kawanaka, M.; Enoki-Konishi, M.; Okuyama, Y.; Takayasu, J.; Nishino, H.; Nishikawa, A.; et al. Sesamin, a lignan of sesame, down-regulates cyclin D1 protein expression in human tumor cells. Cancer Sci. 2007, 98, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Lamoral-Theys, D.; Andolfi, A.; Van Goietsenoven, G.; Cimmino, A.; Le Calvé, B.; Wauthoz, N.; Mégalizzi, V.; Gras, T.; Bruyère, C.; Dubois, J.; et al. Lycorine, the main phenanthridine Amaryllidaceae alkaloid, exhibits significant antitumor activity in cancer cells that display resistance to proapoptotic stimuli: An investigation of structure− activity relationship and mechanistic insight. J. Med. Chem. 2009, 52, 6244–6256. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto, H.; Kondo, S.; Mukudai, Y.; Nagumo, T.; Yasuda, A.; Kurihara, Y.; Kamatani, T.; Shintani, S. Evaluation of anticancer activities of benzo [c] phenanthridine alkaloid sanguinarine in oral squamous cell carcinoma cell line. Anticancer Res. 2011, 31, 2841–2846. [Google Scholar]
- Chang, Y.C.; Hsieh, P.W.; Chang, F.R.; Wu, R.R.; Liaw, C.C.; Lee, K.H.; Wu, Y.C. Two new protopines argemexicaines A and B and the anti-HIV alkaloid 6-acetonyldihydrochelerythrine from formosan Argemone mexicana. Planta Med. 2003, 69, 148–152. [Google Scholar] [CrossRef]
- Sreelekha, M.; Anto, N.P.; Anto, R.J.; Shafi, P.M. Cytotoxicity of 6-acetonyldihydro-chelerythrin, arnottianamide and 6-(2-hydoxypropyl)-dihydrochelerythrine towards human cancer cell lines. Indian J. Chem. 2014, 53B, 647–651. [Google Scholar]
- Oberlies, N.H.; Kroll, D.J. Camptothecin and taxol: Historic achievements in natural products research. J. Nat. Prod. 2004, 67, 129–135. [Google Scholar] [CrossRef]
- Dai, J.; Li, N.; Wang, J.; Schneider, U. Fruitful decades for canthin-6-ones from 1952 to 2015: Biosynthesis, chemistry, and biological activities. Molecules 2016, 21, 493. [Google Scholar] [CrossRef] [Green Version]
- Murakami, C.; Fukamiya, N.; Tamura, S.; Okano, M.; Bastow, K.F.; Tokuda, H.; Mukainaka, T.; Nishino, H.; Lee, K.H. Multidrug-resistant cancer cell susceptibility to cytotoxic quassinoids, and cancer chemopreventive effects of quassinoids and canthin alkaloids. Bioorg. Med. Chem. 2004, 12, 4963–4968. [Google Scholar] [CrossRef]
- Valgas, C.; Souza, S.M.; Smânia, E.F.; Smânia, A., Jr. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Mbugua, R.W.; Njagi, E.M.; Ngule, C.M.; Mwitari, P. Gene Expression Mediated Antiproliferative Potential and Safety of Selected Medicinal Plants Against Cancerous and Normal Cells. BioRxiv 2019. [Google Scholar] [CrossRef]
- Nemati, F.; Dehpouri, A.A.; Eslami, B.; Mahdavi, V.; Mirzanejad, S. Cytotoxic properties of some medicinal plant extracts from Mazandaran, Iran. Iran. Red Crescent Med. J. 2013, 15, e8871. [Google Scholar] [CrossRef] [Green Version]
- Wilson, A.P. Cytotoxicity and viability assays. Anim. Cell Cult. Pract. Approach 2000, 1, 175–219. [Google Scholar]




| Sample Name | Microbial Organism | |||
|---|---|---|---|---|
| MRSA | S. aureus | E. coli | C. albicans | |
| myristic acid (1) | >1000 | >1000 | >1000 | >1000 |
| stigmasterol (2) | >1000 | 62.50 | 15.63 | >1000 |
| sesamin (3) | >1000 | 500 | >1000 | >1000 |
| 8- acetonyldihydrochelerythrine (4) | 31.25 | 15.63 | 15.63 | 62.50 |
| arnottianamide (5) | >1000 | >1000 | >1000 | >1000 |
| 10-methoxycanthin-6-one (6) | 3.91 | 1.95 | 3.91 | 7.81 |
| canthin-6-one (7) | 0.98 | 0.49 | 1.95 | 3.91 |
| 8-oxochelerythrine (8) | 62.50 | 7.81 | 3.91 | 15.63 |
| Z. paracanthum root bark extract | 3.91 | 0.98 | 1.95 | 7.81 |
| Omacilin | 0.98 | 0.49 | 0.98 | - |
| Fluconazole | - | - | - | 1.95 |
| Sample Names | CC50 Normal Cell Line | IC50 Cancerous Cell Lines | Selectivity Indexes | ||
|---|---|---|---|---|---|
| Vero E6 | HCC 1395 | DU 145 | HCC 1395 | DU 145 | |
| myristic acid (1) | 64.86 ± 0.51 c | 57.71 ± 1.2 a | 80.24 ± 0.12 d | 1.12 | 0.81 |
| stigmasterol (2) | 123.88 ± 0.00 b | 0.42 ± 0.1 i | 140.49 ± 1.27 a | 294.94 | 0.88 |
| sesamin (3) | 135.31 ± 0.12 a | 3.39 ± 1.0 h | 115.06 ± 0.03 b | 39.97 | 1.18 |
| 8-acetonyldihydrochelerythrine (4) | 47.83 ± 1.15 e | 9.99 ± 0.6 e | 66.82 ± 0.58 e | 4.79 | 0.72 |
| arnottianamide (5) | 2.77 ± 0.12 h | 38.34 ± 0.1 b | 84.31 ± 0.64 c | 0.07 | 0.03 |
| 10-methoxycanthin-6-one (6) | 53.95 ± 0.38 d | 14.70 ± 0.5 c | 1.58 ± 0.00 i | 3.67 | 34.15 |
| canthin-6-one (7) | 41.81 ± 0.64 f | 8.12 ± 0.6 f | 9.43 ± 0.01 h | 5.15 | 4.43 |
| 8-oxochelerythrine (8) | 135.32 ± 0.12 a | 14.09 ± 0.3 d | 63.41 ± 1.10 f | 9.60 | 2.13 |
| Z. paracanthum root bark extract | 28.28 ± 0.34 g | 7.27 ± 0.0 g | 53.21 ± 1.21 g | 3.89 | 0.53 |
| Doxorubicin (Positive control) | 0.30 ± 0.12 i | 0.21 ± 0.2 i | 0.59 ± 0.01 i | 1.41 | 0.51 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaigongi, M.M.; Lukhoba, C.W.; Yaouba, S.; Makunga, N.P.; Githiomi, J.; Yenesew, A. In Vitro Antimicrobial and Antiproliferative Activities of the Root Bark Extract and Isolated Chemical Constituents of Zanthoxylum paracanthum Kokwaro (Rutaceae). Plants 2020, 9, 920. https://doi.org/10.3390/plants9070920
Kaigongi MM, Lukhoba CW, Yaouba S, Makunga NP, Githiomi J, Yenesew A. In Vitro Antimicrobial and Antiproliferative Activities of the Root Bark Extract and Isolated Chemical Constituents of Zanthoxylum paracanthum Kokwaro (Rutaceae). Plants. 2020; 9(7):920. https://doi.org/10.3390/plants9070920
Chicago/Turabian StyleKaigongi, Magrate M., Catherine W. Lukhoba, Souaibou Yaouba, Nokwanda P. Makunga, Joseph Githiomi, and Abiy Yenesew. 2020. "In Vitro Antimicrobial and Antiproliferative Activities of the Root Bark Extract and Isolated Chemical Constituents of Zanthoxylum paracanthum Kokwaro (Rutaceae)" Plants 9, no. 7: 920. https://doi.org/10.3390/plants9070920