Quantifying Metal Contamination and Potential Uptake by Phragmites australis Adans. (Poaceae) Along a Subtropical River System
Abstract
:1. Introduction
2. Results
2.1. Basic water Parameters
2.2. Sediment
2.3. Sediment Quality Indices
2.4. Relationship between Sediment Nutrient and Metal Variables
2.5. Macrophytes
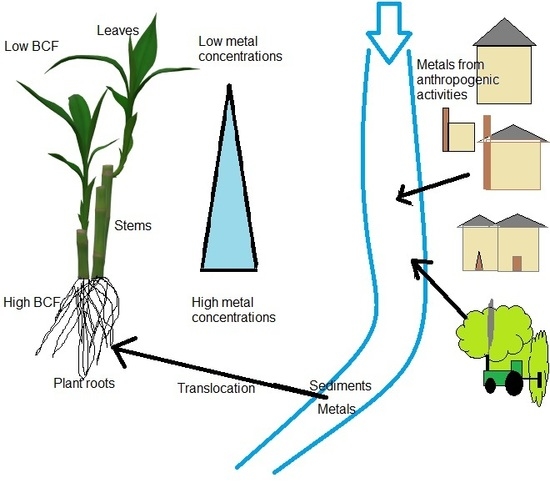
2.6. Bio-Concentration and Translocation of Metals in Phragmites Australis
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Sediments
4.3. Macrophytes
4.4. Data Analysis
4.5. Pollution Indices
4.5.1. Sediment
4.5.2. Macrophytes
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Declaration
References
- Bharti, P.K. Heavy metals in the environment. Aquat. Environ. Toxicol. 2013, 1, 241–266. [Google Scholar]
- Atkinson, K.; Miller, G.T. Living in the Environment: An Introduction to Environmental Science. J. Anim. Ecol. 1991, 60, 1101. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, D.; Xu, Z.; Yuan, S.; Li, Y.; Wang, L. Effect of overlying water pH, dissolved oxygen, and temperature on heavy metal release from river sediments under laboratory conditions. Arch. Environ. Prot. 2017, 43, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Ding, S.; Lin, J.; Fu, Z.; Tang, W.; Fan, X.; Gong, M.; Wang, Y. Seasonal changes of lead mobility in sediments in algae- and macrophyte-dominated zones of the lake. Sci. Total. Environ. 2019, 660, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.I.N.; Hiren, S.; Rita, K.N.; Ira, B. Macrophytes in Phytoremediation of Heavy Metal Contaminated Water and Sediments in Pariyej Community Reserve, Gujarat, India. Turk. J. Fish. Aquat. Sci. 2008, 6, 193–200. [Google Scholar]
- Rajan, S.; Nurul-Nadiah, M.F.; Mahenderan, A.; Kalavathy, R. Effects of climate changes on dissolved heavy metal concentrations among recreational park tributaries in Pahang, Malaysia. Biomed. Res. 2012, 23, 23–30. [Google Scholar]
- Li, Y.; Li, L.; Zhang, Q.; Yang, Y.; Wang, H.; Wang, R.; Zhang, J. Influence of temperature on the heavy metal’s accumulation of five vegetable species in semiarid area of northwest China. Chem. Ecol. 2013, 29, 353–365. [Google Scholar] [CrossRef]
- Radomyski, A.; Lei, K.; Giubilato, E.; Critto, A.; Lin, C.; Marcomini, A. Bioaccumulation of trace metals in aquatic food web. A case study, Liaodong Bay, NE China. Mar. Pollut. Bull. 2018, 137, 555–565. [Google Scholar] [CrossRef]
- Guo, Z.; Ni, Z.; Ye, H.; Xiao, J.; Chen, L.; Green, I.; Zhang, L. Simultaneous uptake of Cd from sediment, water, and diet in a demersal marine goby Mugilogobius chulae. J. Hazard. Mater. 2019, 364, 143–150. [Google Scholar] [CrossRef]
- Uthe, J.F.; Bligh, E.G. Preliminary Survey of Heavy Metal Contamination of Canadian Freshwater Fish. J. Fish. Res. Board Can. 1971, 28, 786–788. [Google Scholar] [CrossRef]
- Søndergaard, M.; Phillips, G.; Hellsten, S.; Kolada, A.; Ecke, F.; Måemets, H.; Mjelde, M.; Azzella, M.M.; Oggioni, A. Maximum growing depth of submerged macrophytes in European lakes. Hydrobiology 2012, 704, 165–177. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Wang, Y.; Luo, G.; Chen, X.; Yang, X.; Gao, B.; He, X. Integrated Assessment of Heavy Metal Contamination in Sediments from a Coastal Industrial Basin, NE China. PLoS ONE 2012, 7, e39690. [Google Scholar] [CrossRef] [PubMed]
- Bere, T.; Dalu, T.; Mwedzi, T. Detecting the impact of heavy metal contaminated sediment on benthic macroinvertebrate communities in tropical streams. Sci. Total. Environ. 2016, 572, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Fosu-Mensah, B.Y.; Addae, E.; Yirenya-Tawiah, D.; Nyame, F. Heavy metals concentration and distribution in soils and vegetation at Korle Lagoon area in Accra, Ghana. Cogent Environ. Sci. 2017, 3, 1405887. [Google Scholar] [CrossRef] [Green Version]
- Ramachandra, T.; Sudarshan, P.; Mahesh, M.; Vinay, S. Spatial patterns of heavy metal accumulation in sediments and macrophytes of Bellandur wetland, Bangalore. J. Environ. Manag. 2018, 206, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Foy, C.D.; Chaney, R.L.; White, M.C. The Physiology of Metal Toxicity in Plants. Annu. Rev. Plant Physiol. 1978, 29, 511–566. [Google Scholar] [CrossRef]
- Sochacki, A.; Guy, B.; Faure, O.; Surmacz-Górska, J. Accumulation of metals and boron in Phragmites australis planted in constructed wetlands polishing real electroplating wastewater. Int. J. Phytoremediat. 2015, 17, 1068–1072. [Google Scholar] [CrossRef]
- Liphadzi, M.; Kirkham, M.; Musil, C. Phytoremediation of soil contaminated with heavy metals: A technology for rehabilitation of the environment. South Afr. J. Bot. 2005, 71, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Mojiri, A. Phytoremediation of heavy metals from municipal wastewater by Typha domingensis. Afr. J. Microbiol. Res. 2012, 6, 643–647. [Google Scholar]
- Dalu, T.; Wasserman, R.J.; Wu, Q.; Froneman, P.W.; Weyl, O.L.F. River sediment metal and nutrient variations along an urban–agriculture gradient in an arid austral landscape: Implications for environmental health. Environ. Sci. Pollut. Res. 2017, 25, 2842–2852. [Google Scholar] [CrossRef]
- Dube, T.; Mhangwa, G.; Makaka, C.; Parirenyatwa, B.; Muteveri, T. Spatial variation of heavy metals and uptake potential by Typha domingensis in a tropical reservoir in the midland’s region, Zimbabwe. Environ. Sci. Pollut. Res. 2019, 26, 10097–10105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, X. Heavy metals in surface sediments of the intertidal Laizhou Bay, Bohai Sea, China: Distributions, sources, and contamination assessment. Mar. Pollut. Bull. 2015, 98, 320–327. [Google Scholar] [CrossRef]
- Ramos, T.; Gonçalves, M.; Branco, M.A.; Brito, D.; Rodrigues, S.; Sánchez-Pérez, J.; Sauvage, S.; Prazeres, Â.; Martins, J.C.; Fernandes, M.L.; et al. Sediment and nutrient dynamics during storm events in the Enxoé temporary river, southern Portugal. Catena 2015, 127, 177–190. [Google Scholar] [CrossRef]
- Zhuang, Q.; Li, G.; Liu, Z. Distribution, source, and pollution level of heavy metals in river sediments from South China. Catena 2018, 170, 386–396. [Google Scholar] [CrossRef]
- O’Brien, J.M.; Lessard, J.L.; Plew, D.; Graham, S.E.; McIntosh, A.R. Aquatic Macrophytes Alter Metabolism and Nutrient Cycling in Lowland Streams. Ecosystems 2013, 17, 405–417. [Google Scholar] [CrossRef]
- Pettit, N.E.; Ward, D.; Adame, F.; Valdez, D.; Bunn, S.E. Influence of aquatic plant architecture on epiphyte biomass on a tropical river floodplain. Aquat. Bot. 2016, 129, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Wang, D.; Ding, S.; Fan, X.; Jin, Z.; Wu, Y.; Wang, Y.; Zhang, C. Zinc pollution in zones dominated by algae and submerged macrophytes in Lake Taihu. Sci. Total. Environ. 2019, 670, 361–368. [Google Scholar] [CrossRef]
- Dalu, T.; Clegg, B.; Nhiwatiwa, T. Aquatic macrophytes in a tropical African reservoir: Diversity, communities, and the impact of reservoir-level fluctuations. Trans. R. Soc. South Afr. 2012, 67, 117–125. [Google Scholar] [CrossRef]
- Dar, N.A.; Pandit, A.K.; Ganai, B.A. Factors affecting the distribution patterns of aquatic macrophytes. Limnol. Rev. 2014, 14, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Chander, S.; Pompapathi, V.; Gujrati, A.; Singh, R.P.; Chaplot, N.; Patel, U.D. Growth of Invasive Aquatic Macrophytes Over Tapi River. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2018, 829–833. [Google Scholar] [CrossRef] [Green Version]
- Świerk, D.; Barbara, S. Occurrence of heavy metals in aquatic macrophytes colonising small aquatic ecosystem. Ecol. Chem. Eng. 2011, 18, 369–384. [Google Scholar]
- Dong, B.; Qin, B.; Li, W.; Gao, G. Growth and Community Composition of Submerged Macrophytes in Lake Taihu (China): Assessment of Changes in Response to Sediment Characteristics. Wetlands 2016, 37, 233–243. [Google Scholar] [CrossRef]
- Gerber, R.; Smit, N.J.; van Vuren, J.; Nakayama, S.M.M.; Yohannes, Y.B.; Ikenaka, Y.; Ishizuka, M.; Wepener, V. Application of a Sediment Quality Index for the assessment and monitoring of metals and organochlorines in a premier conservation area. Environ. Sci. Pollut. Res. 2015, 22, 19971–19989. [Google Scholar] [CrossRef]
- Dahms, S.; Baker, N.; Greenfield, R. Ecological risk assessment of trace elements in sediment: A case study from Limpopo, South Africa. Ecotoxicol. Environ. Saf. 2017, 135, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Edokpayi, J.N.; Odiyo, J.; Popoola, O.E.; Msagati, T.A.M. Assessment of Trace Metals Contamination of Surface Water and Sediment: A Case Study of Mvudi River, South Africa. Sustainability 2016, 8, 135. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ye, S.; Laws, E.A.; Yuan, H.; Ding, X.; Zhao, G. Surface sediment properties and heavy metal pollution assessment in the Shallow Sea Wetland of the Liaodong Bay, China. Mar. Pollut. Bull. 2017, 120, 347–354. [Google Scholar] [CrossRef]
- Osibote, O.; Oputu, O. Fate and partitioning of heavy metals in soils from landfill sites in Cape Town, South Africa: A health risk approach to data interpretation. Environ. Geochem. Health 2019, 42, 283–312. [Google Scholar] [CrossRef] [PubMed]
- Marrugo-Negrete, J.; Durango-Hernández, J.; Ramos, C.R.C.; Urango-Cárdenas, I.; Díez, S. Mercury levels and genotoxic effect in caimans from tropical ecosystems impacted by gold mining. Sci. Total Environ. 2019, 664, 899–907. [Google Scholar] [CrossRef]
- Dalu, T.; Wasserman, R.J.; Magoro, M.L.; Froneman, P.W.; Weyl, O.L. River nutrient water and sediment measurements inform on nutrient retention, with implications for eutrophication. Sci. Total Environ. 2019, 684, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, M.; Theuring, P.; Rode, M.; Borchardt, D. Suspended sediments in the Kharaa River catchment (Mongolia) and its impact on hyporheic zone functions. Environ. Earth Sci. 2011, 65, 1535–1546. [Google Scholar] [CrossRef]
- Barbieri, M. The Importance of Enrichment Factor (EF) and Geoaccumulation Index (Igeo) to Evaluate the Soil Contamination. J. Geol. Geophys. 2016, 5, 1–4. [Google Scholar] [CrossRef]
- Suif, Z.; Fleifle, A.; Yoshimura, C.; Saavedra, O. Spatio-temporal patterns of soil erosion and suspended sediment dynamics in the Mekong River Basin. Sci. Total Environ. 2016, 568, 933–945. [Google Scholar] [CrossRef] [Green Version]
- Koomklang, J.; Yamaguchi, H.; Ichimi, K.; Tada, K. A role for a superfcial sediment layer in upward nutrient fuxes across the overlying water–sediment interface. J. Oceanogr. 2018, 74, 13–21. [Google Scholar] [CrossRef]
- Ali, N.A.; Bernal, M.; Ater, M. Tolerance and bioaccumulation of copper in Phragmites australis and Zea mays. Plant Soil 2002, 239, 103–111. [Google Scholar] [CrossRef]
- Markert, B. Plants as Biomonitors—Potential Advantages and Problems. In Biogeochemistry of Trace Elements; Adriano, D.C., Chen, Z.S., Yang, S.S., Eds.; Science and Technology Letters: Northwood, NY, USA, 1994. [Google Scholar]
- Persaud, D.; Jaagumagi, R.; Hayton, A. Guidelines for the Protection and Management of Aquatic Sediment Quality in Ontario; Ministry of Environment and Energy: Toronto, ON, Canada, 1993.
- Zhu, D.; Schwab, P.; Banks, M.K. Heavy Metal Leaching from Mine Tailings as Affected by Plants. J. Environ. Qual. 1999, 28, 1727–1732. [Google Scholar] [CrossRef]
- Kushwaha, A.; Rani, R.; Kumar, S.; Gautam, A. Heavy metal detoxification and tolerance mechanisms in plants: Implications for phytoremediation. Environ. Rev. 2016, 24, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; He, Z.-L.; Graetz, D.A.; Stoffella, P.J.; Yang, X. Uptake, and distribution of metals by water lettuce (Pistia stratiotes L.). Environ. Sci. Pollut. Res. 2011, 18, 978–986. [Google Scholar] [CrossRef]
- Vymazal, J.; Kröpfelová, L.; Švehla, J.; Chrastný, V.; Štíchová, J. Trace elements in Phragmites australis growing in constructed wetlands for treatment of municipal wastewater. Ecol. Eng. 2009, 35, 303–309. [Google Scholar] [CrossRef]
- Obarska-Pemkowiak, H.; Gajewska, M.; Wojciechowska, E.; Obarska-Pempkowiak, H. Application of Vertical Flow Constructed Wetlands for Highly Contaminated Wastewater Treatment: Preliminary Results. In Water and Nutrient Management in Natural and Constructed Wetlands; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2010; pp. 37–50. [Google Scholar]
- Prajapati, M.; van Bruggen, J.J.A.; Dalu, T.; Malla, R. Assessing the effectiveness of pollutant removal by macrophytes in a floating wetland for wastewater treatment. Appl. Water Sci. 2017, 7, 4801–4809. [Google Scholar] [CrossRef] [Green Version]
- Olszewska, J.P.; Meharg, A.A.; Heal, K.V.; Carey, M.; Gunn, I.D.M.; Searle, K.; Winfield, I.J.; Spears, B.M.; Heal, K.V. Assessing the Legacy of Red Mud Pollution in a Shallow Freshwater Lake: Arsenic Accumulation and Speciation in Macrophytes. Environ. Sci. Technol. 2016, 50, 9044–9052. [Google Scholar] [CrossRef] [Green Version]
- Dalu, T.; Murudi, T.T.; Dondofema, F.; Wasserman, R.J.; Chari, L.D.; Murungweni, F.M.; Cuthbert, R.N. Balloon milkweed Gomphocarpus physocarpus distribution and drivers in an internationally protected wetland. BioInvas. Records 2020, 9, in press. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Agriculture Laboratory Association of Southern Africa (AgriLASA). Soil Handbook; Agri Laboratory Association of Southern Africa: Pretoria, South Africa, 2004. [Google Scholar]
- Chan, K.Y.; Bowman, A.; Oates, A. Oxidizible organiccarbon fractions and soil quality changes in an oxic paleustalfunder different pasture leys. Soil Sci. Soc. Am. J. 2011, 166, 61–67. [Google Scholar] [CrossRef]
- Hering, D.; Johnson, R.K.; Kramm, S.; Schmutz, S.; Szoszkiewicz, K.; Verdonschot, P.F.M. Assessment of European streams with diatoms, macrophytes, macroinvertebrates and fish: A comparative metric-based analysis of organism response to stress. Freshw. Boil. 2006, 51, 1757–1785. [Google Scholar] [CrossRef]
- Campbell, C.R.; Plank, C.O. Preparation of Plant Tissue for Laboratory Analysis. Handb. Ref. Methods Plant Anal. 1997, 37. [Google Scholar] [CrossRef]
- Muller, G. Index of Geoaccumulation in sediments of the Rhine River. Geojournal 1969, 2, 108–118. [Google Scholar]
- Buat-Menard, P.; Chesselet, R. Variable influence of atmospheric flux on the heavy metal chemistry of oceanic suspended matter. Earth Planet. Sci. Lett. 1979, 42, 398–411. [Google Scholar] [CrossRef]
- Li, F.; Zeng, X.-Y.; Wu, C.-H.; Duan, Z.-P.; Wen, Y.-M.; Huang, G.-R.; Long, X.-L.; Li, M.-J.; Li, M.-J.; Xu, J.-Y. Ecological Risks Assessment and Pollution Source Identification of Trace Elements in Contaminated Sediments from the Pearl River Delta, China. Boil. Trace Element Res. 2013, 155, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgol. Mar. Res. 1980, 33, 566–575. [Google Scholar] [CrossRef] [Green Version]
- Barron, M.G. Bioconcentration. Will water-borne organic chemicals accumulate in aquatic animals? Environ. Sci. Technol. 1990, 24, 1612–1618. [Google Scholar] [CrossRef]
- Ghosh, M.; Singh, S.P. A comparative study of cadmium phytoextraction by accumulator and weeds species. Environ. Pollut. 2005, 133, 365–371. [Google Scholar] [CrossRef] [PubMed]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MjM Software: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- SPSS Inc. SPSS Release 16.0.0 for Windows. Polar Engineering and Consulting; SPSS Inc.: Chicago, IL, USA, 2007. [Google Scholar]




| Sites | P | Na | K | Ca | Mg | Cu | Zn | Mn | B | Fe | S | TOC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | % | |
| Cool-dry | ||||||||||||
| M1 | 0.03 ± 0.01 | 240.7 ± 0.3 | 17.6 ± 5.9 | 155 ± 33 | 21.6 ± 8.4 | 76.6 ± 57.7 | 46.4 ± 17.9 | 1307.9 ± 997.8 | 35.1 ± 21.7 | 38,894 ± 21,643 | 22.9 ± 0.02 | 7.1 ± 1.6 |
| M2 | 0.02 ± 0.02 | 179.2 ± 9.7 | 9.8 ± 0.1 | 27 ± 1 | 10.2 ± 1.8 | 39.2 ± 14.8 | 42.5 ± 2.8 | 1858.7 ± 541.1 | 19.7 ± 21.7 | 28,333 ± 35,721 | 5.5 ± 3.7 | 9.9 ± 2.5 |
| M3 | 0.03 ± 0.01 | 187.8 ± 31.2 | 9.8 ± 2.0 | 33 ± 1 | 15.6 ± 2.4 | 50.3 ± 3.9 | 33.9 ± 4.6 | 743.6 ± 199.3 | 34.6 ± 21.1 | 55,361 ± 17,421 | 11.1 ± 1.7 | 8.5 ± 0.8 |
| M4 | 0.01 ± 0.01 | 159.2 ± 8.3 | 7.8 ± 0.1 | 23 ± 1 | 9.0 ± 0.6 | 29.6 ± 2.3 | 25.8 ± 0.1 | 582.1 ± 44.6 | 10.4 ± 8.3 | 16,269 ± 15,009 | 7.3 ± 1.6 | 6.8 ± 0.9 |
| M5 | 0.02 ± 0.01 | 239.1 ± 53 | 11.7 ± 3.9 | 30 ± 12 | 12.6 ± 5.4 | 39.2 ± 8.2 | 24.5 ± 2.9 | 459.8 ± 8.0 | 18.5 ± 2.8 | 30,723 ± 4956 | 14.3 ± 2.8 | 9.4 ± 3.8 |
| Hot-dry | ||||||||||||
| M1 | 10.4 ± 2.4 | 87.4 ± 11.5 | 42.9 ± 23.4 | 3780 ± 758 | 97.8 ± 39.0 | 6.3 ± 3.4 | 2.5 ± 0.4 | 80.6 ± 19.0 | 0.5 ± 0.1 | 124.6 ± 50.8 | 126.4 ± 90.6 | 4.1 ± 2.8 |
| M2 | 10.2 ± 3.0 | 62.1 ± 4.6 | 27.3 ± 0.1 | 711.0 ± 29.0 | 215.4 ± 15 | 7.0 ± 0.2 | 2.8 ± 0.1 | 1140.1 ± 79.2 | 0.3 ± 0.01 | 247.3 ± 18.1 | 14.2 ± 2.5 | 5.3 ± 1.7 |
| M3 | 17.1 ± 6.2 | 62.1 ± 11.5 | 21.5 ± 5.9 | 704 ± 166 | 240.6 ± 60.6 | 5.8 ± 2.1 | 2.6 ± 0.5 | 972.8 ± 477.8 | 0.3 ± 0.04 | 176.5 ± 17.9 | 15.5 ± 1.6 | 9.0 ± 2.0 |
| M4 | 50.6 ± 1.2 | 86.3 ± 10.4 | 37.1 ± 2.0 | 470 ± 82.0 | 147 ± 31.8 | 4.0 ± 0.6 | 3.6 ± 0.1 | 417.5 ± 36.2 | 0.4 ± 0.02 | 367.6 ± 9.6 | 23.4 ± 2.3 | 5.6 ± 1.0 |
| M5 | 22.4 ± 1.6 | 67.9 ± 3.5 | 35.1 ± 0.1 | 420 ± 8.0 | 137.4 ± 0.6 | 4.1 ± 0.5 | 2.1 ± 0.3 | 200.0 ± 15.2 | 0.3 ± 0.04 | 209.8 ± 14.7 | 18.3 ± 4.0 | 4.9 ± 1.1 |
| Hot-wet | ||||||||||||
| M1 | 9.0 ± 2.5 | 81.7 ±5.8 | 56.5 ± 0.5 | 4260 ± 20.0 | 138 ± 6.0 | 12.1 ± 2.3 | 4.7 ± 1.8 | 159.5 ± 0.5 | 0.5 ± 0.01 | 193 ± 21.0 | 54.0 ± 2.4 | 3.7 ± 0.7 |
| M2 | 7.0 ± 0.8 | 42.6 ± 1.2 | 19.4 ± 0.8 | 740.0 ± 20.0 | 192 ± 12.0 | 6.1 ± 1.2 | 3.0 ± 0.9 | 1195 ± 35.0 | 0.2 ± 0.02 | 214 ± 30.0 | 6.6 ± 2.0 | 4.1 ± 0.3 |
| M3 | 4.3 ± 1.4 | 93.2 ± 26.5 | 37.5 ± 0.1 | 1390 ± 190.0 | 438 ± 78.0 | 9.6 ± 2.5 | 2.8 ± 1.5 | 462 ± 61.0 | 0.3 ± 0.01 | 193 ± 21.0 | 4.2 ± 1.0 | 7.3 ± 4.0 |
| M4 | 13.9 ± 1.4 | 74.8 ± 17.3 | 40.7 ± 9.0 | 900.0 ± 220.0 | 252 ± 48.0 | 5.6 ± 2.2 | 3.2 ± 0.8 | 386.5 ± 111.5 | 0.2 ± 0.03 | 231.5 ± 66.5 | 11.1 ± 2.7 | 5.9 ± 2.2 |
| M5 | 4.6 ± 1.3 | 70.2 ± 10.4 | 40.9 ± 15 | 1610 ± 31.0 | 654 ± 138.0 | 4.0 ± 1.9 | 0.9 ± 0..03 | 963 ± 187.0 | 0.2 ± 0.01 | 213.5 ± 70.5 | 5.9 ± 3.3 | 3.5 ± 0.2 |
| Axis 1 | Axis 2 | |
|---|---|---|
| Eigenvalue | 6.22 | 1.30 |
| Variance (%) | 69.13 | 14.47 |
| Cum variance (%) | 69.13 | 83.60 |
| Metals | Factor loadings | |
| Na | −0.91 | 0.26 |
| K | 0.83 | 0.39 |
| Ca | 0.62 | 0.60 |
| Mg | 0.65 | −0.39 |
| Cu | −0.94 | 0.19 |
| Zn | −0.97 | 0.07 |
| Mn | −0.51 | −0.69 |
| B | −0.96 | 0.16 |
| Fe | −0.94 | 0.16 |
| Season | N | P | K | Ca | Mg | Na | Mn | Fe | Cu | Zn | B |
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | |
| Root | |||||||||||
| Cool-dry | 0.9 ± 0.1 | 0.07 ± 0.01 | 0.3 ± 0.1 | 0.21 ± 0.02 | 0.09 ± 0.01 | 1020.8 ± 269.4 | 3763.4 ± 222.6 | 32,182 ± 6464 | 39.4 ± 4.4 | 37.0 ± 4.4 | 30.8 ± 7.7 |
| Hot-dry | 1.0 ± 0.1 | 0.08 ± 0.01 | 0.61 ± 0.1 | 0.28 ± 0.03 | 0.11 ± 0.01 | 2062.2 ± 268.9 | 2193.8 ± 689.7 | 27,857 ± 6263 | 32.0 ± 5.6 | 38.8 ± 5.6 | 32.4 ± 7.6 |
| Hot-wet | 0.7 ± 0.2 | 0.05 ± 0.01 | 0.5 ± 0.2 | 0.12 ± 0.02 | 0.07 ± 0.01 | 1563.4 ± 238.5 | 725.4 ± 259.9 | 17,352 ± 471.7 | 21.0 ± 5.2 | 20.4 ± 5.2 | 11.1 ± 0.6 |
| Stem | |||||||||||
| Cool-dry | 1.1 ± 0.2 | 0.06 ± 0.02 | 1.7 ± 0.4 | 0.17 ± 0.07 | 0.07 ± 0.03 | 1312.6 ± 299.1 | 583.4 ± 224.0 | 1589.6 ± 541.7 | 5.8 ± 0.9 | 18.4 ± 2.1 | 3.4 ± 0.8 |
| Hot-dry | 1.0 ± 0.2 | 0.08 ± 0.02 | 1.7 ± 0.4 | 0.15 ± 0.11 | 0.08 ± 0.03 | 2364.8 ± 266.1 | 518.2 ± 238.6 | 1081 ± 511.88 | 3.8 ± 1.1 | 15.8 ± 2.5 | 3.0 ± 1.2 |
| Hot-wet | 1.0 ± 0.2 | 0.11 ± 0.02 | 1.6 ± 0.3 | 0.15 ± 0.07 | 0.08 ± 0.03 | 1730.0 ± 300.0 | 431.0 ± 227.1 | 2470 ± 391.0 | 4.2 ± 1.0 | 17.7 ± 1.8 | 3.4 ± 0.7 |
| Leaves | |||||||||||
| Cool-dry | 2.4 ± 0.2 | 0.19 ± 0.03 | 1.8 ± 0.1 | 0.43 ± 0.06 | 0.13 ± 0.01 | 295.4 ± 51.8 | 612.8 ± 81.3 | 1336.6 ± 198.3 | 27.6 ± 1.0 | 23.0 ± 3.4 | 12.2 ± 2.3 |
| Hot-dry | 2.3 ± 0.3 | 0.18 ± 0.02 | 1.5 ± 0.2 | 0.62 ± 0.06 | 0.14 ± 0.01 | 348.6 ± 46.8 | 852.0 ± 122.3 | 1477.4 ± 211.6 | 4.0 ± 23.4 | 18.0 ± 4.0 | 16.6 ± 8.3 |
| Hot-wet | 2.5 ± 0.2 | 0.17 ± 0.04 | 1.5 ± 0.2 | 0.30 ± 0.07 | 0.12 ± 0.02 | 386.8 ± 68.1 | 305.0 ± 100.3 | 1147.8 ± 224.6 | 2.8 ± 1.1 | 14.4 ± 3.9 | 5.0 ± 2.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Netshiongolwe, N.R.; Cuthbert, R.N.; Maenetje, M.M.; Chari, L.D.; Motitsoe, S.N.; Wasserman, R.J.; Munyai, L.F.; Dalu, T. Quantifying Metal Contamination and Potential Uptake by Phragmites australis Adans. (Poaceae) Along a Subtropical River System. Plants 2020, 9, 846. https://doi.org/10.3390/plants9070846
Netshiongolwe NR, Cuthbert RN, Maenetje MM, Chari LD, Motitsoe SN, Wasserman RJ, Munyai LF, Dalu T. Quantifying Metal Contamination and Potential Uptake by Phragmites australis Adans. (Poaceae) Along a Subtropical River System. Plants. 2020; 9(7):846. https://doi.org/10.3390/plants9070846
Chicago/Turabian StyleNetshiongolwe, Ndivhuwo R., Ross N. Cuthbert, Mokgale M. Maenetje, Lenin D. Chari, Samuel N. Motitsoe, Ryan J. Wasserman, Linton F. Munyai, and Tatenda Dalu. 2020. "Quantifying Metal Contamination and Potential Uptake by Phragmites australis Adans. (Poaceae) Along a Subtropical River System" Plants 9, no. 7: 846. https://doi.org/10.3390/plants9070846