Insecticidal Activity of Hyoscyamus niger L. on Lucilia sericata Causing Myiasis
Abstract
:1. Introduction

2. Results
3. Discussion
4. Materials and Methods
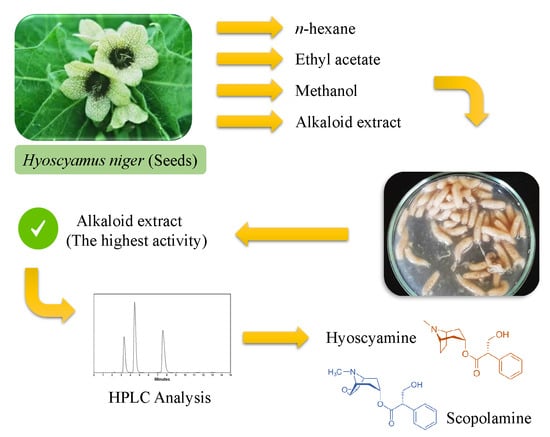
4.1. Plant Material
4.2. Preparation of n-Hexane, Ethyl Acetate and Methanol Extracts
4.3. Preparation of Alkaloid Extract
4.4. Obtaining First, Second and Third Instars
4.5. Mounting of L. sericata Larvae
4.6. Identification of L. sericata Larvae and Adult
4.7. Ingestion Assay
4.8. HPLC Conditions
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baytop, A. Hyoscyamus L. In Flora of Turkey and the East Aegean Islands; Davis, P.H., Ed.; University of Edinburgh: Edinburgh, UK, 1978; Volume 6, pp. 453–456. [Google Scholar]
- Ghorbanpour, M.; Hatami, M.; Hatami, M. Activating antioxidant enzymes, hyoscyamine and scopolamine biosynthesis of Hyoscyamus niger L. plants with nano-sized titanium dioxide and bulk application. Acta Agric. Slov. 2015, 105, 23–32. [Google Scholar] [CrossRef]
- Honda, G.; Yesilada, E.; Tabata, M.; Sezik, E.; Fujita, T.; Takeda, Y.; Takaishi, Y.; Tanaka, T. Traditional medicine in Turkey. VI. Folk medicine in west Anatolia: Afyon, Kutahya, Denizli, Mugla, Aydin provinces. J. Ethnopharmacol. 1996, 53, 75–87. [Google Scholar] [PubMed]
- Sezik, E.; Tabata, M.; Yesilada, E.; Honda, G.; Goto, K.; Ikeshiro, Y. Traditional medicine in Turkey. I. Folk medicine in northeast Anatolia. J. Ethnopharmacol. 1991, 35, 191–196. [Google Scholar] [CrossRef]
- Fujita, T.; Sezik, E.; Tabata, M.; Yesilada, E.; Honda, G.; Takeda, Y.; Tanaka, T.; Takaishi, Y. Traditional medicine in Turkey VII. Folk medicine in middle and west Black Sea regions. Econ. Bot. 1995, 49, 406. [Google Scholar] [CrossRef]
- Sezik, E.; Yesilada, E.; Honda, G.; Takaishi, Y.; Takeda, Y.; Tanaka, T. Traditional medicine in Turkey, X. Folk medicine in Central Anatolia. J. Ethnopharmacol. 2001, 75, 95–115. [Google Scholar] [CrossRef]
- Sezik, E.; Yeşİlada, E.; Tabata, M.; Honda, G.; Takaishi, Y.; Fujita, T.; Tanaka, T.; Takeda, Y. Traditional medicine in Turkey VIII. folk medicine in east Anatolia; Erzurum, Erzíncan, Ağri, Kars, Iğdir provinces. Econ. Bot. 1997, 51, 195–211. [Google Scholar] [CrossRef]
- Simsek, I.; Aytekin, F.; Yesilada, E.; Yildirimli, Ş. An ethnobotanical survey of the Beypazari, Ayas, and Güdül district towns of Ankara Province (Turkey). Econ. Bot. 2004, 58, 705–720. [Google Scholar]
- Yesilada, E.; Honda, G.; Sezik, E.; Tabata, M.; Fujita, T.; Tanaka, T.; Takeda, Y.; Takaishi, Y. Traditional medicine in Turkey. V. Folk medicine in the inner Taurus Mountains. J. Ethnopharmacol. 1995, 46, 133–152. [Google Scholar] [CrossRef]
- Yesilada, E.; Sezik, E.; Honda, G.; Takaishi, Y.; Takeda, Y.; Tanaka, T. Traditional medicine in Turkey IX: Folk medicine in north-west Anatolia. J. Ethnopharmacol. 1999, 64, 195–210. [Google Scholar] [CrossRef]
- Cuneyt, C.; Kevseroglu, K.; Sağlam, B. Physical and physiological dormancy in black henbane (Hyoscyamus niger L.) seeds. J. Plant Biol. 2004, 47, 391–395. [Google Scholar]
- Ma, C.Y.; Liu, W.K.; Che, C.T. Lignanamides and nonalkaloidal components of Hyoscyamus niger seeds. J. Nat. Prod. 2002, 65, 206–209. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Therapeutic importance of Hyoscyamus species grown in Iraq (Hyoscyamus albus, Hyoscyamus niger and Hyoscyamus reticulates)- A review. Iosr J. Pharm. 2018, 8, 18–32. [Google Scholar]
- Dulger, B.; Hacioglu, N.; Goncu, B.S.; Gucin, F. Antifungal activity of seeds of Hyoscyamus niger L. (Henbane) against some clinically relevant fungal pathogens. Asian J. Chem. 2010, 22, 6321–6324. [Google Scholar]
- Horobin, A.J.; Shakesheff, K.M.; Woodrow, S.; Robinson, C.; Pritchard, D.I. Maggots and wound healing: An investigation of the effects of secretions from Lucilia sericata larvae upon interactions between human dermal fibroblasts and extracellular matrix components. Br. J. Derm. 2003, 148, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Zumpt, F. Myiasis in Man and Animals in the Old World: A Textbook for Physicians, Veterinarians and Zoologists; Butterworths Publishing Company: London, UK, 1965. [Google Scholar]
- Service, M.W. Medical Entomology for Students; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Markell, E.K.; Krotoski, W.A. Markell and Voge’s Medical Parasitology, 8th ed.; Elsevier: Philadelphia, PA, USA, 1999. [Google Scholar]
- Yaghoubi, R.; Tirgari, S.; Sina, N. Human auricular myiasis caused by Lucilia sericata: Clinical and parasitological considerations. Acta Med. Iran. 2005, 43, 155–157. [Google Scholar]
- Daniel, M.; Šrámová, H.; Zalabska, E. Lucilia sericata (Diptera: Calliphoridae) causing hospital-acquired myiasis of a traumatic wound. J. Hosp. Infect. 1994, 28, 149–152. [Google Scholar] [CrossRef]
- Khater, H.F.; Khater, D.F. The insecticidal activity of four medicinal plants against the blowfly Lucilia sericata (Diptera: Calliphoridae). Int. J. Derm. 2009, 48, 492–497. [Google Scholar] [CrossRef]
- Francesconi, F.; Lupi, O. Myiasis. Clin. Microbiol. Rev. 2012, 25, 79–105. [Google Scholar] [CrossRef] [Green Version]
- Cavusoglu, T.; Apan, T.; Eker, E.; Vargel, I.; Saray, A. Massive oculofacial myiasis infestation with Lucilia sericata. J. Am. Acad. Derm. 2009, 61, 169–170. [Google Scholar] [CrossRef]
- Huynh, N.; Dolan, B.; Lee, S.; Whitcher, J.P.; Stanley, J. Management of phaeniciatic ophthalmomyiasis externa. Br. J. Ophthalmol. 2005, 89, 1377–1378. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Seo, P.W.; Kim, J.W.; Go, J.H.; Jang, S.C.; Lee, H.J.; Seo, M. A nasal myiasis in a 76-year-old female in Korea. Korean J. Parasitol. 2009, 47, 405–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behravan, M.; Vaezi-Kakhki, M.R.; Baharshahi, A. Comparing larvicidal effect of methanol extract of the aerial parts of henbane (Hyoscyamus niger L.) and oleander (Nerium oleander L.) plants on Anopheles spp. larvae (Diptera: Culicidae) in vitro. Zahedan J. Res. Med. Sci. 2017, 19, e8631. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.J.; Zhou, J.; Li, W.J.; Yan, Y.J.; Dai, B.; Fan, Z.J. Insecticidal activity of extracts from plant recourses in Xinjiang. Mod. Agrochem. 2006, 5, 34. [Google Scholar]
- Khater, H.F.; Hanafy, A.; Abdel-Mageed, A.D.; Ramadan, M.Y.; El-Madawy, R.S. Control of the myiasis-producing fly, Lucilia sericata, with Egyptian essential oils. Int. J. Derm. 2011, 50, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Morsy, T.A.; Mazyad, S.A.; el-Sharkawy, I.M. The larvicidal activity of solvent extracts of three medicinal plants against third instar larvae of Chrysomyia albiceps. J. Egypt. Soc. Parasitol. 1998, 28, 699–709. [Google Scholar] [PubMed]
- Khater, H.F. Biocontrol of Some Insects; Zagazig University: Benha, Egypt, 2003. [Google Scholar]
- Meurant, K.; Sernia, C.; Rembold, H. The effects of azadirachtin A on the morphology of the ring complex of Lucilia cuprina (Wied) larvae (Diptera: Insecta). Cell Tissue Res. 1994, 275, 247–254. [Google Scholar] [CrossRef]
- Begum, A.S. Bioactive non-alkaloidal secondary metabolites of Hyoscyamus niger Linn. seeds: A review. Res. J. Seed Sci. 2010, 3, 210–217. [Google Scholar] [CrossRef]
- Frohne, D.; Pfander, H.J. A Colour Atlas of Poisonous Plants; Wolfe Publishing: London, UK, 1983. [Google Scholar]
- Alizadeh, A.; Moshiri, M.; Alizadeh, J.; Balali-Mood, M. Black henbane and its toxicity—A descriptive review. Avicenna J. Phytomed. 2014, 4, 297–311. [Google Scholar]
- Swathi, S.; Murugananthan, G.; Ghosh, S.; Pradeep, A. Larvicidal and repellent activities of ethanolic extract of Datura stramonium leaves against mosquitoes. Int. J. Pharm. Phytochem. Res. 2012, 4, 25–27. [Google Scholar]
- Myers, J.H.; Moro-Sutherland, D.; Shook, J.E. Anticholinergic poisoning in colicky infants treated with hyoscyamine sulfate. Am. J. Emerg. Med. 1997, 15, 532–535. [Google Scholar] [CrossRef]
- Kamada, H.; Okamura, N.; Satake, M.; Harada, H.; Shimomura, K. Alkaloid production by hairy root cultures in Atropa belladonna. Plant Cell Rep. 1986, 5, 239–242. [Google Scholar] [CrossRef] [PubMed]
- El-Khateeb, R.M.; Abdel-Shafy, S.; Zayed, A.A. Insecticidal effects of neem seed and vegetable oils on larval and pupal stages of sheep blowfly, Lucilia sericata (Diptera: Calliphoridae). J. Egypt Vet. Med. Assoc. 2003, 63, 255–268. [Google Scholar]
- Hoda, S.M.; Fahmy, M.M.; Attia, M.M.; Rabab, M.; Shalaby, H.A.; Massoud, A.M. The insecticidal activity of two medicinal plants (Commiphora molmol) and (Balanites aegyptiaca) against the blowfly Lucilia sericata (Diptera: Calliphoridae). Int. J. Adv. Res. Biol. Sci 2016, 3, 144–158. [Google Scholar]
- Pritchard, M.H.; Kruse, G.O.W. The Collection and Preservation of Animal Parasites; University of Nebraska Press: Lincoln, NE, USA, 1982. [Google Scholar]
- Holloway, B.A. Morphological characters to identify adult Lucilia sericata (Meigen, 1826) and L. cuprina (Wiedemann, 1830)(Diptera: Calliphoridae). N. Zeal. J. Zool. 1991, 18, 413–420. [Google Scholar] [CrossRef]
- Smith, K.; Wall, R.; Howard, J.; Strong, L.; Marchiondo, A.; Jeannin, P. In vitro insecticidal effects of fipronil and β-cyfluthrin on larvae of the blowfly Lucilia sericata. Vet. Parasitol. 2000, 88, 261–268. [Google Scholar] [CrossRef]
- Firoozfar, F.; Mosa Kazemi, S.H.; Shemshad, K.; Baniardalani, M.; Abolhasani, M.; Biglarian, A.; Enayati, A.; Rafinejad, J. Laboratory colonization of Lucilia sericata Meigen (Diptera: Caliphoridae) strain from Hashtgerd, Iran. J. Vector Borne. Dis. 2012, 49, 23–26. [Google Scholar]
- Mroczek, T.; Głowniak, K.; Kowalska, J. Solid–liquid extraction and cation-exchange solid-phase extraction using a mixed-mode polymeric sorbent of Datura and related alkaloids. J. Chromatogr. A 2006, 1107, 9–18. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Am. Mosq. Control. Assoc. 1987, 3, 302–303. [Google Scholar] [CrossRef]



| Extract Type | Conc. (μg/mL) | 1st Instars | Pupa Number | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | p Value | Molted to 2nd | p Value | Molted to 3rd | p Value | ||||||
| No. | M.% | No. | M.% | No. | M.% | ||||||
| n-Hexane | 2 | 100 | 5 ± 1.9 a | ns | 95 | 5.3 ± 1.1 a | ns | 90 | 5.6 ± 2.3 a | ns | 85 |
| 4 | 100 | 12 ± 3.2 a | ns | 88 | 5.7 ± 1.4 a | ns | 83 | 9.6 ± 3.7 a | ns | 75 | |
| 8 | 100 | 13 ± 5.5 a | ns | 87 | 6.9 ± 2.4 a | ns | 81 | 12.3 ± 6.9 a | ns | 71 | |
| 16 | 100 | 18 ± 10.8 a | ns | 82 | 9.8 ± 2.8 a | ns | 74 | 9.5 ± 5.2 a | ns | 67 | |
| 32 | 100 | 24 ± 16.5 b | 0.038 | 76 | 11.8 ± 3.7 a | ns | 67 | 10.4 ± 3.1 a | ns | 60 | |
| Ethyl acetate | 2 | 100 | 2 ± 1.1 a | ns | 98 | 5.1 ± 2.7 a | ns | 93 | 3.2 ± 1.7 a | ns | 90 |
| 4 | 100 | 7 ± 4.3 a | ns | 93 | 7.5 ± 2.9 a | ns | 86 | 5.8 ± 2.8 a | ns | 81 | |
| 8 | 100 | 14 ± 9.6 a | ns | 86 | 8.1 ± 3.1 a | ns | 79 | 11.4 ± 4.5 a | ns | 70 | |
| 16 | 100 | 19 ± 11.3 a | ns | 81 | 12.3 ± 4.2 a | ns | 71 | 12.7 ± 3.6 a | ns | 62 | |
| 32 | 100 | 21 ± 13.2 a,b | 0.049 | 79 | 15.2 ± 3.6 a | ns | 67 | 22.4 ± 9.4 b | 0.037 | 52 | |
| Methanol | 2 | 100 | 12 ± 4.6 a | ns | 88 | 13.6 ± 4.7 a | ns | 76 | 19.7 ± 8.7 a | ns | 61 |
| 4 | 100 | 17 ± 7.5 a | ns | 83 | 18.1 ± 6.3 a | ns | 68 | 20.6 ± 7.3 a | ns | 54 | |
| 8 | 100 | 22 ± 9.9 b | 0.042 | 78 | 20.5 ± 7.8 a,b | 0.047 | 62 | 27.4 ± 8.1 b | 0.044 | 45 | |
| 16 | 100 | 25 ± 9.2 b | 0.037 | 75 | 26.7 ± 6.1 b,c | 0.034 | 55 | 32.7 ± 10.5 c | 0.005 | 37 | |
| 32 | 100 | 33 ± 10.6 b | 0.021 | 67 | 100.0 ± 0.0 e | 0.000 | 0 | 0.0 ± 0.0 a | ns | 0 | |
| Alkaloid extract | 2 | 100 | 26 ± 9.8 b | 0.028 | 74 | 81.1 ± 13.5 d | 0.019 | 14 | 100.0 ± 0.0 e | 0.000 | 0 |
| 4 | 100 | 38 ± 8.5 b | 0.018 | 62 | 88.7 ± 11.4 d | 0.014 | 7 | 100.0 ± 0.0 e | 0.000 | 0 | |
| 8 | 100 | 60 ± 10.4 c | 0.011 | 40 | 100.0 ± 0.0 e | 0.000 | 0 | 0.0 a | - | 0 | |
| 16 | 100 | 81 ± 14.3 d | 0.000 | 19 | 100.0 ± 0.0 e | 0.000 | 0 | 0.0 a | - | 0 | |
| 32 | 100 | 100 ± 0.0 e | 0.000 | 0 | 100.0 ± 0.0 e | 0.000 | 0 | 0.0 a | - | 0 | |
| Control | 100 | 1 ± 0.0 a | ns | 99 | 3.0 ± 1.6 a | ns | 96 | 0.0 a | ns | 96 | |
| Extract Type | Conc. (μg/mL) | 2nd Instars | Pupa Number | |||||
|---|---|---|---|---|---|---|---|---|
| 2nd | p Value | Molted to 3rd | p Value | |||||
| No. | M.% | No. | M.% | |||||
| n-Hexane | 2 | 100 | 4 ± 1.9 a | ns | 96 | 4.2 ± 2.7 a | ns | 92 |
| 4 | 100 | 7 ± 2.3 a | ns | 93 | 9.7 ± 3.1 a | ns | 84 | |
| 8 | 100 | 10 ± 2.1 a | ns | 90 | 12.2 ± 2.3 a | ns | 79 | |
| 16 | 100 | 13 ± 5.2 a | ns | 87 | 14.9 ± 5.4 a | ns | 74 | |
| 32 | 100 | 16 ± 4.9 a | ns | 84 | 22.6 ± 8.6 b | 0.028 | 65 | |
| Ethyl acetate | 2 | 100 | 12 ± 2.6 a | ns | 88 | 17.0 ± 9.2 a | ns | 73 |
| 4 | 100 | 15 ± 6.2 a | ns | 85 | 20.0 ± 12.9 a,b | 0.039 | 68 | |
| 8 | 100 | 21 ± 8.3 a,b | 0.035 | 79 | 22.7 ± 10.8 b | 0.020 | 61 | |
| 16 | 100 | 25 ± 7.5 b | 0.014 | 75 | 32.0 ± 18.2 b | 0.008 | 51 | |
| 32 | 100 | 32 ± 9.8 b | 0.020 | 68 | 41.2 ± 15.3 b,c | 0.000 | 40 | |
| Methanol | 2 | 100 | 22 ± 9.1 a,b | 0.024 | 78 | 23.1 ± 10.4 b | 0.018 | 60 |
| 4 | 100 | 27 ± 6.4 b | 0.007 | 73 | 32.9 ± 17.1 b | 0.000 | 49 | |
| 8 | 100 | 34 ± 8.0 b | 0.003 | 66 | 45.5 ± 14.8 c | 0.000 | 36 | |
| 16 | 100 | 45 ± 11.6 c | 0.000 | 55 | 47.3 ± 18.9 c | 0.000 | 29 | |
| 32 | 100 | 52 ± 19.3 c | 0.000 | 48 | 52.1 ± 17.3 c | 0.000 | 23 | |
| Alkaloid extract | 2 | 100 | 25 ± 8.5 b | 0.017 | 75 | 25.3 ± 10.8 b | 0.011 | 56 |
| 4 | 100 | 38 ± 10.9 b | 0.000 | 62 | 51.6 ± 13.4 c | 0.000 | 30 | |
| 8 | 100 | 47 ± 9.3 c | 0.000 | 53 | 75.5 ± 9.6 d | 0.000 | 13 | |
| 16 | 100 | 89 ± 11.2 d | 0.000 | 11 | 0.0 ± 0.0 a | - | 0 | |
| 32 | 100 | 100 ± 0.0 e | 0.000 | 0 | 0.0 ± 0.0 a | - | 0 | |
| Control | 100 | 0 ± 0.0 a | ns | 100 | 2.0 ± 0.6 a | ns | 98 | |
| Extract Type | Conc. (μg/mL) | 3rd Instars | p Value | Pupa Number | |
|---|---|---|---|---|---|
| No. | M.% | ||||
| n-Hexane | 2 | 100 | 3 ± 1.8 a | ns | 97 |
| 4 | 100 | 12 ± 6.4 a | ns | 88 | |
| 8 | 100 | 14 ± 4.9 a | ns | 86 | |
| 16 | 100 | 20 ± 12.3 a | ns | 80 | |
| 32 | 100 | 23 ± 11.8 a,b | 0.045 | 77 | |
| Ethyl acetate | 2 | 100 | 18 ± 7.3 a | ns | 82 |
| 4 | 100 | 25 ± 9.6 b | 0.031 | 75 | |
| 8 | 100 | 28 ± 8.0 b | 0.024 | 72 | |
| 16 | 100 | 32 ± 10.4 b | 0.011 | 68 | |
| 32 | 100 | 41 ± 13.7 c | 0.000 | 59 | |
| Methanol | 2 | 100 | 17 ± 18.2 a | ns | 73 |
| 4 | 100 | 36 ± 15.1 b | 0.003 | 64 | |
| 8 | 100 | 48 ± 19.7 c | 0.000 | 52 | |
| 16 | 100 | 54 ± 16.5 c | 0.000 | 46 | |
| 32 | 100 | 62 ± 27.4 c | 0.000 | 38 | |
| Alkaloid extract | 2 | 100 | 29 ± 5.6 b | 0.020 | 71 |
| 4 | 100 | 40 ± 9.5 c | 0.000 | 60 | |
| 8 | 100 | 69 ± 10.7 d | 0.000 | 31 | |
| 16 | 100 | 100 ± 0.0 e | 0.000 | 0 | |
| 32 | 100 | 100 ± 0.0 e | 0.000 | 0 | |
| Control | 100 | 0 ± 0.0 a | ns | 100 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Küpeli Akkol, E.; Ilhan, M.; Kozan, E.; Gürağaç Dereli, F.T.; Sak, M.; Sobarzo-Sánchez, E. Insecticidal Activity of Hyoscyamus niger L. on Lucilia sericata Causing Myiasis. Plants 2020, 9, 655. https://doi.org/10.3390/plants9050655
Küpeli Akkol E, Ilhan M, Kozan E, Gürağaç Dereli FT, Sak M, Sobarzo-Sánchez E. Insecticidal Activity of Hyoscyamus niger L. on Lucilia sericata Causing Myiasis. Plants. 2020; 9(5):655. https://doi.org/10.3390/plants9050655
Chicago/Turabian StyleKüpeli Akkol, Esra, Mert Ilhan, Esma Kozan, Fatma Tuğçe Gürağaç Dereli, Mustafa Sak, and Eduardo Sobarzo-Sánchez. 2020. "Insecticidal Activity of Hyoscyamus niger L. on Lucilia sericata Causing Myiasis" Plants 9, no. 5: 655. https://doi.org/10.3390/plants9050655