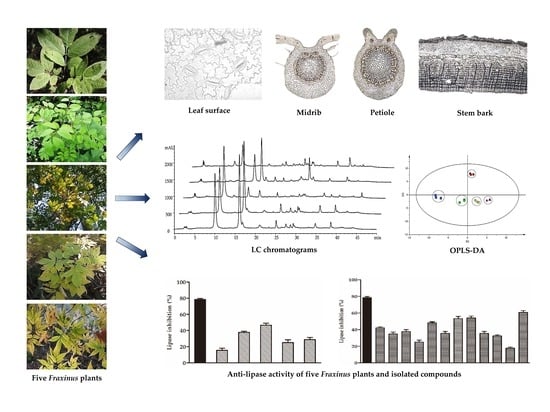
Comparative Studies of Fraxinus Species from Korea Using Microscopic Characterization, Phytochemical Analysis, and Anti-Lipase Enzyme Activity
Abstract
:1. Introduction
2. Results
2.1. Anatomical Characteristics of the Leaf
2.2. Anatomical Characteristics of the Petiole
2.3. Anatomical Characteristics of the Midrib
2.4. Anatomical Characteristics of the Stem Bark
2.5. HPLC-DAD Profiles of Five Fraxinus Plants
2.6. Orthogonal Projections to Latent Structures-Discriminant Analysis (OPLS-DA) Multivariate Statistical Analysis
2.7. Anti-lipase Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Materials
4.3. Anatomical Analysis
4.4. Sample Preparation
4.5. HPLC-DAD Analysis
4.6. Pancreatic Lipase Inhibition Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wallander, E. Systematics of Fraxinus (Oleaceae) and evolution of dioec. Plant. Syst. Evol. 2007, 273, 25–49. [Google Scholar] [CrossRef]
- Kim, S.C.; Baek, S.H.; Lee, M.W.; Nak, K. The complete chloroplast genome of Fraxinus chiisanensis (Oleaceae). Mitochondrial DNA B 2017, 2, 823–824. [Google Scholar] [CrossRef] [Green Version]
- Hinsinger, D.D.; Basak, J.; Gaudeul, M.; Cruaud, C.; Bertolino, P.; Frascaria-Lacoste, N.; Bousquet, J. The phylogeny and biogeographic history of ashes (Fraxinus, Oleaceae) highlight the roles of migration and vicariance in the diversification of temperate trees. PLoS ONE 2013, 8, e80431. [Google Scholar] [CrossRef]
- MacFarlane, D.W.; Meyer, S.P. Characteristics and distribution of potential ash tree hosts for emerald ash borer. Forest Ecol. Manag. 2005, 213, 15–24. [Google Scholar] [CrossRef]
- Wallander, E.; Albert, V.A. Phylogeny and classification of Oleaceae based on rps16 and trnL-F sequence data. Am. J. Bot. 2000, 1827–1841. [Google Scholar] [CrossRef]
- Kim, C. The distribution of the woody plants of South Korea based on herbarium (SNUA) material of The arboretum (VII)—Rhamnaceae. Bull. Seoul Natl. Univ. Arbor. 2001, 21, 1–15. [Google Scholar]
- Calis, I.; Hosny, M.; Khalifa, T.; Nishibe, S. Secoiridoids from Fraxinus angustifolia. Phytochemistry 1993, 33, 1453–1456. [Google Scholar] [CrossRef]
- Kim, H.C.; An, R.B.; Jeong, G.S.; Oh, S.H. 1,1-diphenyl-1-picrylhydrazyl radical scavenging compounds from Cortex Fraxini. Nat. Prod. Sci. 2005, 11, 150–154. [Google Scholar]
- Guo, S.; Guo, T.; Cheng, N.; Liu, Q.; Zhang, Y.; Bai, L.; Zhang, L.; Cao, W.; Ho, C.T.; Bai, N. Hepatoprotective standardized EtOH-water extract from the seeds of Fraxinus rhynchophylla Hance. J. Tradit. Complement. Med. 2017, 7, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Xue, G.; Liu, F.; Gong, X. Immunosuppressive effect of extracts from leaves of Fraxinus mandshurica Rupr. Bioengineered 2017, 8, 212–216. [Google Scholar] [CrossRef] [Green Version]
- Hiroki, T.; Sueo, H.; Sansei, N. Coumarins from bark of Fraxinus japonica and F. mandshurica var. japonica. Chem. Pharm. Bull 1985, 33, 4069–4073. [Google Scholar]
- Kostova, I.; Iossifova, T. Chemical components of Fraxinus species. Fitoterapia 2007, 78, 85–106. [Google Scholar] [CrossRef]
- Sarfraz, I.; Rasul, A.; Jabeen, F.; Younis, T.; Zahoor, M.K.; Arshad, M.; Ali, M. Fraxinus: A plant with versatile pharmacological and biological activities. Evid. Based Complementary Altern. Med. 2017, 2017, 4269868. [Google Scholar] [CrossRef] [Green Version]
- Vandal, J.; Abou-Zaid, M.M.; Ferroni, G.; Leduc, L.G. Antimicrobial activity of natural products from the flora of Northern Ontario, Canada. Pharma. Biol. 2015, 53, 800–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, J.W.; Simon, M. British Herbal Pharmacopoeia, 4th ed.; British Herbal Medicine Association press: Dorset, UK, 1996; p. 212. [Google Scholar]
- Upton, R.; Graff, A.; Jolliffe, G.; Langer, R.; Williamson, W. American Herbal Pharmacopoeia: Botanical Pharmacognosy—Microscopic Characterization of Botanical Medicines, 1st ed.; CRC Press: Boca Raton, FL, USA, 2011; p. 800. [Google Scholar]
- State Pharmacopoeia Commission of the PRC. Pharmacopoeia of the People’s Republic of China, Part I, 10th ed.; China Medical Science Press: Beijing, China, 2015; p. 271. [Google Scholar]
- The Ministry of Health, Labour and Welfare. Japanese Pharmacopoeia (English Version), 17th ed.; The Stationery Office: Tokyo, Japan, 2016; p. 134.
- Obradovic, M.; Krajsek, S.S.; Dermastia, M.; Kreft, S. A new method for the authentication of plant samples by analyzing fingerprint chromatograms. Phytochem. Anal. 2007, 18, 123–132. [Google Scholar] [CrossRef]
- Kothavade, R.J.; Dhurat, R.S.; Mishra, S.N.; Kothavade, U.R. Clinical and laboratory aspects of the diagnosis and management of cutaneous and subcutaneous infections caused by rapidly growing mycobacteria. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 161–188. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Shin, E.; Liu, Q.; Kim, S.B.; Choi, K.M.; Yoo, H.S.; Hwang, B.Y.; Lee, M.K. Secoiridoids from the stem barks of Fraxinus rhynchophylla with pancreatic lipase inhibitory activity. Nat. Prod. Res. 2013, 27, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Shin, E.; Liu, Q.; Kim, S.B.; Choi, K.M.; Yoo, H.S.; Hwang, B.Y.; Lee, M.K. Lignan Derivatives from Fraxinus rhynchophylla and inhibitory activity on pancreatic lipase. Nat. Prod. Sci. 2012, 18, 116–120. [Google Scholar]
- Lunagariya, N.A.; Patel, N.K.; Jagtap, S.C.; Bhutani, K.K. Inhibitors of pancreatic lipase: State of the art and clinical perspectives. EXCLI J. 2014, 13, 897–921. [Google Scholar]
- Kim, G.N.; Shin, M.R.; Shin, S.H.; Lee, A.R.; Lee, J.Y.; Seo, B.I.; Kim, M.Y.; Kim, T.H.; Noh, J.S.; Rhee, M.H. Study of antiobesity effect through inhibition of pancreatic lipase activity of diospyros kaki Fruit and Citrus unshiu peel. Biomed Res. Int. 2016, 2016, 1723042. [Google Scholar] [CrossRef] [Green Version]
- De la Garza, A.L.; Milagro, F.I.; Boque, N.; Campion, J.; Martinez, J.A. Natural inhibitors of pancreatic lipase as new players in obesity treatment. Planta Med. 2011, 77, 773–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akter, K.-M.; Kim, H.-J.; Park, W.S.; Khalil, A.A.K.; Ahn, M.-J. Anti-Helicobacter pylori activity of compounds isolated from Fraxinus mandshurica bark. Nat. Prod. Sci. 2020, in press. [Google Scholar]
- Smillie, T.J.; Khan, I.A. A comprehensive approach to identifying and authenticating botanical products. Clin. Pharmacol. Ther. 2010, 87, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Chang, C.-S.; Kim, H.; Choi, D.Y. A preliminary population genetic study of an overlooked endemic ash, Fraxinus chiisanensis in Korea using allozyme variation. J. Korean For. Soc. 2011, 98, 531–538. [Google Scholar]
- Kim, Y.-S.; Kim, H.; Son, S.-W. The IUCN Red List of Threatened Species. 2016. [Google Scholar] [CrossRef]
- Min, W.K.; Jeon, J.I.; Chang, C.S. A taxonomic reconsideration of Fraxinus chiisanensis (Oleaceae) in South Korea. J. Korean For. Soc. 2001, 90, 266–276. [Google Scholar]
- Kang, J.G.; Park, C.Y. Anti-obesity drugs: A review about their effects and safety. Diabetes Metab. J. 2012, 36, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Glazer, G. Long-term pharmacotherapy of obesity 2000: A review of efficacy and safety. Arch. Intern. Med. 2001, 161, 1814–1824. [Google Scholar] [CrossRef] [Green Version]
- Christensen, R.; Kristensen, P.K.; Bartels, E.M.; Bliddal, H.; Astrup, A. Efficacy and safety of the weight-loss drug rimonabant: A meta-analysis of randomised trials. Lancet 2007, 370, 1706–1713. [Google Scholar] [CrossRef]
- Buchholz, T.; Melzig, M.F. Polyphenolic compounds as pancreatic lipase inhibitors. Planta Med. 2015, 81, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Kuem, N.; Song, S.J.; Yu, R.; Yun, J.W.; Park, T. Oleuropein attenuates visceral adiposity in high-fat diet-induced obese mice through the modulation of WNT10b- and galanin-mediated signalings. Mol. Nutr. Food Res. 2014, 58, 2166–2176. [Google Scholar] [CrossRef]
- Karkovic Markovic, A.; Toric, J.; Barbaric, M.; Jakobusic Brala, C. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, T.H.; Kim, S.B.; Ahn, J.H.; Liu, Q.; Hwang, B.Y.; Lee, M.K. Inhibitory activity of benzophenones from Anemarrhena asphodeloides on pancreatic lipase. Nat. Prod. Commun. 2013, 8, 481–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beladjila, K.A.; Berrehal, D.; De Tommasi, N.; Granchi, C.; Bononi, G.; Braca, A.; De Leo, M. New phenylethanoid glycosides from Cistanche phelypaea and their activity as inhibitors of monoacylglycerol lipase (MAGL). Planta Med. 2018, 84, 710–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.S.; Lee, Y.M.; Kim, H.; Kim, J.; Jang, D.S.; Kim, J.H.; Kim, J.S. Anti-obesity effect of Morus bombycis root extract: Anti-lipase activity and lipolytic effect. J. Ethnopharmacol. 2010, 130, 621–624. [Google Scholar] [CrossRef]









| Parameters | FC | FM | FR | FS | FSV |
|---|---|---|---|---|---|
| Number of glandular trichome (in 500 × 500 μm2) | 1.7 ± 0.2c | 2.4 ± 0.4b | 2.5 ± 0.4b | 3.6 ± 0.9b | 7.0 ± 0.4a |
| Diameter of glandular trichome (μm) | 41.1 ± 1.9a | 42.8 ± 3.7a | 36.3 ± 0.8b | 42.3 ± 2.8a | 32.3 ± 1.5c |
| Number of epidermal cells (in 300 × 300 μm2) | 78.4 ± 11.3c | 148.4 ± 12.5a | 98.5 ± 14.2c | 92.3 ± 4.8c | 122.8 ± 8.2b |
| Number of stomata (in 300 × 300 μm2) | 11.7 ± 0.4e | 33.9 ± 4.0a | 19.3 ± 1.8c | 14.7 ± 1.3d | 24.6 ± 3.0b |
| Stomatal length (μm) | 37.5 ± 2.0a | 22.8 ± 0.6c | 33.4 ± 0.7b | 34.7 ± 2.3ab | 32.5 ± 1.6b |
| Stomatal width (μm) | 26.0 ± 2.4a | 17.1 ± 0.5b | 23.9 ± 1.5a | 24.2 ± 2.5a | 25.0 ± 1.5a |
| Stomatal index | 13.0 ± 1.1b | 18.7 ± 1.5a | 16.4 ± 1.8a | 13.7 ± 1.3b | 16.7 ± 0.9a |
| Parameters | FC | FM | FR | FS | FSV |
|---|---|---|---|---|---|
| Size of epidermal cell of adaxial portion (length × width, μm) | 17.4 ± 1.3a × 17.8 ± 1a | 11.2 ± 1c × 11.8 ± 1b | 19.4 ± 2.3a × 15.6 ± 1.7ab | 13.8 ± 1.3b × 12.4 ± 2.2b | 12.9 ± 1b × 11.6 ± 0.9b |
| Size of epidermal cell of abaxial portion (length × width, μm) | 13.3 ± 1.4b × 13.6 ± 1.6b | 10.2 ± 1.0c × 11.5 ± 1b | 21.1 ± 2.3a × 18.7 ± 3.5a | 17.5 ± 1.9a × 14.9 ± 1.0ab | 13.6 ± 0.9b × 12.4 ± 0.8b |
| Ratio of cortex thickness to radius in abaxial portion | 0.23 ± 0.03b | 0.29 ± 0.01a | 0.30 ± 0.04a | 0.28 ± 0.02a | 0.27 ± 0.01a |
| Diameter of parenchyma cell of cortex in adaxial portion (μm) | 35.3 ± 2.5a | 24.8 ± 1.9b | 24.9 ± 2.0b | 24.7 ± 1.5b | 20.0 ± 0.4c |
| Diameter of parenchyma cell in abaxial portion (μm) | 51.6 ± 4.6a | 42.5 ± 1.8b | 40.4 ± 2.7b | 33.4 ± 2.0c | 27.7 ± 1.2d |
| Number of sclerenchyma cell (in 300 × 300 μm2) | 40.9 ± 5.3d | 51.3 ± 3.2c | 70.5 ± 4.7b | 54.9 ± 3.0c | 80.3 ± 3.4a |
| Diameter of parenchyma cell in pith (μm) | 81.3 ± 2.6a | 54.3 ± 3.0b | 54.5 ± 10.9b | 37.7 ± 2.7c | 30.9 ± 2.3d |
| Parameters | FC | FM | FR | FS | FSV |
|---|---|---|---|---|---|
| Size of epidermal cell of adaxial portion (length × width, μm) | 21.8 ± 1.5a × 19.1 ± 1.6a | 17.0 ± 2.5b × 14.9 ± 0.3b | 21.8 ± 4.0ab × 16.6 ± 2.1ab | 15.0 ± 1.1b × 13.5 ± 1.6b | 13.1 ± 0.4c × 11.9 ± 0.9b |
| Size of epidermal cell of abaxial portion (length × width, μm) | 13.6 ± 1.1ab × 14.3 ± 1.0b | 11.3 ± 1.8b × 13.6 ± 1.4b | 16.3 ± 2.0a × 18.8 ± 2.4a | 13.1 ± 1.0b × 15.1 ± 2.2ab | 12.4 ± 0.6b × 13.2 ± 0.5b |
| Number of sclerenchyma cell (in 300 × 300 μm2) | 38.6 ± 3.0c | 52.7 ± 9.2b | 70.1 ± 6.5b | 75.5 ± 17.1b | 114.8 ± 18.7a |
| Diameter of parenchyma cell in adaxial portion (μm) | 28.4 ± 1.9a | 23.8 ± 1.4ab | 29.9 ± 5.4a | 20.7 ± 2.3b | 18.1 ± 0.8b |
| Diameter of parenchyma cell in abaxial portion (μm) | 53.4 ± 2.5a | 43.8 ± 1.2b | 45.2 ± 2.4b | 35.0 ± 2.7c | 29.2 ± 0.7d |
| Diameter of parenchyma cell in pith (μm) | 75.7 ± 3.7a | 61.4 ± 4.4b | 49.2 ± 4.1c | 28.4 ± 4.0d | 22.2 ± 3.5d |
| Size of palisade cell (length × width, μm) | 48.6 ± 4.6a × 17.4 ± 4.7a | 44.3 ± 4.5ab × 12.5 ± 0.4a | 44.4 ± 6.4ab × 14.2 ± 0.7a | 46.2 ± 3.0a × 14.4 ± 1.5a | 40.8 ± 1.8b × 12.5 ± 0.5a |
| Palisade ratio | 1.7 ± 0.1b | 2.0 ± 0.2a | 1.9 ± 0.2ab | 2.1 ± 0.2a | 2.2 ± 0.2a |
| Parameters | FC | FM | FR | FS | FSV |
|---|---|---|---|---|---|
| Size of cork cell (length × width, μm) | 21.2 ± 3.4ab × 53.6 ± 4.9a | 20.1 ± 4.8ab × 40.0 ± 3.6b | 13.6 ± 1.3b × 33.1 ± 2.3c | 16.2 ± 0.9b × 36.8 ± 1.8bc | 22.1 ± 1.1a × 36.2 ± 1.6bc |
| Diameter of parenchyma cell (in cortex length × width, μm) | 28.1 ± 2.0a × 55.3 ± 2.2a | 24.8 ± 1.4ab × 44.5 ± 1.9b | 24.5 ± 2.7ab × 51.6 ± 1.5a | 23.0 ± 1.2b × 42.9 ± 2.5b | 22.8 ± 0.7b × 40.6 ± 1.7b |
| Number of phloem fiber (in 500 × 500 μm2) | 83.7 ± 4.6c | 85.4 ± 3.2c | 123.0 ± 16.5ab | 138.4 ± 3.5a | 122.7 ± 4.2b |
| Number of phloem ray (in 500 × 500 μm2) | 3.4 ± 0.3b | 4.5 ± 0.5ab | 3.3 ± 0.2b | 3.6 ± 0.5b | 4.8 ± 0.2a |
| Size of phloem ray (length × width, μm) | 40.5 ± 0.3a × 21.1 ± 2.0a | 34.8 ± 0.8b × 15.0 ± 1.5b | 34.5 ± 4.3ab × 20.9 ± 1.2a | 32.1 ± 4.5b × 15.5 ± 2.1b | 28.3 ± 0.4b × 14.7 ± 0.4b |
| Compounds | Season | FC | FM | FR | FS | FSV |
|---|---|---|---|---|---|---|
| Esculin (1) | Summer | ND* | 0.31 ± 0.04c | 32.5 ± 2.4b | 31.6 ± 2.5b | 38.7 ± 3.1a |
| Autumn | ND | 0.24 ± 0.09c | 24.8 ± 0.8a | 28.8 ± 4.8a | 20.8 ± 2.5b | |
| Tyrosol (2) | Summer | ND | 0.56 ± 0.11a | ND | ND | ND |
| Autumn | ND | 0.48 ± 0.09a | ND | ND | ND | |
| Isofraxidin-7-O-β-D-glucopyranoside (3) | Summer | ND | 2.39 ± 0.41a | 0.23 ± 0.04b | ND | ND |
| Autumn | ND | 1.66 ± 0.14a | 0.22 ± 0.01b | ND | ND | |
| Fraxin (4) | Summer | ND | 14.8 ± 2.2b | 10.8 ± 1.4c | 58.1 ± 3.9a | 61.7 ± 3.9a |
| Autumn | ND | 8.32 ± 1.61c | ND | 37.0 ± 2.4a | 24.8 ± 3.0b | |
| Mandshurin (5) | Summer | ND | 20.6 ± 0.7a | ND | ND | ND |
| Autumn | ND | 10.2 ± 0.5a | ND | ND | ND | |
| Fraxetin (6) | Summer | ND | 0.27 ± 0.03b | 0.08 ± 0.00c | 1.39 ± 0.12a | 1.53 ± 0.15a |
| Autumn | ND | 0.26 ± 0.01a | ND | ND | ND | |
| Calceolarioside A (7) | Summer | 10.9 ± 1.0b | 4.26 ± 1.50c | 2.23 ± 0.42d | 13.5 ± 1.2a | 10.7 ± 0.4b |
| Autumn | 7.44 ± 1.23a | 1.96 ± 0.27c | ND | 7.69 ± 1.42a | 2.72 ± 0.53b | |
| Calceolarioside B (8) | Summer | ND | 30.4 ± 2.9a | 10.3 ± 0.7b | 10.6 ± 1.0b | 9.11 ± 1.32b |
| Autumn | ND | 9.94 ± 1.45a | 4.04 ± 0.27b | 8.90 ± 1.74a | 7.91 ± 1.13a | |
| Pinoresinol-4′-O-β-D-glucopyranoside (9) | Summer | ND | 12.1 ± 0.4a | 0.62 ± 0.17d | 1.74 ± 0.06b | 0.82 ± 0.05c |
| Autumn | ND | 9.74 ± 0.56a | 0.61 ± 0.06c | 0.77 ± 0.04b | 0.24 ± 0.00d | |
| Fraxinol (10) | Summer | ND | 0.53 ± 0.05a | ND | ND | ND |
| Autumn | ND | 0.47 ± 0.02a | ND | ND | ND | |
| Oleuropein (11) | Summer | ND | 16.9 ± 0.4a | 10.2 ± 2.0b | 5.34 ± 0.99d | 6.77 ± 0.25c |
| Autumn | ND | 6.98 ± 0.32a | 5.10 ± 0.97b | 2.97 ± 0.59c | 6.26 ± 0.95ab | |
| Ligstroside (12) | Summer | 27.9 ± 1.8a | 15.5 ± 0.9b | 2.11 ± 0.41c | 2.74 ± 0.47c | 1.97 ± 0.22cd |
| Autumn | 23.9 ± 0.8a | 14.7 ± 0.7b | ND | 2.51 ± 0.50c | 1.80 ± 0.31c | |
| Total coumarins | Summer | ND | 38.9 ± 3.4c | 43.6 ± 3.8c | 91.1 ± 6.5b | 101.9 ± 7.2a |
| Autumn | ND | 21.2 ± 2.3d | 25.5 ± 0.8c | 65.8 ± 7.2a | 45.5 ± 5.5b | |
| Total phenylethanoids | Summer | 10.9 ± 1.0d | 35.3 ± 4.5a | 12.5 ± 1.1d | 24.1 ± 2.2b | 19.8 ± 1.7c |
| Autumn | 7.44 ± 1.23c | 12.4 ± 1.7b | 4.04 ± 0.27d | 16.6 ± 3.2a | 10.6 ± 1.7bc | |
| Total secoiridoids | Summer | 27.9 ± 1.8b | 32.4 ± 1.3a | 12.3 ± 2.4c | 8.08 ± 1.46d | 8.74 ± 0.47d |
| Autumn | 23.9 ± 0.8a | 21.7 ± 1.0b | 5.08 ± 0.97d | 5.48 ± 1.09d | 8.06 ± 1.26c | |
| Total | Summer | 38.8 ± 2.8c | 118.6 ± 9.6a | 69.1 ± 7.5b | 125.0 ± 10.2a | 131.3 ± 9.4a |
| Autumn | 31.3 ± 2.0c | 65.0 ± 5.7b | 34.8 ± 2.1c | 88.6 ± 11.5a | 64.5 ± 8.4b |
| Botanical Name | Collection Places (Latitude, Longitude) | Collection Year | Specimen No. | Abbreviations |
|---|---|---|---|---|
| Fraxinus chiisanensis Nakai | Sancheong (35°27′55.9″N, 127°56′06.7″E) (35°27′55.4″N, 127°56′07.9″E) Jiri Mt. (35°14′58.8″N, 127°42′31.2″E) (35°14′58.0″N, 127°42′29.8″E) Pocheon (37°45′07.1″N, 127°09′58.7″E) | 2016–2018 | PGSC-560-1~7 | FC |
| F. mandshurica Rupr. | Sancheong (35°14′00.1″N, 127°47′53.9″E) Jinju (35°10′53.3″N, 128°05′42.8″E) (35°13′59.9″N, 127°47′54.1″E) Pocheon (37°44′57.2″N, 127°09′55.7″E) | 2016–2018 | PGSC-561-1~12 | FM |
| F. rhynchophylla Hance | Jinju (35°10′53.1″N, 128°05′42.6″E) Jiri Mt. (35°15′02.1″N, 127°42′30.1″E) Youngwol (37°12′18.4″N, 128°26′05.3″E) Pocheon (37°45′10.2″N, 127°09′49.6″E) | 2016–2018 | PGSC-562-1~20 | FR |
| F. sieboldiana Blume | Jiri Mt. (35°14′54.5″N, 127°42′18.3″E) Miryang (35°34′06.6″N, 128°59′34.4″E) (35°34′04.7″N, 128°59′34.9″E) Pocheon (37°45′11.9″N, 127°09′54.9″E) | 2016–2019 | PGSC-563-1~15 | FS |
| F. sieboldiana var. angustata Blume | Jiri Mt. (35°14′53.7″N, 127°42′30.0″E) Miryang (35°34′05.2″N, 128°59′34.4″E) (35°34′05.7″N, 128°59′31.8″E) Sancheong (35°17′46.8″N, 127°58′29.6″E) Pocheon (37°45′11.4″N, 127°09′56.2″E) | 2016–2019 | PGSC-564-1~15 | FSV |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akter, K.-M.; Park, W.S.; Kim, H.-J.; Khalil, A.A.K.; Ahn, M.-J. Comparative Studies of Fraxinus Species from Korea Using Microscopic Characterization, Phytochemical Analysis, and Anti-Lipase Enzyme Activity. Plants 2020, 9, 534. https://doi.org/10.3390/plants9040534
Akter K-M, Park WS, Kim H-J, Khalil AAK, Ahn M-J. Comparative Studies of Fraxinus Species from Korea Using Microscopic Characterization, Phytochemical Analysis, and Anti-Lipase Enzyme Activity. Plants. 2020; 9(4):534. https://doi.org/10.3390/plants9040534
Chicago/Turabian StyleAkter, Kazi-Marjahan, Woo Sung Park, Hye-Jin Kim, Atif Ali Khan Khalil, and Mi-Jeong Ahn. 2020. "Comparative Studies of Fraxinus Species from Korea Using Microscopic Characterization, Phytochemical Analysis, and Anti-Lipase Enzyme Activity" Plants 9, no. 4: 534. https://doi.org/10.3390/plants9040534