Agro-Techniques for Lodging Stress Management in Maize-Soybean Intercropping System—A Review
Abstract
:1. Introduction
2. Lodging; A Serious Threat to Intercropping System
2.1. Stem Development and Lignin Metabolism under Intercropping
2.2. Role of Carbohydrates and Lignin Biosynthesis
2.3. Plant Hormonal Activities and Shade Avoidance under Intercropping SYSTEM
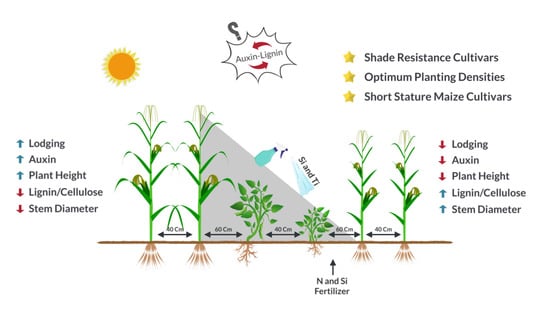
3. Agro-Techniques for the Management of Lodging Stress
3.1. Genetic Manipulation for Increasing Lodging Resistance
3.2. Proper Sowing Time and Planting Density
3.3. Efficient Use of Fertilizers
3.4. Development of Lodging Resistant Cultivars
3.5. Role of Silicon and Titanium
3.6. Chemical Approaches
4. Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oelbermann, M.; Echarte, L. Evaluating soil carbon and nitrogen dynamics in recently established maize-soyabean inter-cropping systems. Eur. J. Soil Sci. 2011, 62, 35–41. [Google Scholar] [CrossRef]
- Tscharntke, T.; Clough, Y.; Wanger, T.C.; Jackson, L.; Motzke, I.; Perfecto, I.; Vandermeer, J.; Whitbread, A. Global food security, biodiversity conservation and the future of agricultural intensification. Biol. Conserv. 2012, 151, 53–59. [Google Scholar] [CrossRef]
- Rosenberg, M. Current World Population. 2011. Available online: www.about.com (accessed on 1 February 2011).
- Vogel, E.; Meyer, R. Climate Change, Climate Extremes, and Global Food Production—Adaptation in the Agricultural Sector. In Resilience; Elsevier: Amsterdam, The Netherlands, 2018; pp. 31–49. [Google Scholar]
- Yang, F.; Liao, D.; Fan, Y.; Gao, R.; Wu, X.; Rahman, T.; Yong, T.; Liu, W.; Liu, J.; Du, J.; et al. Effect of narrow-row planting patterns on crop competitive and economic advantage in maize–soybean relay strip intercropping system. Plant Prod. Sci. 2016, 20, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kermah, M.; Franke, A.C.; Adjei-Nsiah, S.; Ahiabor, B.D.K.; Abaidoo, R.C.; Giller, K.E. Maize-grain legume intercropping for enhanced resource use efficiency and crop productivity in the Guinea savanna of northern Ghana. Field Crops Res. 2017, 213, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Hussain, S.; Ahmed, Z.; Yang, F.; Wang, X.; Liu, W.; Yong, T.; Du, J.; Shu, K.; Yang, W.; et al. Comparative analysis of maize–soybean strip intercropping systems: A review. Plant Prod. Sci. 2019, 22, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Knörzer, H.; Graeff-Hönninger, S.; Guo, B.; Wang, P.; Claupein, W. The rediscovery of intercropping in China: A traditional cropping system for future Chinese agriculture—A review. In Climate Change, Intercropping, Pest Control and Beneficial Microorganisms; Springer: Berlin/Heidelberg, Germany, 2009; pp. 13–44. [Google Scholar]
- Hong, Y.; Heerink, N.; Zhao, M.; van der Werf, W. Intercropping contributes to a higher technical efficiency in smallholder farming: Evidence from a case study in Gaotai County, China. Agric. Syst. 2019, 173, 317–324. [Google Scholar] [CrossRef]
- Li, L.; Li, S.-M.; Sun, J.-H.; Zhou, L.-L.; Bao, X.-G.; Zhang, H.-G.; Zhang, F.-S. Diversity enhances agricultural productivity via rhizosphere phosphorus facilitation on phosphorus-deficient soils. Proc. Natl. Acad. Sci. USA 2007, 104, 11192–11196. [Google Scholar] [CrossRef] [Green Version]
- Du, J.-B.; Han, T.-F.; Gai, J.-Y.; Yong, T.-W.; Xin, S.; Wang, X.-C.; Feng, Y.; Jiang, L.; Kai, S.; Liu, W.-G. Maize-soybean strip intercropping: Achieved a balance between high productivity and sustainability. J. Integr. Agric. 2018, 17, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Chen, J.; Cheng, Y.; Raza, M.A.; Wu, X.; Wang, Z.; Liu, Q.; Wang, R.; Wang, X.; Yong, T. Effect of shading and light recovery on the growth, leaf structure, and photosynthetic performance of soybean in a maize-soybean relay-strip intercropping system. PLoS ONE 2018, 13, e0198159. [Google Scholar] [CrossRef]
- Ahmed, S.; Raza, M.A.; Zhou, T.; Hussain, S.; Khalid, M.H.B.; Feng, L.; Wasaya, A.; Iqbal, N.; Ahmed, A.; Liu, W. Responses of soybean dry matter production, phosphorus accumulation, and seed yield to sowing time under relay intercropping with maize. Agronomy 2018, 8, 282. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Raza, M.A.; Li, Z.; Chen, Y.; Khalid, M.H.B.; Du, J.; Liu, W.; Wu, X.; Song, C.; Yu, L. The influence of light intensity and leaf movement on photosynthesis characteristics and carbon balance of soybean. Front. Plant Sci. 2019, 9, 1952. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liao, D.; Wu, X.; Gao, R.; Fan, Y.; Raza, M.A.; Wang, X.; Yong, T.; Liu, W.; Liu, J. Effect of aboveground and belowground interactions on the intercrop yields in maize-soybean relay intercropping systems. Field Crops Res. 2017, 203, 16–23. [Google Scholar] [CrossRef]
- Raza, M.A.; Feng, L.Y.; van Der Werf, W.; Iqbal, N.; Khalid, M.H.B.; Chen, Y.K.; Wasaya, A.; Ahmed, S.; Din, A.M.U.; Khan, A. Maize leaf-removal: A new agronomic approach to increase dry matter, flower number and seed-yield of soybean in maize soybean relay intercropping system. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardiner, B.; Berry, P.; Moulia, B. Wind impacts on plant growth, mechanics and damage. Plant Sci. 2016, 245, 94–118. [Google Scholar] [CrossRef]
- Wassmann, R.; Jagadish, S.; Sumfleth, K.; Pathak, H.; Howell, G.; Ismail, A.; Serraj, R.; Redona, E.; Singh, R.; Heuer, S. Regional vulnerability of climate change impacts on Asian rice production and scope for adaptation. Adv. Agron. 2009, 102, 91–133. [Google Scholar]
- Liu, W.; Deng, Y.; Hussain, S.; Zou, J.; Yuan, J.; Luo, L.; Yang, C.; Yuan, X.; Yang, W. Relationship between cellulose accumulation and lodging resistance in the stem of relay intercropped soybean [Glycine max (L.) Merr.]. Field Crops Res. 2016, 196, 261–267. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Sayama, T.; Yamazaki, H.; Miyoshi, T.; Ishimoto, M.; Funatsuki, H. Quantitative trait loci associated with lodging tolerance in soybean cultivar ‘Toyoharuka’. Breed. Sci. 2014, 64, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Mizuta, K.; Araki, H.; Takahashi, T. Shifting timing of intensive nitrogen topdressing later to the stem-elongation phase reduced lower internodes length and lodging risk of wheat. Plant Prod. Sci. 2020, 1–9. [Google Scholar] [CrossRef]
- Nagashima, H.; Hikosaka, K. Plants in a crowded stand regulate their height growth so as to maintain similar heights to neighbours even when they have potential advantages in height growth. Ann. Bot. 2011, 108, 207–214. [Google Scholar] [CrossRef]
- Hubbart, S.; Smillie, I.R.; Heatley, M.; Swarup, R.; Foo, C.C.; Zhao, L.; Murchie, E.H. Enhanced thylakoid photoprotection can increase yield and canopy radiation use efficiency in rice. Commun. Biol. 2018, 1, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Wang, J.; Wang, Z.; Li, W.; Wang, C.; Yan, S.; Li, H.; Zhang, A.; Tang, Z.; Wei, M. Optimized nitrogen fertilizer application mode increased culms lignin accumulation and lodging resistance in culms of winter wheat. Field Crops Res. 2018, 228, 31–38. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Shi, Y.; Li, Y.; Yin, Y.; Yang, D.; Luo, Y.; Pang, D.; Xu, X.; Li, W. Manipulation of lignin metabolism by plant densities and its relationship with lodging resistance in wheat. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Weng, F.; Zhang, W.; Wu, X.; Xu, X.; Ding, Y.; Li, G.; Liu, Z.; Wang, S. Impact of low-temperature, overcast and rainy weather during the reproductive growth stage on lodging resistance of rice. Sci. Rep. 2017, 7, 46596. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Dorairaj, D.; Ismail, M.R.; Sinniah, U.R.; Kar Ban, T. Influence of silicon on growth, yield, and lodging resistance of MR219, a lowland rice of Malaysia. J. Plant Nutr. 2017, 40, 1111–1124. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.; Tran, L.-S.P. Impacts of priming with silicon on the growth and tolerance of maize plants to alkaline stress. Front. Plant Sci. 2016, 7, 243. [Google Scholar] [CrossRef] [Green Version]
- Berry, P.M.; Spink, J. Predicting yield losses caused by lodging in wheat. Field Crops Res. 2012, 137, 19–26. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, Z.; Cai, Z.; Zeng, W.; Lai, Z.; Chen, H.; Yang, S.; Tang, X.; Yanzhu, S.; Gai, J. Establishment of an evaluation system of shade tolerance in soybean and its variation in southern China germplasm population. Sci. Agric. Sin. 2017, 50, 729–801. [Google Scholar]
- Haghighi, M.; Heidarian, S.; Da Silva, J.A.T. The effect of titanium amendment in N-withholding nutrient solution on physiological and photosynthesis attributes and micronutrient uptake of tomato. Biol. Trace Elem. Res. 2012, 150, 381–390. [Google Scholar] [CrossRef]
- Boykov, I.N.; Shuford, E.; Zhang, B. Nanoparticle titanium dioxide affects the growth and microRNA expression of switchgrass (Panicum virgatum). Genomics 2019, 111, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Samadi, N.; Yahyaabadi, S.; Rezayatmand, Z. Effect of TiO2 and TiO2 nanoparticle on germination, root and shoot Length and photosynthetic pigments of Mentha piperita. Int. J. Plant Soil Sci. 2014, 3, 408–418. [Google Scholar] [CrossRef]
- Assefa, T.; Otyama, P.I.; Brown, A.V.; Kalberer, S.R.; Kulkarni, R.S.; Cannon, S.B. Genome-wide associations and epistatic interactions for internode number, plant height, seed weight and seed yield in soybean. BMC Genom. 2019, 20, 527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longin, C.F.H.; Würschum, T. Genetic variability, heritability and correlation among agronomic and disease resistance traits in a diversity panel and elite breeding material of spelt wheat. Plant Breed. 2014, 133, 459–464. [Google Scholar] [CrossRef]
- Specht, J.; Chase, K.; Macrander, M.; Graef, G.; Chung, J.; Markwell, J.; Germann, M.; Orf, J.H.; Lark, K. Soybean response to water: A QTL analysis of drought tolerance. Crop Sci. 2001, 41, 493–509. [Google Scholar] [CrossRef]
- Piñera-Chavez, F.; Berry, P.; Foulkes, M.; Jesson, M.; Reynolds, M. Avoiding lodging in irrigated spring wheat. I. Stem and root structural requirements. Field Crops Res. 2016, 196, 325–336. [Google Scholar] [CrossRef]
- Shah, A.N.; Tanveer, M.; ur Rehman, A.; Anjum, S.A.; Iqbal, J.; Ahmad, R. Lodging stress in cereal—Effects and management: An overview. Environ. Sci. Pollut. Res. 2017, 24, 5222–5237. [Google Scholar] [CrossRef]
- Su, B.; Song, Y.; Song, C.; Cui, L.; Yong, T.; Yang, W. Growth and photosynthetic responses of soybean seedlings to maize shading in relay intercropping system in Southwest China. Photosynthetica 2014, 52, 332–340. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, R.; Hou, Z.; Yan, C.; Xia, X.; Ma, C.; Dong, S.; Gong, Z. Mechanical properties of soybean plants under various plant densities. Crop Pasture Sci. 2020, 71, 249–259. [Google Scholar] [CrossRef]
- Gommers, C.M.; Visser, E.J.; St Onge, K.R.; Voesenek, L.A.; Pierik, R. Shade tolerance: When growing tall is not an option. Trends Plant Sci. 2013, 18, 65–71. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Green-Tracewicz, E.; Page, E.R.; Swanton, C.J. Shade avoidance in soybean reduces branching and increases plant-to-plant variability in biomass and yield per plant. Weed Sci. 2011, 59, 43–49. [Google Scholar] [CrossRef]
- Casal, J.J. Shade avoidance. Arab. Book/Am. Soc. Plant Biol. 2012, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.-J.; Wu, L.-M.; Ding, Y.-F.; Fei, W.; Wu, X.-R.; Li, G.-H.; Liu, Z.-H.; She, T.; Ding, C.-Q.; Wang, S.-H. Top-dressing nitrogen fertilizer rate contributes to decrease culm physical strength by reducing structural carbohydrate content in japonica rice. J. Integr. Agric. 2016, 15, 992–1004. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Rahman, T.; Song, C.; Su, B.; Yang, F.; Yong, T.; Wu, Y.; Zhang, C.; Yang, W. Changes in light environment, morphology, growth and yield of soybean in maize-soybean intercropping systems. Field Crops Res. 2017, 200, 38–46. [Google Scholar] [CrossRef]
- Liu, W.-G.; Hussain, S.; Ting, L.; Zou, J.-L.; Ren, M.-L.; Tao, Z.; Jiang, L.; Feng, Y.; Yang, W.-Y. Shade stress decreases stem strength of soybean through restraining lignin biosynthesis. J. Integr. Agric. 2019, 18, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Jin, L.; Li, B.; Zhang, J.; Zhao, B.; Dong, S.; Liu, P. Effects of shading on stalks morphology, structure and lodging of summer maize in field. Sci. Agric. Sin. 2012, 45, 3497–3505. [Google Scholar]
- Weng, J.-K.; Li, X.; Stout, J.; Chapple, C. Independent origins of syringyl lignin in vascular plants. Proc. Natl. Acad. Sci. USA 2008, 105, 7887–7892. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Ma, J.; Wang, L. A hemicellulose-bound form of silicon with potential to improve the mechanical properties and regeneration of the cell wall of rice. New Phytol. 2015, 206, 1051–1062. [Google Scholar] [CrossRef]
- Koyama, K.; Ikeda, H.; Poudel, P.R.; Goto-Yamamoto, N. Light quality affects flavonoid biosynthesis in young berries of Cabernet Sauvignon grape. Phytochemistry 2012, 78, 54–64. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, L.; Shan, Y.; Liu, Y.; Tian, Y.; Xia, T. Influence of shade on flavonoid biosynthesis in tea (Camellia sinensis (L.) O. Kuntze). Sci. Horticult. 2012, 141, 7–16. [Google Scholar] [CrossRef]
- Yu, J.-K.; Graznak, E.; Breseghello, F.; Tefera, H.; Sorrells, M.E. QTL mapping of agronomic traits in tef [Eragrostis tef (Zucc) Trotter]. BMC Plant Biol. 2007, 7, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, S.; Iqbal, N.; Rahman, T.; Liu, T.; Brestic, M.; Safdar, M.E.; Asghar, M.A.; Farooq, M.U.; Shafiq, I.; Ali, A. Shade effect on carbohydrates dynamics and stem strength of soybean genotypes. Environ. Exp. Bot. 2019, 162, 374–382. [Google Scholar] [CrossRef]
- Zhang, J.; Li, G.; Song, Y.; Liu, Z.; Yang, C.; Tang, S.; Zheng, C.; Wang, S.; Ding, Y. Lodging resistance characteristics of high-yielding rice populations. Field Crops Res. 2014, 161, 64–74. [Google Scholar] [CrossRef]
- Acreche, M.M.; Slafer, G.A. Lodging yield penalties as affected by breeding in Mediterranean wheats. Field Crops Res. 2011, 122, 40–48. [Google Scholar] [CrossRef]
- Arai-Sanoh, Y.; Ida, M.; Zhao, R.; Yoshinaga, S.; Takai, T.; Ishimaru, T.; Maeda, H.; Nishitani, K.; Terashima, Y.; Gau, M. Genotypic variations in non-structural carbohydrate and cell-wall components of the stem in rice, sorghum, and sugar vane. Biosci. Biotechnol. Biochem. 2011, 1105072478. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, W.; Ding, Y.; Zhang, J.; Cambula, E.D.; Weng, F.; Liu, Z.; Ding, C.; Tang, S.; Chen, L. Shading contributes to the reduction of stem mechanical strength by decreasing cell wall synthesis in japonica rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 881. [Google Scholar] [CrossRef] [Green Version]
- Loades, C.J. Manipulation of Defence Related Lignification in Wheat; University of Southampton: Southampton, UK, 2003. [Google Scholar]
- Boudet, A.M.; Kajita, S.; Grima-Pettenati, J.; Goffner, D. Lignins and lignocellulosics: A better control of synthesis for new and improved uses. Trends Plant Sci. 2003, 8, 576–581. [Google Scholar] [CrossRef]
- Wang, C.; Hu, D.; Liu, X.; She, H.; Ruan, R.; Yang, H.; Yi, Z.; Wu, D. Effects of uniconazole on the lignin metabolism and lodging resistance of culm in common buckwheat (Fagopyrum esculentum M.). Field Crops Res. 2015, 180, 46–53. [Google Scholar] [CrossRef]
- Ahmad, I.; Kamran, M.; Guo, Z.; Meng, X.; Ali, S.; Zhang, P.; Liu, T.; Cai, T.; Han, Q. Effects of uniconazole or ethephon foliar application on culm mechanical strength and lignin metabolism, and their relationship with lodging resistance in winter wheat. Crop Pasture Sci. 2020, 71, 12–22. [Google Scholar] [CrossRef]
- Liu, W.-G.; Ren, M.-L.; Ting, L.; Du, Y.-L.; Tao, Z.; Liu, X.-M.; Jiang, L.; Hussain, S.; Yang, W.-Y. Effect of shade stress on lignin biosynthesis in soybean stems. J. Integr. Agric. 2018, 17, 1594–1604. [Google Scholar] [CrossRef]
- Moura, J.C.M.S.; Bonine, C.A.V.; de Oliveira Fernandes Viana, J.; Dornelas, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Li, C.; Li, S.; Zhu, Q.; Zhang, H.; Wang, H.; Yu, C.; Martin, S.K.S.; Xie, F. Effect of shade on leaf photosynthetic capacity, light-intercepting, electron transfer and energy distribution of soybeans. Plant Growth Regul. 2017, 83, 409–416. [Google Scholar] [CrossRef]
- Peng, D.; Chen, X.; Yin, Y.; Lu, K.; Yang, W.; Tang, Y.; Wang, Z. Lodging resistance of winter wheat (Triticum aestivum L.): Lignin accumulation and its related enzymes activities due to the application of paclobutrazol or gibberellin acid. Field Crops Res. 2014, 157, 1–7. [Google Scholar] [CrossRef]
- Huber, H.; de Brouwer, J.; von Wettberg, E.J.; During, H.J.; Anten, N.P. More cells, bigger cells or simply reorganization? Alternative mechanisms leading to changed internode architecture under contrasting stress regimes. New Phytol. 2014, 201, 193–204. [Google Scholar] [CrossRef]
- Xue, J.; Gou, L.; Zhao, Y.; Yao, M.; Yao, H.; Tian, J.; Zhang, W. Effects of light intensity within the canopy on maize lodging. Field Crops Res. 2016, 188, 133–141. [Google Scholar] [CrossRef]
- Okuno, A.; Hirano, K.; Asano, K.; Takase, W.; Masuda, R.; Morinaka, Y.; Ueguchi-Tanaka, M.; Kitano, H.; Matsuoka, M. New approach to increasing rice lodging resistance and biomass yield through the use of high gibberellin producing varieties. PLoS ONE 2014, 9, e86870. [Google Scholar] [CrossRef]
- Hirano, K.; Ordonio, R.L.; Matsuoka, M. Engineering the lodging resistance mechanism of post-Green Revolution rice to meet future demands. Proc. Jpn. Acad. Ser. B 2017, 93, 220–233. [Google Scholar] [CrossRef] [Green Version]
- Spielmeyer, W.; Ellis, M.H.; Chandler, P.M. Semidwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene. Proc. Natl. Acad. Sci. USA 2002, 99, 9043–9048. [Google Scholar] [CrossRef] [Green Version]
- Xiang, D.B.; Zhao, G.; Wan, Y.; Tan, M.L.; Song, C.; Song, Y. Effect of planting density on lodging-related morphology, lodging rate, and yield of tartary buckwheat (Fagopyrum tataricum). Plant Prod. Sci. 2016, 19, 479–488. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Shuxian, L.; Mumtaz, M.; Shafiq, I.; Iqbal, N.; Brestic, M.; Shoaib, M.; Sisi, Q.; Li, W.; Mei, X.; et al. Foliar application of silicon improves stem strength under low light stress by regulating lignin biosynthesis genes in soybean (Glycine max (L.) Merr.). J. Hazard. Mater. 2020, 401, 123256. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Zhang, Y.; Hussain, S.; Wang, S.; Zhang, X.; Yang, J.; Xu, M.; Qin, S.; Yang, W.; Liu, W. Slight Shading Stress at Seedling Stage Does not Reduce Lignin Biosynthesis or Affect Lodging Resistance of Soybean Stems. Agronomy 2020, 10, 544. [Google Scholar] [CrossRef] [Green Version]
- Raza, M.A.; Feng, L.Y.; Iqbal, N.; Ahmed, M.; Chen, Y.K.; Khalid, M.H.B.; Din, A.M.U.; Khan, A.; Ijaz, W.; Hussain, A. Growth and development of soybean under changing light environments in relay intercropping system. PeerJ 2019, 7, e7262. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-S.; Feng, Y.; Gong, W.-Z.; Ahmed, S.; Fan, Y.-F.; Wu, X.-L.; Yong, T.-W.; Liu, W.-G.; Kai, S.; Jiang, L. Shade adaptive response and yield analysis of different soybean genotypes in relay intercropping systems. J. Integr. Agric. 2017, 16, 1331–1340. [Google Scholar] [CrossRef]
- Luo, Y.; Ni, J.; Pang, D.; Jin, M.; Chen, J.; Kong, X.; Li, W.; Chang, Y.; Li, Y.; Wang, Z. Regulation of lignin composition by nitrogen rate and density and its relationship with stem mechanical strength of wheat. Field Crops Res. 2019, 241, 107572. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; Yin, Y.; Wang, Z.; Shi, Y.; Peng, D.; Ni, Y.; Cai, T. Relationship between lignin metabolism and lodging resistance in wheat. Acta Agron.Sin. 2011, 37, 1616–1622. [Google Scholar] [CrossRef]
- Del Río, J.C.; Rencoret, J.; Prinsen, P.; Martínez, A.n.T.; Ralph, J.; Gutiérrez, A. Structural characterization of wheat straw lignin as revealed by analytical pyrolysis, 2D-NMR, and reductive cleavage methods. J. Agric. Food Chem. 2012, 60, 5922–5935. [Google Scholar] [CrossRef] [Green Version]
- Hyles, J.; Vautrin, S.; Pettolino, F.; MacMillan, C.; Stachurski, Z.; Breen, J.; Berges, H.; Wicker, T.; Spielmeyer, W. Repeat-length variation in a wheat cellulose synthase-like gene is associated with altered tiller number and stem cell wall composition. J. Exp. Bot. 2017, 68, 1519–1529. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Zhang, D.; Hu, J.; Zhou, X.; Ye, X.; Reichel, K.L.; Stewart, N.R.; Syrenne, R.D.; Yang, X.; Gao, P. Comparative genome analysis of lignin biosynthesis gene families across the plant kingdom. BMC Bioinform. 2009, 10, S3. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Ruan, R.; Yuan, X.; Hu, D.; Yang, H.; Li, Y.; Yi, Z. Relationship between lignin metabolism and lodging resistance of culm in buckwheat. J. Agric. Sci. 2014, 6, 29. [Google Scholar] [CrossRef] [Green Version]
- Dranski, J.A.L.; Malavasi, U.C.; Malavasi, M.D.M. Relationship between lignin content and quality of Pinus taeda seedlings1. Rev. Árvore 2015, 39, 905–913. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Zhao, Y.-l.; Jiang, X.-N. Stable and specific expression of 4-coumarate: Coenzyme A ligase gene (4CL1) driven by the xylem-specific Pto4CL1 promoter in the transgenic tobacco. Biotechnol. Lett. 2004, 26, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.A. Lignin biosynthesis: Tyrosine shortcut in grasses. Nat.Plants 2016, 2, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Fagerstedt, K.V.; Kukkola, E.M.; Koistinen, V.V.; Takahashi, J.; Marjamaa, K. Cell wall lignin is polymerised by class III secretable plant peroxidases in Norway spruce. J. Integr. Plant Biol. 2010, 52, 186–194. [Google Scholar] [CrossRef]
- Berthet, S.; Thevenin, J.; Baratiny, D.; Demont-Caulet, N.; Debeaujon, I.; Bidzinski, P.; Leple, J.-C.; Huis, R.; Hawkins, S.; Gomez, L.-D. Role of plant laccases in lignin polymerization. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2012; Volume 61, pp. 145–172. [Google Scholar]
- Ma, Q.-H. Characterization of a cinnamoyl-CoA reductase that is associated with stem development in wheat. J. Exp. Bot. 2007, 58, 2011–2021. [Google Scholar] [CrossRef]
- Nguyen, T.-N.; Son, S.; Jordan, M.C.; Levin, D.B.; Ayele, B.T. Lignin biosynthesis in wheat (Triticum aestivum L.): Its response to waterlogging and association with hormonal levels. BMC Plant Biol. 2016, 16, 28. [Google Scholar] [CrossRef] [Green Version]
- Tamaoki, D.; Karahara, I.; Nishiuchi, T.; Wakasugi, T.; Yamada, K.; Kamisaka, S. Involvement of auxin dynamics in hypergravity-induced promotion of lignin-related gene expression in Arabidopsis inflorescence stems. J. Exp. Bot. 2011, 62, 5463–5469. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, J.; Núñez-Flores, M.J.L.; Gómez-Ros, L.V.; Uzal, E.N.; Carrasco, A.E.; Díaz, J.; Sottomayor, M.; Cuello, J.; Barceló, A.R. Hormonal regulation of the basic peroxidase isoenzyme from Zinnia elegans. Planta 2009, 230, 767–778. [Google Scholar] [CrossRef]
- Cai, T.; Meng, X.; Liu, X.; Liu, T.; Wang, H.; Jia, Z.; Yang, D.; Ren, X. Exogenous hormonal application regulates the occurrence of wheat tillers by changing endogenous hormones. Front. Plant Sci. 2018, 9, 1886. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Gu, D.; Ding, Y.; Wang, Q.; Li, G.; Wang, S. The relationship between nitrogen, auxin and cytokinin in the growth regulation of rice (Oryza sativa L.) Tiller buds. Aust. J. Crop Sci. 2011, 5, 1019. [Google Scholar]
- Chatfield, S.P.; Stirnberg, P.; Forde, B.G.; Leyser, O. The hormonal regulation of axillary bud growth in Arabidopsis. Plant J. 2000, 24, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A. Shade avoidance. New Phytol. 2008, 179, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Keuskamp, D.H.; Sasidharan, R.; Pierik, R. Physiological regulation and functional significance of shade avoidance responses to neighbors. Plant Signal. Behav. 2010, 5, 655–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, S.; Liu, T.; Iqbal, N.; Brestic, M.; Pang, T.; Mumtaz, M.; Shafiq, I.; Li, S.; Wang, L.; Gao, Y.; et al. Effects of lignin, cellulose, hemicellulose, sucrose and monosaccharide carbohydrates on soybean physical stem strength and yield in intercropping. Photochem. Photobiol. Sci. 2020, 19, 462–472. [Google Scholar] [CrossRef]
- Boomsma, C.R.; Santini, J.B.; Tollenaar, M.; Vyn, T.J. Maize morphophysiological responses to intense crowding and low nitrogen availability: An analysis and review. Agron. J. 2009, 101, 1426–1452. [Google Scholar] [CrossRef] [Green Version]
- Carriedo, L.G.; Maloof, J.N.; Brady, S.M. Molecular control of crop shade avoidance. Curr. Opin. Plant Biol. 2016, 30, 151–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, W.; Song, Q.; Zuo, B.; Han, Q.; Jia, Z. Effects of Gibberellin (GA4+7) in Grain Filling, Hormonal Behavior, and Antioxidants in High-Density Maize (Zea mays L.). Plants 2020, 9, 978. [Google Scholar] [CrossRef]
- Wang, D.; Graef, G.L.; Procopiuk, A.M.; Diers, B.W. Identification of putative QTL that underlie yield in interspecific soybean backcross populations. Theor. Appl. Genet. 2004, 108, 458–467. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, T.G. Integration of lodging resistance QTL in soybean. Sci. Rep. 2019, 9, 6540. [Google Scholar] [CrossRef] [Green Version]
- Berry, P.; Berry, S. Understanding the genetic control of lodging-associated plant characters in winter wheat (Triticum aestivum L.). Euphytica 2015, 205, 671–689. [Google Scholar] [CrossRef]
- Yadav, S.; Singh, U.M.; Naik, S.M.; Venkateshwarlu, C.; Ramayya, P.J.; Raman, K.A.; Sandhu, N.; Kumar, A. Molecular mapping of QTLs associated with lodging resistance in dry direct-seeded rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 1431. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Ookawa, T.; Aya, K.; Ochiai, Y.; Hirasawa, T.; Ebitani, T.; Takarada, T.; Yano, M.; Yamamoto, T.; Fukuoka, S. Isolation of a novel lodging resistance QTL gene involved in strigolactone signaling and its pyramiding with a QTL gene involved in another mechanism. Mol. Plant 2015, 8, 303–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Wang, P.; Zhang, X.; Li, X.; Yan, X.; Fu, D.; Wu, G. The genetic and molecular basis of crop height based on a rice model. Planta 2018, 247, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.K.; Bansal, K.C.; Aggarwal, P.K.; Datta, S.K.; Craufurd, P.Q. Agricultural biotechnology for crop improvement in a variable climate: Hope or hype? Trends Plant Sci. 2011, 16, 363–371. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Ma, B.L. A new method for assessing plant lodging and the impact of management options on lodging in canola crop production. Sci. Rep. 2016, 6, 31890. [Google Scholar] [CrossRef] [Green Version]
- Cui, F.; Li, J.; Ding, A.; Zhao, C.; Wang, L.; Wang, X.; Li, S.; Bao, Y.; Li, X.; Feng, D. Conditional QTL mapping for plant height with respect to the length of the spike and internode in two mapping populations of wheat. Theor. Appl. Genet. 2011, 122, 1517–1536. [Google Scholar] [CrossRef]
- Hai, L.; Guo, H.; Xiao, S.; Jiang, G.; Zhang, X.; Yan, C.; Xin, Z.; Jia, J. Quantitative trait loci (QTL) of stem strength and related traits in a doubled-haploid population of wheat (Triticum aestivum L.). Euphytica 2005, 141, 1–9. [Google Scholar] [CrossRef]
- Ellis, M.; Rebetzke, G.; Azanza, F.; Richards, R.; Spielmeyer, W. Molecular mapping of gibberellin-responsive dwarfing genes in bread wheat. Theor. Appl. Genet. 2005, 111, 423–430. [Google Scholar] [CrossRef]
- Griffiths, S.; Simmonds, J.; Leverington, M.; Wang, Y.; Fish, L.; Sayers, L.; Alibert, L.; Orford, S.; Wingen, L.; Snape, J. Meta-QTL analysis of the genetic control of crop height in elite European winter wheat germplasm. Mol. Breed. 2012, 29, 159–171. [Google Scholar] [CrossRef]
- Spielmeyer, W.; Hyles, J.; Joaquim, P.; Azanza, F.; Bonnett, D.; Ellis, M.; Moore, C.; Richards, R. A QTL on chromosome 6A in bread wheat (Triticum aestivum) is associated with longer coleoptiles, greater seedling vigour and final plant height. Theor. Appl. Genet. 2007, 115, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Shearman, V.J. Changes in the Yield Limiting Processes Associated with the Genetic Improvement of Wheat; University of Nottingham: Nottingham, UK, 2001. [Google Scholar]
- Rebetzke, G.; Ellis, M.; Bonnett, D.; Mickelson, B.; Condon, A.; Richards, R. Height reduction and agronomic performance for selected gibberellin-responsive dwarfing genes in bread wheat (Triticum aestivum L.). Field Crops Res. 2012, 126, 87–96. [Google Scholar] [CrossRef]
- Daoura, B.G.; Chen, L.; Du, Y.; Hu, Y.-G. Genetic effects of dwarfing gene Rht-5 on agronomic traits in common wheat (Triticum aestivum L.) and QTL analysis on its linked traits. Field Crops Res. 2014, 156, 22–29. [Google Scholar] [CrossRef]
- Daoura, B.G.; Chen, L.; Hu, Y.-G. Agronomic traits affected by dwarfing gene’Rht-5’in common wheat (‘Triticum aestivum’ L.). Aust. J. Crop Sci. 2013, 7, 1270. [Google Scholar]
- Chen, L.; Yang, Y.; Cui, C.; Lu, S.; Lu, Q.; Du, Y.; Su, R.; Chai, Y.; Li, H.; Chen, F. Effects of Vrn-B1 and Ppd-D1 on developmental and agronomic traits in Rht5 dwarf plants of bread wheat. Field Crops Res. 2018, 219, 24–32. [Google Scholar] [CrossRef]
- Huang, C.; Liu, Q.; Li, H.; Li, X.; Zhang, C.; Zhang, F. Optimised sowing date enhances crop resilience towards size-asymmetric competition and reduces the yield difference between intercropped and sole maize. Field Crops Res. 2018, 217, 125–133. [Google Scholar] [CrossRef]
- Egbe, O. Effects of plant density of intercropped soybean with tall sorghum on competitive ability of soybean and economic yield at Otobi, Benue State, Nigeria. J. Cereals Oilseeds 2010, 1, 1–10. [Google Scholar]
- Zhou, T.; Du, Y.; Ahmed, S.; Liu, T.; Ren, M.; Liu, W.; Yang, W. Genotypic differences in phosphorus efficiency and the performance of physiological characteristics in response to low phosphorus stress of soybean in southwest of China. Front. Plant Sci. 2016, 7, 1776. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Wang, Y.; Dong, X.; Qian, T.; Yin, L.; Dong, S.; Chu, J.; He, M. Delayed sowing can increase lodging resistance while maintaining grain yield and nitrogen use efficiency in winter wheat. Crop J. 2017, 5, 541–552. [Google Scholar] [CrossRef]
- Spink, J.; Semere, T.; Sparkes, D.; Whaley, J.; Foulkes, M.; Clare, R.; Scott, R. Effect of sowing date on the optimum plant density of winter wheat. Ann. Appl. Biol. 2000, 137, 179–188. [Google Scholar] [CrossRef]
- Ahmed, S.; Raza, M.A.; Yuan, X.; Du, Y.; Iqbal, N.; Chachar, Q.; Soomro, A.A.; Ibrahim, F.; Hussain, S.; Wang, X.; et al. Optimized planting time and co-growth duration reduce the yield difference between intercropped and sole soybean by enhancing soybean resilience toward size-asymmetric competition. Food Energy Secur. 2020, 9. [Google Scholar] [CrossRef]
- Yu, Y.; Stomph, T.-J.; Makowski, D.; van der Werf, W. Temporal niche differentiation increases the land equivalent ratio of annual intercrops: A meta-analysis. Field Crops Res. 2015, 184, 133–144. [Google Scholar] [CrossRef]
- Zhang, F.; Li, L. Using competitive and facilitative interactions in intercropping systems enhances crop productivity and nutrient-use efficiency. Plant Soil 2003, 248, 305–312. [Google Scholar] [CrossRef]
- Song, D.; Tariq, A.; Pan, K.; Khan, S.U.; Saleh, T.A.; Gong, S.; Zhang, A.; Wu, X. Influence of planting distance and density on the yield and photosynthetic traits of sweet potato (Ipomoea balatas L.) under an intercropping system with walnut (Juglans regia) saplings. Soil Tillage Res. 2020, 196, 104484. [Google Scholar] [CrossRef]
- Wang, C.; Wu Ruan, R.; Hui Yuan, X.; Hu, D.; Yang, H.; Li, Y.; Lin Yi, Z. Effects of nitrogen fertilizer and planting density on the lignin synthesis in the culm in relation to lodging resistance of buckwheat. Plant Prod. Sci. 2015, 18, 218–227. [Google Scholar] [CrossRef] [Green Version]
- Sher, A.; Khan, A.; Ashraf, U.; Liu, H.H.; Li, J.C. Characterization of the effect of increased plant density on canopy morphology and stalk lodging risk. Front. Plant Sci. 2018, 9, 1047. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, Z.; Liao, D.; Raza, M.A.; Wang, B.; Zhang, J.; Chen, J.; Feng, L.; Wu, X.; Liu, C. Uptake and utilization of nitrogen, phosphorus and potassium as related to yield advantage in maize-soybean intercropping under different row configurations. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Khan, S.; Anwar, S.; Kuai, J.; Noman, A.; Shahid, M.; Din, M.; Ali, A.; Zhou, G. Alteration in yield and oil quality traits of winter rapeseed by lodging at different planting density and nitrogen rates. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Cheng, B.; Raza, A.; Wang, L.; Xu, M.; Lu, J.; Gao, Y.; Qin, S.; Zhang, Y.; Ahmad, I.; Zhou, T.; et al. Effects of Multiple Planting Densities on Lignin Metabolism and Lodging Resistance of the Strip Intercropped Soybean Stem. Agronomy 2020, 10, 1177. [Google Scholar] [CrossRef]
- Chen, P.; Du, Q.; Liu, X.; Zhou, L.; Hussain, S.; Lei, L.; Song, C.; Wang, X.; Liu, W.; Yang, F. Effects of reduced nitrogen inputs on crop yield and nitrogen use efficiency in a long-term maize-soybean relay strip intercropping system. PLoS ONE 2017, 12, e0184503. [Google Scholar] [CrossRef] [Green Version]
- Said, M.; Hamd-Alla, W. Impact of foliar spraying with antioxidant and intercropping pattern of maize and soybean on yields and its attributes. J. Plant Prod. 2018, 9, 1069–1073. [Google Scholar] [CrossRef] [Green Version]
- Kebebew, S.; Belete, K.; Tana, T. Productivity evaluation of maize-soybean intercropping system under rainfed condition at Bench-Maji Zone, Ethiopia. Eur. Res. 2014, 79, 1301–1309. [Google Scholar]
- Addo-Quaye, A.; Darkwa, A.; Ocloo, G. Yield and productivity of component crops in a maize-soybean intercropping system as affected by time of planting and spatial arrangement. J. Agric. Biol. Sci. 2011, 6, 50–57. [Google Scholar]
- Yogesh, S.; Halikatti, S.; Hiremath, S.; Potdar, M.; Harlapur, S.; Venkatesh, H. Light use efficiency, productivity and profitability of maize and soybean intercropping as influenced by planting geometry and row proportion. Karnataka J. Agric. Sci. 2014, 27, 1–4. [Google Scholar]
- Khatri, N.; Dahal, K.; Amgain, L.; Karki, T. Productivity and economic assessment of maize and soybean intercropping under various tillage and residue levels in Chitwan, Nepal. World J. Agric. Res. 2014, 2, 6–12. [Google Scholar] [CrossRef]
- Ijoyah, M.; Fanen, F. Effects of different cropping pattern on performance of maize-soybean mixture in Makurdi, Nigeria. Sci. J. Crop Sci. 2012, 1, 39–47. [Google Scholar]
- Mahmoudi, R.; Jamshidi, K.; Pouryousef, M. Evaluation of grain yield of maize (Zea mays L.) and soybean (Glycine max L.) instrip intercropping. Int. J. Agron. Plant Prod. 2013, 4, 2388–2392. [Google Scholar]
- Zaman, U.; Ahmad, Z.; Farooq, M.; Saeed, S.; Ahmad, M.; Wakeel, A. Potassium fertilization may improve stem strength and yield of Basmati rice grown on nitrogen-fertilized soils. Pak. J. Agric. Sci. 2015, 52, 437–443. [Google Scholar]
- Zhang, M.; Wang, H.; Yi, Y.; Ding, J.; Zhu, M.; Li, C.; Guo, W.; Feng, C.; Zhu, X. Effect of nitrogen levels and nitrogen ratios on lodging resistance and yield potential of winter wheat (Triticum aestivum L.). PLoS ONE 2017, 12, e0187543. [Google Scholar] [CrossRef]
- Mariani, L.; Ferrante, A. Agronomic management for enhancing plant tolerance to abiotic stresses—Drought, salinity, hypoxia, and lodging. Horticulturae 2017, 3, 52. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.; Sun, M.; Wang, F.; Liu, J.; Feng, B.; Si, J.; Zhang, B.; Li, S.; Li, H. Effects of high NH+ 4 on K+ uptake, culm mechanical strength and grain filling in wheat. Front. Plant Sci. 2014, 5, 703. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhao, J.; Liu, Y.; Huang, N.; Tian, K.; Shah, F.; Liang, K.; Zhong, X.; Liu, B. Optimized nitrogen management enhances lodging resistance of rice and its morpho-anatomical, mechanical, and molecular mechanisms. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, F.-Z.; Li, J.-C.; Wang, C.-Y.; Qu, H.-J.; Shen, X.-S. Effects of nitrogenous fertilizer application model on culm lodging resistance in winter wheat. Acta Agron. Sin. 2008, 34, 1080–1085. [Google Scholar] [CrossRef]
- Berry, P.; Griffin, J.; Sylvester-Bradley, R.; Scott, R.; Spink, J.; Baker, C.; Clare, R. Controlling plant form through husbandry to minimise lodging in wheat. Field Crops Res. 2000, 67, 59–81. [Google Scholar] [CrossRef]
- Peake, A.; Bell, K.; Carberry, P.; Poole, N.; Raine, S. Vegetative nitrogen stress decreases lodging risk and increases yield of irrigated spring wheat in the subtropics. Crop Pasture Sci. 2016, 67, 907–920. [Google Scholar] [CrossRef]
- Yousaf, M.; Li, J.; Lu, J.; Ren, T.; Cong, R.; Fahad, S.; Li, X. Effects of fertilization on crop production and nutrient-supplying capacity under rice-oilseed rape rotation system. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bhiah, K.; Guppy, C.; Lockwood, P.; Jessop, R. Effect of potassium on rice lodging under high nitrogen nutrition. In Proceedings of the Brisbane (AU): 19th World Congress of Soil Science “Soil Solution for a Changing World, Brisbane, Australia, 1–6 August 2010; pp. 1–6. [Google Scholar]
- Kong, E.; Liu, D.; Guo, X.; Yang, W.; Sun, J.; Li, X.; Zhan, K.; Cui, D.; Lin, J.; Zhang, A. Anatomical and chemical characteristics associated with lodging resistance in wheat. Crop J. 2013, 1, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [Green Version]
- Hafsi, C.; Debez, A.; Abdelly, C. Potassium deficiency in plants: Effects and signaling cascades. Acta Physiol. Plant. 2014, 36, 1055–1070. [Google Scholar] [CrossRef]
- Pitre, F.E.; Cooke, J.E.; Mackay, J.J. Short-term effects of nitrogen availability on wood formation and fibre properties in hybrid poplar. Trees 2007, 21, 249–259. [Google Scholar] [CrossRef]
- Yong, T.; Liu, X.; Yang, F.; Song, C.; Wang, X.; Liu, W.; Su, B.; Zhou, L.; Yang, W. Characteristics of nitrogen uptake, use and transfer in a wheat-maize-soybean relay intercropping system. Plant Prod. Sci. 2015, 18, 388–397. [Google Scholar] [CrossRef]
- Li, L.; Sun, J.; Zhang, F.; Li, X.; Yang, S.; Rengel, Z. Wheat/maize or wheat/soybean strip intercropping: I. Yield advantage and interspecific interactions on nutrients. Field Crops Res. 2001, 71, 123–137. [Google Scholar] [CrossRef]
- Peoples, M.; Brockwell, J.; Herridge, D.; Rochester, I.; Alves, B.; Urquiaga, S.; Boddey, R.; Dakora, F.; Bhattarai, S.; Maskey, S. The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis 2009, 48, 1–17. [Google Scholar] [CrossRef]
- Yang, F.; Huang, S.; Gao, R.; Liu, W.; Yong, T.; Wang, X.; Wu, X.; Yang, W. Growth of soybean seedlings in relay strip intercropping systems in relation to light quantity and red: Far-red ratio. Field Crops Res. 2014, 155, 245–253. [Google Scholar] [CrossRef]
- Yu, Y.; Makowski, D.; Stomph, T.J.; van der Werf, W. Robust Increases of Land Equivalent Ratio with Temporal Niche Differentiation: A Meta-Quantile Regression. Agron. J. 2016, 108, 2269–2279. [Google Scholar] [CrossRef]
- Yu, Y.; Stomph, T.-J.; Makowski, D.; Zhang, L.; van der Werf, W. A meta-analysis of relative crop yields in cereal/legume mixtures suggests options for management. Field Crops Res. 2016, 198, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Li, C.; Zhang, C.; Yu, Y.; van der Werf, W.; Zhang, F. Intercropping maize and soybean increases efficiency of land and fertilizer nitrogen use; A meta-analysis. Field Crops Res. 2020, 246, 107661. [Google Scholar] [CrossRef]
- Upadhya, D.; Dhakal, R.; Khadka, K.; Rana, S.; Acharya, P.; Rana, R.; Chaudhary, P. Local knowledge on climate-induced traits in rice for improving crop yield, food security and climate resilience. Int. Agric. Innov. Res. J. 2016, 5, 385–396. [Google Scholar]
- Waseem, M.; Ahmad, R.; Randhawa, M.A.; Aziz, T.; Mahmood, N. Influence of silicon application on blast incidence and lodging resistance in rice. J. Agric. Res. 2016, 54, 435–443. [Google Scholar]
- Fallah, A. Studies effect of silicon on lodging parameters in rice plant under hydroponics culture in a greenhouse experiment. In Proceedings of the Silicon in Agriculture Conference, Wild Coast Sun, South Africa, 26–31 October 2008. [Google Scholar]
- Salman, D.; Morteza, S.; Dariush, Z.; Abbas, G.M.; Reza, Y.; Ehsan, G.D.; Reza, N.A. Application of nitrogen and silicon rates on morphological and chemical lodging related characteristics in rice (Oryza sativa L.) at North of Iran. J. Agric. Sci. 2012, 4, 12. [Google Scholar] [CrossRef]
- Kim, S.G.; Kim, K.W.; Park, E.W.; Choi, D. Silicon-induced cell wall fortification of rice leaves: A possible cellular mechanism of enhanced host resistance to blast. Phytopathology 2002, 92, 1095–1103. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zou, W.; Li, Y.; Feng, Y.; Zhang, H.; Wu, Z.; Tu, Y.; Wang, Y.; Cai, X.; Peng, L. Silica distinctively affects cell wall features and lignocellulosic saccharification with large enhancement on biomass production in rice. Plant Sci. 2015, 239, 84–91. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, L.; Liu, J.; Liu, X.; Li, X.; Ma, J.; Lin, Y.; Xu, F. Evidence for ‘silicon’within the cell walls of suspension-cultured rice cells. New Phytol. 2013, 200, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Yingang, L.; Jun, M.; Ying, T.; Junyu, H.; Christie, P.; Lingjia, Z.; Wenjie, R.; Zhang, M.; Shiping, D. Effect of silicon on growth, physiology, and cadmium translocation of tobacco (Nicotiana tabacum L.) in cadmium-contaminated soil. Pedosphere 2018, 28, 680–689. [Google Scholar]
- Wang, Y.; Zhang, B.; Jiang, D.; Chen, G. Silicon improves photosynthetic performance by optimizing thylakoid membrane protein components in rice under drought stress. Environ. Exp. Bot. 2019, 158, 117–124. [Google Scholar] [CrossRef]
- Rehman, B. Silicon elicited varied physiological and biochemical responses in Indian mustard (Brassica juncea): A concentration dependent study. Israel J. Plant Sci. 2016, 63, 158–166. [Google Scholar] [CrossRef]
- Hernandez-Apaolaza, L. Can silicon partially alleviate micronutrient deficiency in plants? A review. Planta 2014, 240, 447–458. [Google Scholar] [CrossRef]
- Hussain, S.; Iqbal, N.; Brestic, M.; Raza, M.A.; Pang, T.; Langham, D.R.; Safdar, M.E.; Ahmed, S.; Wen, B.; Gao, Y.; et al. Changes in morphology, chlorophyll fluorescence performance and Rubisco activity of soybean in response to foliar application of ionic titanium under normal light and shade environment. Sci. Total Environ. 2019, 658, 626–637. [Google Scholar] [CrossRef]
- Xu, C.; Gao, Y.; Tian, B.; Ren, J.; Meng, Q.; Wang, P. Effects of EDAH, a novel plant growth regulator, on mechanical strength, stalk vascular bundles and grain yield of summer maize at high densities. Field Crops Res. 2017, 200, 71–79. [Google Scholar] [CrossRef]
- Berry, P.; Sterling, M.; Spink, J.; Baker, C.; Sylvester-Bradley, R.; Mooney, S.; Tams, A.; Ennos, A. Understanding and reducing lodging in cereals. Adv. Agron. 2004, 84, 215–269. [Google Scholar]
- Rajala, A. Plant Growth Regulators to Manipulate Cereal Growth in Northern Growing Conditions. 2003. Available online: https://helda.helsinki.fi/bitstream/handle/10138/20698/plantgro.pdf?sequence=1 (accessed on 2 June 2012).
- Bhaskaruni, S.V.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A Review on multi-component green synthesis of N-containing heterocycles using mixed oxides as heterogeneous catalysts. Arabian J. Chem. 2020, 13, 1142–1178. [Google Scholar] [CrossRef]
- Kamran, M.; Ahmad, I.; Wu, X.; Liu, T.; Ding, R.; Han, Q. Application of paclobutrazol: A strategy for inducing lodging resistance of wheat through mediation of plant height, stem physical strength, and lignin biosynthesis. Environ. Sci. Pollut. Res. 2018, 25, 29366–29378. [Google Scholar] [CrossRef] [PubMed]
- Shah, L.; Yahya, M.; Shah, S.M.A.; Nadeem, M.; Ali, A.; Ali, A.; Wang, J.; Riaz, M.W.; Rehman, S.; Wu, W. Improving lodging resistance: Using wheat and rice as classical examples. Int. J. Mol. Sci. 2019, 20, 4211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leśniowska-Nowak, J.; Nowak, M.; Zapalska, M.; Dudziak, K.; Kowalczyk, K. Influence of CCC and trinexapac-ethyl on the expression of genes involved in gibberellic biosynthesis and metabolism pathway in isogenic line with Rht12 dwarfing gene. Acta Sci. Polonorum. Hortorum Cultus 2017, 16, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Espindula, M.; Rocha, V.; Grossi, J.; Souza, M.; Souza, L.; Favarato, L. Use of growth retardants in wheat. Planta Daninha 2009, 27, 379–387. [Google Scholar] [CrossRef] [Green Version]



| Trait (s) | Cropping System | Behavior | Reference (s) |
|---|---|---|---|
| Morphological Traits | Maize-soybean Intercropping | ||
| Plant Height | Positively Correlated with lodging | [48] | |
| Internodal-length | Negatively correlated with lodging resistance | [47,73] | |
| Stem Diameter | Strongly correlated with resistance to lodging | [40,74] | |
| Anatomical Features | |||
| Number of vascular bundles | Positively correlated with lodging resistance | [75] | |
| Width of vascular bundle sheath | Strongly associated with resistance to lodging | [51,76] | |
| Cell Wall Thickness | Strongly correlated with lodging resistance | [59] | |
| Physical Aspects | |||
| Wind | Significantly associated with lodging | [40] | |
| Rainfall | Strongly correlated with lodging | [74] | |
| Shade | Negatively associated with lodging resistance | [59,77,78] | |
| Biochemical Features | |||
| Lignin and Cellulose Content | Positively associated with cell wall thickness and lodging resistance | [19,79] |
| Planting Density in Monocropping (×103 Plants ha−1) | Planting Density in Intercropping (×103 Plants ha−1) | ||
|---|---|---|---|
| Country | Maize Soybean | Maize Soybean | Reference (s) |
| China (MSIS) | 59 117 | 59 117 | [124,134,135] |
| Egypt | 71 143 | 24 95 | [136] |
| Ethiopia | 44 500 | 44 375 | [137] |
| Ghana | 56 111 | 56 222 | [138] |
| India | 83 333 | 42 250 | [139] |
| Nepal | 40 200 | 40 200 | [140] |
| Nigeria | 33 200 | 33 200 | [141] |
| Iran | 36 400 | 36 400 | [142] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, A.; Asghar, M.A.; Ahmad, B.; Bin, C.; Iftikhar Hussain, M.; Li, W.; Iqbal, T.; Yaseen, M.; Shafiq, I.; Yi, Z.; et al. Agro-Techniques for Lodging Stress Management in Maize-Soybean Intercropping System—A Review. Plants 2020, 9, 1592. https://doi.org/10.3390/plants9111592
Raza A, Asghar MA, Ahmad B, Bin C, Iftikhar Hussain M, Li W, Iqbal T, Yaseen M, Shafiq I, Yi Z, et al. Agro-Techniques for Lodging Stress Management in Maize-Soybean Intercropping System—A Review. Plants. 2020; 9(11):1592. https://doi.org/10.3390/plants9111592
Chicago/Turabian StyleRaza, Ali, Muhammad Ahsan Asghar, Bushra Ahmad, Cheng Bin, M. Iftikhar Hussain, Wang Li, Tauseef Iqbal, Muhammad Yaseen, Iram Shafiq, Zhang Yi, and et al. 2020. "Agro-Techniques for Lodging Stress Management in Maize-Soybean Intercropping System—A Review" Plants 9, no. 11: 1592. https://doi.org/10.3390/plants9111592