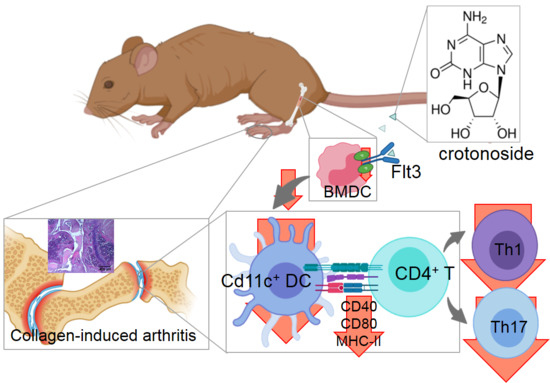
Alleviation of Collagen-Induced Arthritis by Crotonoside through Modulation of Dendritic Cell Differentiation and Activation
Abstract
:1. Introduction
2. Material and Methods
2.1. Ethics Statement
2.2. Animals
2.3. CIA Induction and Crotonoside Treatment
2.4. Scoring for Evaluating Arthritis Severity in CIA
2.5. Histological Assessment
2.6. Cytokine Measurement In Vitro and In Vivo
2.7. CCK-8 Cell Viability Assay
2.8. Lactate Dehydrogenase (LDH) Assay
2.9. Flow Cytometry Analysis of Surface Markers on DCs
2.10. Determination of Splenic T Cell Subtypes
2.11. Assay of Antigen-Specific T Cell Activation in Co-Culture Settings
2.12. Western Blot Analysis
2.13. Statistical Analysis
3. Results
3.1. Crotonoside Reduced Arthritic Severity in CIA Mice
3.2. Reduced Synovial DC Infiltration and Splenic DC Activation by Crotonoside
3.3. Proinflammatory Cytokines and Inflammatory T Cell Subsets Were Suppressed through Crotonoside Administration
3.4. Crotonoside Inhibited BMDC Differentiation
3.5. Crotonoside Inhibited Co-Stimulatory Molecules and Cytokine Production of BMDCs Induced by LPS
3.6. Crotonoside Impaired the Ability of BMDCs to Activate Antigen-Specific T Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Myasoedova, E.; Davis, J.; Matteson, E.L.; Crowson, C.S. Is the epidemiology of rheumatoid arthritis changing? Results from a population-based incidence study, 1985-2014. Ann. Rheum. Dis. 2020, 79, 440–444. [Google Scholar] [CrossRef]
- Okamoto, H.; Kobayashi, A. Tyrosine kinases in rheumatoid arthritis. J. Inflamm. 2011, 8, 21. [Google Scholar] [CrossRef] [Green Version]
- Kurowska, W.; Kuca-Warnawin, E.H.; Radzikowska, A.; Maslinski, W. The role of anti-citrullinated protein antibodies (ACPA) in the pathogenesis of rheumatoid arthritis. Cent. Eur. J. Immunol. 2017, 42, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Karsunky, H.; Merad, M.; Cozzio, A.; Weissman, I.L.; Manz, M.G. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J. Exp. Med. 2003, 198, 305–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.; Greenberg, J.D.; Bhardwaj, N. Dendritic cells as targets for therapy in rheumatoid arthritis. Nat. Rev. Rheumatol. 2009, 5, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.B.; Langridge, W.H.R. The function of myeloid dendritic cells in rheumatoid arthritis. Rheumatol. Int. 2017, 37, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Leung, B.P.; Conacher, M.; Hunter, D.; McInnes, I.B.; Liew, F.Y.; Brewer, J.M. A novel dendritic cell-induced model of erosive inflammatory arthritis: Distinct roles for dendritic cells in T cell activation and induction of local inflammation. J. Immunol. 2002, 169, 7071–7077. [Google Scholar] [CrossRef]
- Santiago-Schwarz, F.; Anand, P.; Liu, S.; Carsons, S.E. Dendritic cells (DCs) in rheumatoid arthritis (RA): Progenitor cells and soluble factors contained in RA synovial fluid yield a subset of myeloid DCs that preferentially activate Th1 inflammatory-type responses. J. Immunol. 2001, 167, 1758–1768. [Google Scholar] [CrossRef]
- Yirga, G.; Teferi, M.; Kasaye, M. Survey of medicinal plants used to treat human ailments in Hawzen district, Northern Ethiopia. Int. J. Biodivers. Conserv. 2011, 3, 6. [Google Scholar] [CrossRef]
- Mangunwidjaja, D.; Raharja, S.; Kardono, L.B.; Iswantini, D. Characteristics, efficacy and safety testing of standardized extract of Croton tiglium seed from Indonesia as laxative material. Pak. J. Biol. Sci. 2008, 11, 618–622. [Google Scholar] [CrossRef] [Green Version]
- Tsai, J.C.; Tsai, S.; Chang, W.C. Effect of ethanol extracts of three Chinese medicinal plants with laxative properties on ion transport of the rat intestinal epithelia. Biol. Pharm. Bull. 2004, 27, 162–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Lan, M.; Wu, H.P.; Shi, Y.Q.; Lu, J.; Ding, J.; Wu, K.C.; Jin, J.P.; Fan, D.M. Direct effect of croton oil on intestinal epithelial cells and colonic smooth muscle cells. World J. Gastroenterol. 2002, 8, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Tyler, V.E.; Brady, L.R.; Robbers, J.E. Pharmacognosy, 9th ed.; Lee & Febiger: Philadelphia, PA, USA, 1988; 519p. [Google Scholar]
- Gupta, D.P.; Park, S.H.; Yang, H.J.; Suk, K.; Song, G.J. Neuroprotective and Anti-Neuroinflammatory Effects of a Poisonous Plant Croton Tiglium Linn. Extract. Toxins 2020, 12, 261. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.Z.; Yu, S.; Yan, P.A.; Gong, D.Y.; Wu, F.L.; He, Z.; Yuan, Y.Y.; Zhao, A.Y.; Tang, X.; Zhang, R.Q.; et al. Crotonoside exhibits selective post-inhibition effect in AML cells via inhibition of FLT3 and HDAC3/6. Oncotarget 2017, 8, 103087–103099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deane, K.D.; O’Donnell, C.I.; Hueber, W.; Majka, D.S.; Lazar, A.A.; Derber, L.A.; Gilliland, W.R.; Edison, J.D.; Norris, J.M.; Robinson, W.H.; et al. The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis Rheum. 2010, 62, 3161–3172. [Google Scholar] [CrossRef] [Green Version]
- Dehlin, M.; Bokarewa, M.; Rottapel, R.; Foster, S.J.; Magnusson, M.; Dahlberg, L.E.; Tarkowski, A. Intra-articular fms-like tyrosine kinase 3 ligand expression is a driving force in induction and progression of arthritis. PLoS ONE 2008, 3, e3633. [Google Scholar] [CrossRef]
- Dehlin, M.; Andersson, S.; Erlandsson, M.; Brisslert, M.; Bokarewa, M. Inhibition of fms-like tyrosine kinase 3 alleviates experimental arthritis by reducing formation of dendritic cells and antigen presentation. J. Leukoc. Biol. 2011, 90, 811–817. [Google Scholar] [CrossRef]
- Brand, D.D.; Latham, K.A.; Rosloniec, E.F. Collagen-induced arthritis. Nat. Protoc. 2007, 2, 1269–1275. [Google Scholar] [CrossRef]
- Tian, H.; Cronstein, B.N. Understanding the mechanisms of action of methotrexate: Implications for the treatment of rheumatoid arthritis. Bull. NYU Hosp. Jt. Dis. 2007, 65, 168–173. [Google Scholar]
- Deng, G.M.; Zheng, L.; Chan, F.K.; Lenardo, M. Amelioration of inflammatory arthritis by targeting the pre-ligand assembly domain of tumor necrosis factor receptors. Nat. Med. 2005, 11, 1066–1072. [Google Scholar] [CrossRef]
- Liu, D.; Li, T.; Luo, H.; Zuo, X.; Liu, S.; Wu, S. The effect of the cholinergic anti-inflammatory pathway on collagen-induced arthritis involves the modulation of dendritic cell differentiation. Arthritis Res. Ther. 2018, 20, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nash, P. 2019 update: EULAR RA management recommendations, efficacy and safety systematic literature reviews. Ann. Rheum. Dis. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurr, E.; Mohr, W.; Pallasch, G. Proteoglycans from human articular cartilage: The effect of joint location on the structure. J. Clin. Chem. Clin. Biochem. 1985, 23, 811–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J. Bone Joint Surg. Am. 1971, 53, 69–82. [Google Scholar] [CrossRef]
- Mihret, A.; Mamo, G.; Tafesse, M.; Hailu, A.; Parida, S. Dendritic Cells Activate and Mature after Infection with Mycobacterium tuberculosis. BMC Res. Notes 2011, 4, 247. [Google Scholar] [CrossRef] [Green Version]
- Boissier, M.C.; Assier, E.; Falgarone, G.; Bessis, N. Shifting the imbalance from Th1/Th2 to Th17/treg: The changing rheumatoid arthritis paradigm. Joint Bone Spine 2008, 75, 373–375. [Google Scholar] [CrossRef]
- Hart, D.N. Dendritic cells: Unique leukocyte populations which control the primary immune response. Blood 1997, 90, 3245–3287. [Google Scholar] [CrossRef]
- Barnden, M.J.; Allison, J.; Heath, W.R.; Carbone, F.R. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 1998, 76, 34–40. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Sivridis, E.; Brekken, R.; Thorpe, P.E.; Anastasiadis, P.; Gatter, K.C.; Harris, A.L.; Koukourakis, M.I. The angiogenic "vascular endothelial growth factor/flk-1(KDR) receptor" pathway in patients with endometrial carcinoma: Prognostic and therapeutic implications. Cancer 2001, 92, 2569–2577. [Google Scholar] [CrossRef]
- Lean, J.M.; Fuller, K.; Chambers, T.J. FLT3 ligand can substitute for macrophage colony-stimulating factor in support of osteoclast differentiation and function. Blood 2001, 98, 2707–2713. [Google Scholar] [CrossRef]
- Ramos, M.I.; Perez, S.G.; Aarrass, S.; Helder, B.; Broekstra, P.; Gerlag, D.M.; Reedquist, K.A.; Tak, P.P.; Lebre, M.C. FMS-related tyrosine kinase 3 ligand (Flt3L)/CD135 axis in rheumatoid arthritis. Arthritis Res Ther. 2013, 15, R209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelov, G.S.; Tomkowiak, M.; Marcais, A.; Leverrier, Y.; Marvel, J. Flt3 ligand-generated murine plasmacytoid and conventional dendritic cells differ in their capacity to prime naive CD8 T cells and to generate memory cells in vivo. J. Immunol. 2005, 175, 189–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavanagh, L.L.; Boyce, A.; Smith, L.; Padmanabha, J.; Filgueira, L.; Pietschmann, P.; Thomas, R. Rheumatoid arthritis synovium contains plasmacytoid dendritic cells. Arthritis Res. Ther. 2005, 7, R230–R240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benson, R.A.; Patakas, A.; Conigliaro, P.; Rush, C.M.; Garside, P.; McInnes, I.B.; Brewer, J.M. Identifying the cells breaching self-tolerance in autoimmunity. J. Immunol. 2010, 184, 6378–6385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuya, K.; Kaku, Y.; Yoshida, K.; Joh, K.; Kurosaka, D. Therapeutic effects of sunitinib, one of the anti-angiogenetic drugs, in a murine arthritis. Mod. Rheumatol. 2014, 24, 487–491. [Google Scholar] [CrossRef]
- Gozel, N.; Cakirer, M.; Karatas, A.; Tuzcu, M.; Ozdemir, F.A.; Dagli, A.F.; Sahin, K.; Koca, S.S. Sorafenib Reveals Anti-Arthritic Potentials in Collagen Induced Experimental Arthritis Model. Arch. Rheumatol. 2018, 33, 309–315. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Liu, F.; Gong, Y.F.; Huang, T.Y.; Zhang, X.M.; Huang, X.Y. Antiarthritic Effects of Sorafenib in Rats with Adjuvant-Induced Arthritis. Anat Rec. 2018, 301, 1519–1526. [Google Scholar] [CrossRef] [Green Version]
- Ewing, P.L.; Schlenk, F.; Emerson, G.A. Comparison of smooth muscle effects of crotonoside (isoguanosine) and adenosine. J. Pharmacol. Exp. Ther. 1949, 97, 379–383. [Google Scholar]
- Hsu, F.C.; Belmonte, P.J.; Constans, M.M.; Chen, M.W.; McWilliams, D.C.; Hiebert, S.W.; Shapiro, V.S. Histone Deacetylase 3 Is Required for T Cell Maturation. J. Immunol. 2015, 195, 1578–1590. [Google Scholar] [CrossRef]
- Angiolilli, C.; Kabala, P.A.; Grabiec, A.M.; Van Baarsen, I.M.; Ferguson, B.S.; Garcia, S.; Malvar Fernandez, B.; McKinsey, T.A.; Tak, P.P.; Fossati, G.; et al. Histone deacetylase 3 regulates the inflammatory gene expression programme of rheumatoid arthritis fibroblast-like synoviocytes. Ann. Rheum. Dis. 2017, 76, 277–285. [Google Scholar] [CrossRef]
- Yan, P.; Zhang, L.; Peng, C.; Zhang, R. Pharmacokinetics and tissue distribution of crotonoside. Xenobiotica 2018, 48, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Madan, B.; Goh, K.C.; Hart, S.; William, A.D.; Jayaraman, R.; Ethirajulu, K.; Dymock, B.W.; Wood, J.M. SB1578, a novel inhibitor of JAK2, FLT3, and c-Fms for the treatment of rheumatoid arthritis. J. Immunol. 2012, 189, 4123–4134. [Google Scholar] [CrossRef] [PubMed] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-C.; Lin, C.-C.; Li, S.; Lin, W.-Y.; Lehman, C.W.; Bracci, N.R.; Tsai, S.-W. Alleviation of Collagen-Induced Arthritis by Crotonoside through Modulation of Dendritic Cell Differentiation and Activation. Plants 2020, 9, 1535. https://doi.org/10.3390/plants9111535
Lin S-C, Lin C-C, Li S, Lin W-Y, Lehman CW, Bracci NR, Tsai S-W. Alleviation of Collagen-Induced Arthritis by Crotonoside through Modulation of Dendritic Cell Differentiation and Activation. Plants. 2020; 9(11):1535. https://doi.org/10.3390/plants9111535
Chicago/Turabian StyleLin, Shih-Chao, Chi-Chien Lin, Shiming Li, Wan-Yi Lin, Caitlin W. Lehman, Nicole R. Bracci, and Sen-Wei Tsai. 2020. "Alleviation of Collagen-Induced Arthritis by Crotonoside through Modulation of Dendritic Cell Differentiation and Activation" Plants 9, no. 11: 1535. https://doi.org/10.3390/plants9111535