Stability Despite Reduction: Flower Structure, Patterns of Receptacle Elongation and Organ Fusion in Eriocaulon (Eriocaulaceae: Poales)
Abstract
:1. Introduction
2. Results
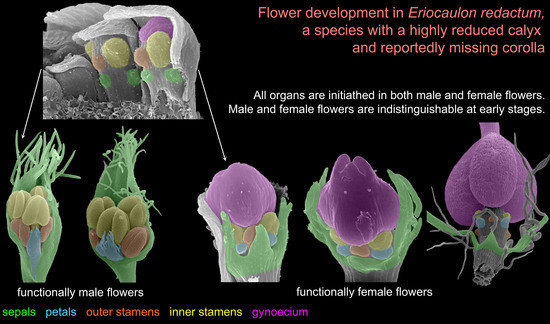
2.1. Eriocaulon redactum
2.2. Species with Petals Conspicuous in Anthetic Female Flowers
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eichler, A.W. Blüthendiagramme; Engelmann: Leipzig, Germany, 1875; Volume 1. [Google Scholar]
- Endress, P.K. Major evolutionary traits of monocot flowers. In Monocotyledons: Systematics and Evolution; Rudall, P.J., Cribb, P.J., Cutler, D.F., Humphries, C.J., Eds.; Royal Botanic Gardens: Kew, London, 1995; pp. 43–79. [Google Scholar]
- Remizowa, M.V.; Sokoloff, D.D.; Rudall, P.J. Evolutionary history of the monocot flower. Ann. Mo. Bot. Gard. 2010, 97, 617–645. [Google Scholar] [CrossRef]
- Ronse De Craene, L.P. Floral Diagrams: An Aid to Understanding Flower Morphology and Evolution; Cambridge University Press: Cambridge, UK, 2010; ISBN 978-1-139-48455-8. [Google Scholar]
- Remizowa, M.V.; Rudall, P.J.; Choob, V.V.; Sokoloff, D.D. Racemose inflorescences of monocots: Structural and morphogenetic interaction at the flower/inflorescence level. Ann. Bot. 2013, 112, 1553–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buzgo, M. Flower structure and development of Araceae compared with alismatids and Acoraceae. Bot. J. Linn. Soc. 2001, 136, 393–425. [Google Scholar] [CrossRef]
- Hieronymus, G. Eriocaulaceae. In Die Natürlichen Pflanzenfamilien Teil II. Abt. 4; Engler, A., Prantl, K., Eds.; Engelmann: Leipzig, Germany, 1888; pp. 21–27. [Google Scholar]
- Ronte, H. Beiträge zur Kenntniss der Blüthengestaltung einiger Tropenpflanzen. Flora 1891, 74, 492–529. [Google Scholar]
- Ruhland, W. Eriocaulaceae. In Das Pflanzenreich, IV. 30 (Heft 13); Engler, A., Ed.; Engelmann: Leipzig, Germany, 1903; pp. 1–294. [Google Scholar]
- Hamann, U. Commelinales. In A. Engler’s Syllabus der Pflanzenfamilien (edn 12). Bd. 2; Melchior, H., Ed.; Bornträger: Berlin, Germany, 1964; Volume 12, pp. 549–561. [Google Scholar]
- Stützel, T. Blüten- und infloreszenzmorphologische Untersuchungen zur Systematik der Eriocaulaceen. Diss. Bot. 1984, 71, 1–108. [Google Scholar]
- Mayo, S.J.; Bogner, J.; Boyce, P.C. The Genera of Araceae; Royal Botanic Gardens: Kew, UK, 1997; ISBN 1 900347 22 9. [Google Scholar]
- Stützel, T. Eriocaulaceae. In The Families and Genera of Vascular Plants. Vol. IV. Flowering Plants. Monocotyledons. Alismatanae and Commelinanae (Except Gramineae); Kubitzki, K., Ed.; Springer: Berlin, Germany, 1998; pp. 197–207. [Google Scholar]
- Stützel, T. Zur Funktion und Evolution köpfchenförmiger Blütenstände, insbesondere der Eriocaulaceen. Beitr. Biol. Pflanz. 1981, 56, 439–468. [Google Scholar]
- Stützel, T. Untersuchungen über Verzweigung und Infloreszenzaufbau von Eriocaulaceen. Flora 1982, 172, 105–112. [Google Scholar] [CrossRef]
- Stützel, T.; Trovó, M. Inflorescences in Eriocaulaceae: Taxonomic relevance and practical implications. Ann. Bot. 2013, 112, 1505–1522. [Google Scholar] [CrossRef]
- Oriani, A.; Scatena, V.L. Floral anatomy of xyrids (Poales): Contributions to their reproductive biology, taxonomy, and phylogeny. Int. J. Plant Sci. 2012, 173, 767–779. [Google Scholar] [CrossRef] [Green Version]
- Givnish, T.J.; Zuluaga, A.; Spalink, D.; Gomez, M.S.; Lam, V.K.Y.; Saarela, J.M.; Sass, C.; Iles, W.J.D.; de Sousa, D.J.L.; Leebens-Mack, J.; et al. Monocot plastid phylogenomics, timeline, net rates of species diversification, the power of multi-gene analyses, and a functional model for the origin of monocots. Am. J. Bot. 2018, 105, 1888–1910. [Google Scholar] [CrossRef]
- Darshetkar, A.M.; Datar, M.N.; Tamhankar, S.; Li, P.; Choudhary, R.K. Understanding evolution in Poales: Insights from Eriocaulaceae plastome. PLoS ONE 2019, 14, e0221423. [Google Scholar] [CrossRef] [Green Version]
- Remizowa, M.V.; Kuznetsov, A.N.; Kuznetsova, S.P.; Rudall, P.J.; Nuraliev, M.S.; Sokoloff, D.D. Flower development and vasculature in Xyris grandis (Xyridaceae, Poales); a case study for examining petal diversity in monocot flowers with a double perianth. Bot. J. Linn. Soc. 2012, 170, 93–111. [Google Scholar] [CrossRef] [Green Version]
- Linder, H.P.; Rudall, P.J. Evolutionary history of Poales. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 107–124. [Google Scholar] [CrossRef]
- Givnish, T.J.; Ames, M.; McNeal, J.R.; McKain, M.R.; Steele, P.R.; dePamphilis, C.W.; Graham, S.W.; Pires, J.C.; Stevenson, D.W.; Zomlefer, W.B.; et al. Assembling the tree of the monocotyledons: Plastome sequence phylogeny and evolution of Poales. Ann. Mo. Bot. Gard. 2010, 97, 584–616. [Google Scholar] [CrossRef]
- Stützel, T. Die systematische Stellung der Gattung Wurdackia (Eriocaulaceae). Flora 1985, 177, 335–344. [Google Scholar] [CrossRef]
- Watanabe, M.T.C.; Hensold, N.; Sano, P.T. Syngonanthus androgynus, a striking new species from South America, its phylogenetic placement and implications for evolution of bisexuality in Eriocaulaceae. PLoS ONE 2015, 10, e0141187. [Google Scholar] [CrossRef] [Green Version]
- Rosa, M.M.; Scatena, V.L. Floral anatomy of Eriocaulon elichrysoides and Syngonanthus caulescens (Eriocaulaceae). Flora 2003, 198, 188–199. [Google Scholar] [CrossRef]
- Rosa, M.M.; Scatena, V.L. Floral anatomy of Paepalanthoideae (Eriocaulaceae, Poales) and their nectariferous structures. Ann. Bot. 2007, 99, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Giulietti, A.M.; Andrade, M.J.G.; Scatena, V.L.; Trovó, M.; Coan, A.I.; Sano, P.T.; Santos, F.A.R.; Borges, R.L.B.; van den Berg, C. Molecular phylogeny, morphology and their implications for the taxonomy of Eriocaulaceae. Rodriguésia 2012, 63. [Google Scholar] [CrossRef]
- De Silva, A.L.; Trovó, M.; Coan, A.I. Floral development and vascularization help to explain merism evolution in Paepalanthus (Eriocaulaceae, Poales). PeerJ 2016, 4, e2811. [Google Scholar] [CrossRef] [Green Version]
- Ansari, R.; Balakrishnan, N.P. The Family Eriocaulaceae in India; Bishen Singh & Mahendra Pal Singh: Dehradun, India, 2009; ISBN 978-81-211-0725-9. [Google Scholar]
- Takahashi, H. A comparative study of floral development in Trillium apetalon and T. kamtschaticum (Liliaceae). J. Plant Res. 1994, 107, 237–243. [Google Scholar] [CrossRef]
- Vislobokov, N.; Sokoloff, D.; Degtjareva, G.; Valiejo-Roman, C.; Kuznetsov, A.; Nuraliev, M.S. Aspidistra paucitepala (Asparagaceae), a new species with occurrence of the lowest tepal number in flowers of Asparagales. Phytotaxa 2014, 161, 270. [Google Scholar] [CrossRef]
- Stützel, T.; Gansser, N. Floral morphology of North American Eriocaulaceae and its taxonomic implications. Feddes Repert. 1996, 106, 495–502. [Google Scholar] [CrossRef]
- Phillips, S.M. Notes on some Eriocaulon species from Ceylon. Kew Bull. 1994, 49, 287–303. [Google Scholar] [CrossRef]
- Ma, W.L.; Zhang, Z.; Stützel, T. Eriocaulaceae. In Flora of China; Science Press: Beijing, China, 2000; Volume 24, pp. 7–17. [Google Scholar]
- Gaikwad, S.P.; Yadav, S.R. Eriocaulaceae in Maharashtra. Biodivers. India 2002, 1, 256–341. [Google Scholar]
- Leach, G.J. A revision of Australian Eriocaulon (Eriocaulaceae). Telopea 2017, 20, 205–259. [Google Scholar] [CrossRef]
- Phillips, S.M. Eriocaulaceae of the Flora Zambesiaca area. Kirkia 1998, 17, 11–67. [Google Scholar]
- Steinberg, E.I. Eriocaulaceae. In Flora of USSR; Komarov, V.L., Ed.; Izdatel’stvo Akademii Nauk SSSR: Leningrad, Russia, 1935; Volume 3, pp. 494–498. [Google Scholar]
- Ghazanfar, S.A. Eriocaulaceae. In Flora of Pakistan; Nasir, E., Ali, S.I., Eds.; University of Karachi: Karachi, Pakistan, 1982; pp. 1–4. [Google Scholar]
- De Oliveira, A.L.R.; Bove, C.P. Eriocaulon L. from Brazil: An annotated checklist and taxonomic novelties. Acta Bot. Brasil. 2015, 29, 175–189. [Google Scholar] [CrossRef] [Green Version]
- Stützel, T. Die epipetalen Drüsen der Gattung Eriocaulon (Eriocaulaceae). Beitr. Biol. Pflanz. 1985, 60, 271–276. [Google Scholar]
- Cook, C.D.K. Aquatic and Wetland Plants of India; Oxford University Press: New York, NY, USA, 1996; ISBN 978-0-19-854821-8. [Google Scholar]
- Joshi, V.C.; Rajkumar, S.; Janarthanam, M.K. New records of the family Eriocaulaceae from Goa. J. Bombay Nat. Hist. Soc. 2001, 98, 155–156. [Google Scholar]
- Larridon, I.; Tanaka, N.; Liang, Y.; Phillips, S.M.; Barfod, A.S.; Cho, S.-H.; Gale, S.W.; Jobson, R.W.; Kim, Y.-D.; Li, J.; et al. First molecular phylogenetic insights into the evolution of Eriocaulon (Eriocaulaceae, Poales). J. Plant Res. 2019, 132, 589–600. [Google Scholar] [CrossRef] [Green Version]
- Kral, R. Eriocaulaceae. In Flora of North America North of Mexico, Vol. 22. Magnoliophyta: Alismatidae, Arecidae, Commelinidae (in part), and Zingiberidae; Flora of North America Editorial Committee, Ed.; Oxford University Press: New York, NY, USA, 2000; pp. 198–210. [Google Scholar]
- Govil, G.M.; Lavania, S. Floral vasculature of Eriocaulon L. J. Indian Bot. Soc. 1982, 61, 371–376. [Google Scholar]
- Bittrich, V. Caryophyllaceae. In The Families and Genera of Vascular Plants. Dicotyledons: Magnoliid, Hamamelid and Caryophyllid Families; Kubitzki, K., Rohwer, J.G., Bittrich, V., Eds.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 206–236. ISBN 978-3-662-02899-5. [Google Scholar]
- Jeiter, J.; Hilger, H.H.; Smets, E.F.; Weigend, M. The relationship between nectaries and floral architecture: A case study in Geraniaceae and Hypseocharitaceae. Ann. Bot. 2017, 120, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Konarska, A.; Masierowska, M. Structure of floral nectaries and female-biased nectar production in protandrous species Geranium macrorrhizum and Geranium phaeum. Protoplasma 2020, 257, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Endress, P.K. Synorganisation without organ fusion in the flowers of Geranium robertianum (Geraniaceae) and its not so trivial obdiplostemony. Ann. Bot. 2010, 106, 687–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remizowa, M.; Sokoloff, D.; Rudall, P. Patterns of floral structure and orientation in Japonolirion, Narthecium, and Tofieldia. Aliso 2006, 22, 159–171. [Google Scholar] [CrossRef]
- Endress, P.K. The whole and the parts: Relationships between floral architecture and floral organ shape, and their repercussions on the interpretation of fragmentary floral fossils. Ann. Mo. Bot. Gard. 2008, 95, 101–120. [Google Scholar] [CrossRef] [Green Version]
- Ronse De Craene, L. Understanding the role of floral development in the evolution of angiosperm flowers: Clarifications from a historical and physico-dynamic perspective. J. Plant Res. 2018, 131, 367–393. [Google Scholar] [CrossRef]
- Čelakovský, L. Ueber die Verwandtschaft von Typha und Sparganium. Österr. Bot. Z. 1891, 41, 266–272. [Google Scholar] [CrossRef]
- Müller-Doblies, D. Über die Verwandtschaft von Typha und Sparganium im Infloreszenz- und Blütenbau. Bot. Jahrb. 1970, 89, 451–562. [Google Scholar]
- Schaffner, J.H. The development of the stamens and carpels of Typha latifolia. Bot. Gaz. 1897, 24, 93–102. [Google Scholar] [CrossRef]
- Carvalho, J.D.T. de Morfoanatomia e Desenvolvimento de Órgãos Reprodutivos em Espécies de Typha L. (Typhaceae). Master’s Thesis, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 2019. [Google Scholar]
- Sokoloff, D.D.; Remizowa, M.V.; Linder, H.P.; Rudall, P.J. Morphology and development of the gynoecium in Centrolepidaceae: The most remarkable range of variation in Poales. Am. J. Bot. 2009, 96, 1925–1940. [Google Scholar] [CrossRef] [Green Version]
- Sokoloff, D.D.; Remizowa, M.V.; Barrett, M.D.; Conran, J.G.; Rudall, P.J. Morphological diversity and evolution of Centrolepidaceae (Poales), a species-poor clade with diverse body plans and developmental patterns. Am. J. Bot. 2015, 102, 1219–1249. [Google Scholar] [CrossRef] [PubMed]
- Vrijdaghs, A.; Goetghebeur, P.; Muasya, A.M.; Caris, P.; Smets, E. Floral ontogeny in Ficinia and Isolepis (Cyperaceae), with focus on the nature and origin of the gynophore. Ann. Bot. 2005, 96, 1247–1264. [Google Scholar] [CrossRef] [Green Version]
- Vrijdaghs, A.; Muasya, A.M.; Goetghebeur, P.; Caris, P.; Nagels, A.; Smets, E. A floral ontogenetic approach to questions of homology within the Cyperoideae (Cyperaceae). Bot. Rev. 2009, 75, 30–51. [Google Scholar] [CrossRef]
- Oriani, A.; Stützel, T.; Scatena, V.L. Contributions to the floral anatomy of Juncaceae (Poales—Monocotyledons). Flora 2012, 207, 334–340. [Google Scholar] [CrossRef]
- Shamrov, I.I.; Anisimova, G.M.; Kotelnikova, N.S. Comparative analysis of gynoecium morphogenesis in Juncus filiformis and Luzula pedemontana (Juncaceae). Bot. Zhurnal 2012, 97, 985–1009. [Google Scholar]
- Remizowa, M.V.; Sokoloff, D.D.; Kondo, K. Early flower and inflorescence development in Dioscorea tokoro (Dioscoreales): Shoot chirality, handedness of cincinni and common tepal-stamen primordia. Wulfenia 2010, 17, 77–97. [Google Scholar]
- Sattler, R. Organogenesis of Flowers: A Photographic Text-Atlas; University of Toronto Press: Toronto, ON, Canada, 1973; ISBN 0-8020-1864-5. [Google Scholar]
- Kodaira, E.; Fukai, S. Floral initiation and development in three field-grown Allium species belonging to different sub-genera. J. Hortic. Sci. Biotechnol. 2005, 80, 765–773. [Google Scholar] [CrossRef]
- Remizowa, M.V.; Sokoloff, D.D.; Moskvicheva, L.A. Morphology and development of flower and shoot system in Tofieldia pusilla (Tofieldiaceae). Bot. Zhurnal 2005, 90, 840–853. [Google Scholar]
- Posluszny, U. Re-evaluation of certain key relationships in the Alismatidae: Floral organogenesis of Scheuchzeria palustris (Scheuchzeriaceae). Am. J. Bot. 1983, 70, 925–933. [Google Scholar] [CrossRef]
- Volkova, O.A.; Remizowa, M.V.; Sokoloff, D.D.; Severova, E.E. A developmental study of pollen dyads and notes on floral development in Scheuchzeria (Alismatales: Scheuchzeriaceae). Bot. J. Linn. Soc. 2016, 182, 791–810. [Google Scholar] [CrossRef] [Green Version]
- Remizowa, M.V. One upward, two steps down: Order of floral organ initiation. Russ. J. Dev. Biol. 2019, 50, 325–340. [Google Scholar] [CrossRef]
- Pande, P.C.; Singh, V. Floral development of Iris decora Wall. (Iridaceae). Bot. J. Linn. Soc. 1981, 83, 41–56. [Google Scholar] [CrossRef]
- Fukai, S.; Goi, M. Floral initiation and development in Freesia. Tech. Bull. Fac. Agric. Kagawa Univ. 1998, 50, 69–72. [Google Scholar]
- Sokoloff, D.D.; Remizowa, M.V.; Timonin, A.C.; Oskolski, A.A.; Nuraliev, M.S. Types of organ fusion in angiosperm flowers (with examples from Chloranthaceae, Araliaceae and monocots). Biol. Serbica 2018, 40, 16–46. [Google Scholar]
- Rudall, P.J. All in a spin: Centrifugal organ formation and floral patterning. Curr. Opin. Plant Biol. 2010, 13, 108–114. [Google Scholar] [CrossRef]
- Sinjushin, A.A. Revisiting the floral structure and ontogeny of Trapa natans L. (Lythraceae). Wulfenia 2018, 25, 57–70. [Google Scholar]
- Decraene, L.P.R.; Smets, E.F. Dédoublement revisited: Towards a renewed interpretation of the androecium of the Magnoliophytina. Bot. J. Linn. Soc. 1993, 113, 103–124. [Google Scholar] [CrossRef]
- Hare, C.L. The structure and development of Eriocaulon septangulare With. Bot. J. Linn. Soc. 1950, 53, 422–448. [Google Scholar] [CrossRef]
- Oriani, A.; Scatena, V.L. Floral organogenesis and vasculature in Mayacaceae, an enigmatic family of Poales. Plant Syst. Evol. 2019, 305, 549–562. [Google Scholar] [CrossRef]
- Igersheim, A.; Buzgo, M.; Endress, P.K. Gynoecium diversity and systematics in basal monocots. Bot. J. Linn. Soc. 2001, 136, 1–65. [Google Scholar] [CrossRef]
- Endress, P.K. The morphological relationship between carpels and ovules in angiosperms: Pitfalls of morphological interpretation. Bot. J. Linn. Soc. 2019, 189, 201–227. [Google Scholar] [CrossRef] [Green Version]
- Fomichev, C.I.; Briggs, B.G.; Macfarlane, T.D.; Sokoloff, D.D. Structure and development of female flowers in early-diverging restiids, Anarthria, Lyginia and Hopkinsia (Restionaceae s.l.): Further evidence of multiple pathways of gynoecium reduction in wind-pollinated lineages of Poales. Bot. J. Linn. Soc. 2019, 190, 117–150. [Google Scholar] [CrossRef]
- Ronse Decraene, L.P.; Linder, P.H.; Smets, E.F. Floral ontogenetic evidence in support of the Willdenowia clade of South African Restionaceae. J. Plant Res. 2001, 114, 329–342. [Google Scholar] [CrossRef]
- Ronse Decraene, L.P.; Linder, H.P.; Smets, E.F. Ontogeny and evolution of the flowers of South African Restionaceae with special emphasis on the gynoecium. Plant Syst. Evol. 2002, 231, 225–258. [Google Scholar] [CrossRef]
- The Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Kircher, P. Untersuchungen zur Blüten- and Infloreszenzmorphologie, Embryologie und Systematik der Restionaceen im Vergleich mit Gramineen und verwandten Familien. Diss. Bot. 1986, 94, 1–219. [Google Scholar]
- Linder, H.P. The gynocecia of Australian Restionaceae: Morphology, anatomy and systematic implications. Aust. Syst. Bot. 1992, 5, 227–245. [Google Scholar] [CrossRef]
- Linder, H.P. The structure and evolution of the female flower of the African Restionaceae. Bot. J. Linn. Soc. 1992, 109, 401–425. [Google Scholar] [CrossRef]
- Rudall, P.J.; Stuppy, W.; Cunniff, J.; Kellogg, E.A.; Briggs, B.G. Evolution of reproductive structures in grasses (Poaceae) inferred by sister-group comparison with their putative closest living relatives, Ecdeiocoleaceae. Am. J. Bot. 2005, 92, 1432–1443. [Google Scholar] [CrossRef] [PubMed]
- Sajo, M.G.; Rudall, P.J. Morphological evolution in the graminid clade: Comparative floral anatomy of the grass relatives Flagellariaceae and Joinvilleaceae. Bot. J. Linn. Soc. 2012, 170, 393–404. [Google Scholar] [CrossRef] [Green Version]
- Reynders, M.; Vrijdaghs, A.; Larridon, I.; Huygh, W.; Leroux, O.; Muasya, A.M.; Goetghebeur, P. Gynoecial anatomy and development in Cyperoideae (Cyperaceae, Poales): Congenital fusion of carpels facilitates evolutionary modifications in pistil structure. Plant Ecol. Evol. 2012, 145, 96–125. [Google Scholar] [CrossRef]















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokoloff, D.D.; Yadav, S.R.; Chandore, A.N.; Remizowa, M.V. Stability Despite Reduction: Flower Structure, Patterns of Receptacle Elongation and Organ Fusion in Eriocaulon (Eriocaulaceae: Poales). Plants 2020, 9, 1424. https://doi.org/10.3390/plants9111424
Sokoloff DD, Yadav SR, Chandore AN, Remizowa MV. Stability Despite Reduction: Flower Structure, Patterns of Receptacle Elongation and Organ Fusion in Eriocaulon (Eriocaulaceae: Poales). Plants. 2020; 9(11):1424. https://doi.org/10.3390/plants9111424
Chicago/Turabian StyleSokoloff, Dmitry D., Shrirang R. Yadav, Arun N. Chandore, and Margarita V. Remizowa. 2020. "Stability Despite Reduction: Flower Structure, Patterns of Receptacle Elongation and Organ Fusion in Eriocaulon (Eriocaulaceae: Poales)" Plants 9, no. 11: 1424. https://doi.org/10.3390/plants9111424