Establishment of a Cell Suspension Culture of Ageratina pichinchensis (Kunth) for the Improved Production of Anti-Inflammatory Compounds
Abstract
:1. Introduction
2. Results and Discussion
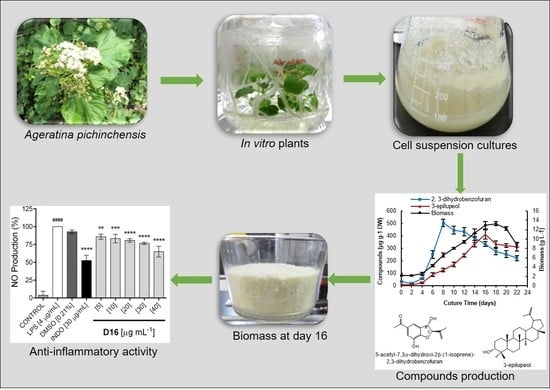
2.1. Cell Suspension Culture and Growth Kinetics
2.2. Cell Viability and pH in the Culture Medium
2.3. Phytochemical Analysis of the Cell Suspension Cultures
2.4. Production of Bioactive Compounds During Growth Kinetics
2.5. In Vitro Anti-Inflammatory Activity
3. Materials and Methods
3.1. General Procedures
3.2. Plant Material
3.3. Establishment of Cell Suspension Cultures
3.3.1. Growth Kinetics
3.3.2. Cell Viability
3.3.3. Sugar Quantification and pH Measurement
3.4. Extraction and Isolation of Compounds from Cell Suspension Cultures
3.4.1. Desmethoxyencecalin (1)
3.4.2. (2S,3R)-5-Acetyl-7,3α-dihydroxy-2β-(1-isopropenyl)-2,3-dihydrobenzofuran (2)
3.4.3. 3-Epilupeol (3)
3.4.4. Stigmasterol (4) and β-Sitosterol (5)
3.4.5. (-)-Artemesinol (6)
3.4.6. (±)-Artemesinol Glucoside (7)
3.5. Quantification of Compounds 2 and 3 by GC-MS
3.6. In Vitro Anti-Inflammatory Activity
3.6.1. Cell Culture
3.6.2. Assay for Cell Viability
3.6.3. Treatment with LPS
3.6.4. Determination of NO Concentration
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bremer, K. Asteraceae. In Cladistics and Classification, 1st ed.; Timber Press: Portland, OR, USA, 1994; p. 752. [Google Scholar]
- Katinas, L.; Gutierrez, D.G.; Grossi, M.A.; Crisci, J.V. Panorama de la familia Asteraceae (Compositae) en la República Argentina. Bol. Soc. Argent. Bot. 2007, 42, 113–129. [Google Scholar]
- Funk, V.A.; Susanna, A.; Steussy, T.F.; Robinson, H.E. Systematics, Evolution and Biogeography of Compositae, 1st ed.; International Association for Plant Taxonomy: Vienna, Austria, 2009; pp. 171–189. [Google Scholar]
- Villaseñor, J.L. Diversidad y distribución de la familia Asteraceae en México. Bot. Sci. 2018, 96, 332–358. [Google Scholar] [CrossRef] [Green Version]
- Villaseñor, J.L.; Rosell, J.A.; Ortíz, E. Plant families as predictors of plant biodiversity in México. Diversity Distrib. 2007, 13, 871–886. [Google Scholar]
- Velasco, J.; Barreto, S.; Buitrago, D.; Vivas, R. Antimicrobial activity of extracts from Ageratina neriifolia (Asteraceae). Ciencia 2006, 14, 411–414. [Google Scholar]
- Lima, T.C.; Santos, A.D.C.; Costa, D.T.M.; Souza, R.J.; Barison, A.; Steindel, M.; Biavatti, M.W. Chromenes from leaves of Calea pinnatifida and evaluation of their leishmanicidal activity. Rev. Bras. Farmacogn. 2015, 25, 7–10. [Google Scholar] [CrossRef] [Green Version]
- Del Barrio, G.; Spengler, I.; García, T.; Roque, A.; Álvarez, A.I.; Calderón, S.J.; Parra, F. Antiviral activity of Ageratina havanensis and major chemical compounds from the most active action. Rev. Bras. Farmacogn. 2011, 21, 915–920. [Google Scholar] [CrossRef]
- Navarro, V.M.; González, A.; Fuentes, M.; Avilez, M.; Ríos, M.Y.; Zepeda, G. Antifungal activities of nine traditional Mexican medicinal plants. J. Ethnopharmacol. 2003, 87, 85–88. [Google Scholar] [CrossRef]
- García, P.; García, S.; Martínez, G.; Scior, R.F.; Salvador, J.L.; Martínez, M.M.; del Río, R.E. Analgesic effect of leaf extract from Ageratina glabrata in the hot plate test. Rev. Bras. Farmacogn. 2011, 21, 928–935. [Google Scholar] [CrossRef] [Green Version]
- Sasikumar, J.M.; Doss, A. Antibacterial activity of Eupatorium glandulosum leaves. Fitoterapia 2005, 76, 240–243. [Google Scholar] [CrossRef]
- Gómez, F.; Quijano, L.; Calderón, J.S.; Perales, A.; Ríos, T. 2,2-Dimethylchromenes from Eupatorium aschembornianum. Phytochemistry 1982, 21, 2095–2097. [Google Scholar] [CrossRef]
- Ríos, M.Y.; Aguilar-Guadarrama, B.; Navarro, V. Two new benzofuranes from Eupatorium aschembornianum and their antimicrobial activity. Planta Med. 2013, 69, 967–970. [Google Scholar]
- Romero-Cerecero, O.; Zamilpa, A.; González-Cortazar, M.; Alonso-Cortés, D.; Jiménez-Ferrer, E.; Nicasio-Torres, P.; Aguilar- Santamaría, L.; Tortoriello, J. Pharmacological and chemical study to identify wound-healing active compounds in Ageratina pichinchensis. Planta Med. 2013, 79, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Guadarrama, B.; Navarro, V.; León-Rivera, I.; Ríos, M.Y. Active compounds against tinea pedis dermatophytes from Ageratina pichinchensis var. bustamenta. Nat. Prod. Res. 2009, 16, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cerecero, O.; Rojas, G.; Navarro, V.; Herrera-Arellano, A.; Zamilpa-Álvarez, A.; Tortoriello, J. Effectiveness and tolerability of a standardized extract from Ageratina pichinchensis on patients with tinea pedis: An explorative pilot study controlled with ketoconazole. Planta Med. 2006, 72, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Mendoza, M.E.; Rodriguez-Silverio, J.; Rivero-Cruz, J.F.; Rocha-González, H.I.; Pineda-Farías, J.B.; Arrieta, J. Antinociceptive effect and gastroprotective mechanisms of 3,5-diprenyl-4-hydroxyacetophenone from Ageratina pichinchensis. Fitoterapia 2013, 87, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Vanisree, M.; Lee, C.Y.; Lo, S.F.; Nalawade, S.M.; Lin, C.Y.; Tsay, H.S. Studies on the production of some important secondary metabolites from medicinal plants by plant tissue cultures. Bot. Bull. Acad. Sin. 2004, 45, 1–22. [Google Scholar]
- Leathers, R.R.; Davin, C.; Zry, J.P. Betalain producing cell cultures of Beta vulgaris L. Var. Bikores monogerm (red beet)1. Vitro Cell. Dev. Biol. 1992, 28, 39–45. [Google Scholar] [CrossRef]
- Ketchum, R.E.B.; Gibson, D.M. Placlitaxel production in suspension cell cultures of Taxus. Plant Cell Tiss. Org. 1996, 46, 9–16. [Google Scholar] [CrossRef]
- Vongpaseuth, K.; Roberts, S.C. Advancements in the understanding of paclitaxel metabolism in tissue culture. Curr. Pharm. Biotechnol. 2007, 8, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Wink, M.; Alfermann, A.W.; Franke, R.; Wetterauer, B.; Distl, M.; Windhövel, J.; Kron, O.; Fuss, E.; Garden, H.; Mohagheghzadeh, A.; et al. Sustainable bioproduction of phytochemicals by plant in vitro cultures: Anticancer agents. Plant Genet. Res. 2005, 3, 90–100. [Google Scholar] [CrossRef]
- Zhou, M.L.; Shao, J.R.; Tang, Y.X. Production and metabolic engineering of terpenoid indole alkaloids in cell cultures of the medicinal plant Catharanthus roseus (L.) G. Don (Madagascar periwinkle). Biotechnol. Appl. Biochem. 2009, 52, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.C.; Ren, Y.G.; Zhao, J.; Long, F.; Chen, J.; Jian, Z. Shikonin enhances sensitization of gefitinib against wild-type EGFR non-small cell lung cancer via inhibition PFM2/stat3/cyclinD1 signal pathway. Life Sci. 2018, 204, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, K. Lithospermum erythrorhizon cell cultures: Present and future aspects. Plant Biotechnol. 2017, 34, 131–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Shanmugasundaram, K.; Rigby, A.; Kung, A.L. Shikonin, a natural product from the root of Lithospermum erythorhizon, is a cytotoxic DNA-bindging agent. Eur. J. Pharm. Sci. 2013, 49, 18–26. [Google Scholar] [CrossRef]
- Sánchez-Ramos, M.; Marquina-Bahena, S.; Romero-Estrada, A.; Bernabé-Antonio, A.; Cruz-Sosa, F.; González-Christen, J.; Acevedo-Fernández, J.J.; Perea-Arango, I.; Álvarez, L. Establishment and phytochemical analysis of a callus culture from Ageratina pichinchensis (Asteraceae) and its anti-inflammatory activity. Molecules 2018, 23, 1258. [Google Scholar] [CrossRef] [Green Version]
- Mathur, S.; Shekhawat, S.G. Establishment and characterization of Stevia rebaudiana (Bertoni) cell suspension culture: An in vitro approach for production of stevioside. Acta Physiol. Plant. 2013, 35, 931–939. [Google Scholar] [CrossRef]
- Nigra, H.M.; Alvarez, M.A.; Giulietti, A.M. Effect of carbon and nitrogen sources on growth and solasodine production in batch suspension cultures of Solanum eleagnifolium Cav. Plant Cell Tiss. Org. Cult. 1990, 21, 55–60. [Google Scholar] [CrossRef]
- Guadarrama-Flores, B.; Rodríguez-Monroy, M.; Cruz-Sosa, F.; García-Carmona, F.; Gandía-Herrero, F. Production of dehydroxylated betaleins and dopamine in cell suspension cultures of Celosia argenta var. plumosa. J. Agric. Food Chem. 2015, 63, 2741–2749. [Google Scholar] [CrossRef]
- Kehie, M.; Kumaria, S.; Tandon, P. Biotechnological enhancement of capsaicin biosynthesis in cell suspension cultures of Naga King Chili (Capsicum chinense Jacq.). Bioproc. Biosyst. Eng. 2016, 39, 205–210. [Google Scholar] [CrossRef]
- Singh, M.; Chaturvedi, R. Evaluation of nutrient uptake and physical parameters on cell biomass growth and production of spilanthol in suspension cultures of Spilanthes acmella Murr. Bioproc. Biosyst. Eng. 2012, 35, 943–951. [Google Scholar] [CrossRef]
- Sahraroo, A.; Mirjalili, H.M.; Corchete, P.; Balabar, M.; Moghadam, F.M.R. Establishment and characterization of a Satureja khuzistanica Jamzad (Lamilaceae) cell suspension culture: A new in vitro source of rosmarinic acid. Cytotechnology 2016, 68, 1415–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlov, A.; Werner, S.; Ilieva, M.; Bley, T. Characteristics of Helianthus annuus plant cell culture as a producer of immunologically active exopolysaccharides. Eng. Life Sci. 2005, 5, 280–283. [Google Scholar] [CrossRef]
- Patil, R.A.; Kolewe, M.E.; Roberts, S.C. Cellular aggregation is a key parameter associated with long term variability in paclitaxel accumulation in Taxus suspension cultures. Plant Cell Tiss. Org. Cult. 2013, 112, 303–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butenko, R.G.; Lipsky, A.K.; Chernyak, N.D.; Arya, H.C. Changes in culture medium pH by cell suspension cultures of Dioscorea deltoidea. Plant Sci. 1984, 35, 207–212. [Google Scholar] [CrossRef]
- An tSaoir, S.M.; Damvoglou, V. The effect of carbohydrate source and pH on in vitro growth of Vitis vinifera cultivars Black Hamburg and Alvarino. In Physiology, Growth and Development of Plants in Culture; Lumsden, P.J., Nicholas, J.R., Davies, W.J., Eds.; Springer: Dodrecht, The Netherlands, 1994; pp. 94–97. [Google Scholar]
- Warhade, I.M.; Badere, S.R. Fusarium oxysporum cell elicitor enhances betalain content in the cell suspension culture of Celosia cristata. Physiol. Mol. Biol. Plant 2018, 24, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Fulkarni, M.M.; Bhimsem, A.N.; Sudhakar, G.D.; Ravindra, N.S. Five chromenes from Bledharispermum subsessile. Phytochemistry 1987, 26, 2969–2971. [Google Scholar] [CrossRef]
- Proksch, P.; Witte, L.; Wray, V. Chromene Glycosides from Ageratina altzssima. Phytochemistry 1988, 27, 3690–3691. [Google Scholar] [CrossRef]
- Tsuno, T.; Niwano, T.; Kakui, Y. Anti-Inflammatory agent containing sterol esters for use in cosmetics, quasi-drugs, pharmaceutical and health food. Jpn. Kokai Tokkyo Koho, JP 2016, 2016196418. [Google Scholar]
- Jianbo, L.; Haiging, Z.; Aisa, B.; Akber, H. Antimicrobial and antitumor activities of crude extracts and isolated compounds from Euphorbia humifusa. Asian J. Chem. 2013, 25, 3957–3960. [Google Scholar]
- Romero-Cerecero, O.; Román-Ramos, R.; Zamilpa, A.; Jiménez-Ferrer, J.E.; Rojas-Bribiesca, G.; Tortoriello, J. Clinical trial to compare the effectiveness of two concentrations of the Ageratina pichinchensis extract in the topical treatment of onychomycosis. J. Ethnopharmacol. 2009, 126, 74–78. [Google Scholar] [CrossRef]
- Nhan, H.N.; Loc, H.N. Production of eurycomanone from cell suspension culture of Eurycoma longifolia. Pharm. Biol. 2017, 55, 2234–2239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradi, A.; Zarinkamar, F.; De Domenico, S.; Mita, G.; Di Sansebastiano, G.P.; Caretto, S. Salycilic acid induces exudation of cronic and phenolics in Saffron suspension-cultured cells. Plants 2020, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Ruta, C.; De Mastro, G.; Ancona, S.; Tagarelli, A.; De Cillis, F.; Benelli, C.; Lambardi, M. Large-scale plant production of Lycium barbarum L. by liquid culture in temporary system and possible application to the synthesis of bioactive substance. Plants 2020, 9, 844. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Bhattacharya, S.; Khosla, P.M.; Puri, S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J. Appl. Res. Med. Aroma 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Chowdhury, B.L.; Hussaini, F.A.; Shoeb, A. Antiviral constituents from Vicoa indica. Int. J. Crude Drug Res. 1990, 2, 121–124. [Google Scholar] [CrossRef]
- Feng, D.; Ling, W.H.; Duan, R.D. Lycopene suppresses LPS-induced NO and IL-6 production by inhibiting the activation of ERK, p38MAPK, and NF-κB in macrophages. Inflamm. Res. 2010, 59, 115–121. [Google Scholar] [CrossRef]
- Nicholas, C.; Batra, S.; Vargo, M.A.; Voss, O.H.; Gavrilin, M.A.; Wewers, M.D.; Guttridge, D.C.; Grotewold, E.; Doseff, A.I. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-κB through the suppression of p65 phosphorylation. J. Immunol. 2007, 179, 7121–7127. [Google Scholar] [CrossRef] [Green Version]
- Yasukawa, K.; Yu, S.Y.; Yamanouchi, S.; Takido, M.; Akihisa, T.; Tamura, T. Some lupane-type triterpenes inhibit tumor proportion by 12-O-tetradecanoylphorbol-13-acetate in two stage carcinogenesis in mouse skin. Phytomedicine 1995, 4, 309–313. [Google Scholar] [CrossRef]
- Akihisa, T.; Franzblau, S.G.; Ukiva, M.; Okuda, H.; Zhang, F.; Yasukawa, K.; Suzuki, T.; Kimura, Y. Antitubercular activity of triterpenoinds from Asteraceae flowers. Biol. Pharm. Bull. 2005, 28, 158–160. [Google Scholar] [CrossRef] [Green Version]
- Puapairoj, J.; Naegchomnong, W.; Kijjoa, A.; Pinto, M.M.; Pedro, M.; Nascimento, M.S.J.; Silva, A.M.S.; Herz, W. Cytotoxic activity of lupine-type triterpenes from Glochidion sphaerogynum and Glochidion eriocarpum two of which induce apoptosis. Planta Med. 2005, 71, 208–213. [Google Scholar] [CrossRef]
- Romero-Estrada, A.; Maldonado-Magaña, A.; González-Christen, J.; Marquina-Bahena, S.; Garduño-Ramírez, M.L.; Rodríguez-López, V.; Alvarez, L. Anti-inflammatory and antioxidative effects of six pentacyclic triterpenes isolated from the Mexican copal resin of Bursera copallifera. BMC Complem. Altern. Med. 2016, 16, 422–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, T.; Sowndhararajan, K.; Hong, S.; Lee, J.; Park, S.Y.; Kim, S.; Jhoo, J.W. Inhibition of nitric oxide production in LPS-stimulated RAW 264.7 cells by stem bark of Ulmus pumila L. Saudi J. Biol. Sci. 2014, 21, 427–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ando, Y.; Oku, T.; Tsuji, T. Platelet supernatant suppresses LPS-induced nitric oxide production from macrophages accompanied by inhibition of NF-κB signaling and increased arginase-1 expression. PLoS ONE 2016, 11, e0162208–e0162223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, R.; Yuan, J.; Wu, S.; Huang, J.; Xu, X.; Wu, Z.; Gao, H. Anti-inflammation furanoditerpenoids from Caesalpinia minax Hance. Phytochemistry 2015, 117, 325–331. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Kanwar, R.K.; Burrow, H.; Baratchi, S. Recent advances on the roles of NO in cancer and chronic inflammatory disorders. Curr. Med. Chem. 2009, 16, 2373–2394. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Melis, M.P.; Corona, G.; Deiana, M. Modulation of LPS-induced nitric oxide production in intestinal cells by hydroxytyrosol and tyrosol metabolites: Insight into the mechanism of action. Food Chem. Toxicol. 2019, 125, 520–527. [Google Scholar] [CrossRef] [Green Version]
- Assanga, I.; Gil-Salido, A.A.; Luján, L.; Rosas-Durazo, A.; Acosta-Silva, A.; Rivera-Castañeda, E.G.; Rubio-Pino, J.L. Cell growth curves for different cell lines and their relationship with biological activities. J. Biotechnol. Mol. Biol. Res. 2013, 4, 60–70. [Google Scholar]
- Rodríguez-Monroy, M.; Galindo, E. Broth rheology, growth and metabolite production of Beta vulgaris suspension culture: A comparative study between cultures grown in flasks and in a stirred tank. Enzyme Microb. Tech. 1999, 24, 687–693. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugar and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Ramos, M.; Alvarez, L.; Romero-Estrada, A.; Bernabé-Antonio, A.; Marquina-Bahena, S.; Cruz-Sosa, F. Establishment of a Cell Suspension Culture of Ageratina pichinchensis (Kunth) for the Improved Production of Anti-Inflammatory Compounds. Plants 2020, 9, 1398. https://doi.org/10.3390/plants9101398
Sánchez-Ramos M, Alvarez L, Romero-Estrada A, Bernabé-Antonio A, Marquina-Bahena S, Cruz-Sosa F. Establishment of a Cell Suspension Culture of Ageratina pichinchensis (Kunth) for the Improved Production of Anti-Inflammatory Compounds. Plants. 2020; 9(10):1398. https://doi.org/10.3390/plants9101398
Chicago/Turabian StyleSánchez-Ramos, Mariana, Laura Alvarez, Antonio Romero-Estrada, Antonio Bernabé-Antonio, Silvia Marquina-Bahena, and Francisco Cruz-Sosa. 2020. "Establishment of a Cell Suspension Culture of Ageratina pichinchensis (Kunth) for the Improved Production of Anti-Inflammatory Compounds" Plants 9, no. 10: 1398. https://doi.org/10.3390/plants9101398