Successional Categorization of European Hemi-boreal Forest Tree Species
Abstract
:1. Background
2. Successional Categorization of Forest Tree Species in Lithuania
3. Four Types of Forest Successional Groups for Lithuania
4. General Suggestions for Forest Management
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Angelstam, P.; Kuuluvainen, T. Boreal Forest Disturbance Regimes, Successional Dynamics and Landscape Structures: A European Perspective. Ecol. Bull. 2004, 51, 117–136. [Google Scholar] [CrossRef]
- Shipley, B.; Vile, D.; Garnier. E. From plant traits to plant communities: A statistical mechanistic approach to biodiversity. Science 2006, 314, 812–814. [Google Scholar] [CrossRef] [PubMed]
- Van Teeffelen, A.J.A.; Vos, C.C.; Opdam, P.F.M. Species in a dynamic world: Consequences of habitat network dynamics on conservation planning. Biol. Conserv. 2012, 153, 239–253. [Google Scholar] [CrossRef] [Green Version]
- Aubry, C.; DeVine, W.; Shoal, R.; Bower, A.; Miller, J.; Maggiulli, N. Climate Change and Forest Biodiversity: A Vulnerability Assessment and Action Plan for National Forests in Western Washington; USDA Forest Service, Pacific Northwest Region: Portland, OR, USA, 2011; p. 308. [Google Scholar]
- Franklin, J. Regeneration and growth of pioneer and shade-tolerant rain forest trees in Tonga. N. Z. J. Bot. 2003, 41, 669–684. [Google Scholar] [CrossRef]
- Millet, J.; Bouchard, A.; Édelin, C. Relationship between architecture and successional status of trees in the temperate deciduous forest. Écoscience 1999, 6, 187–203. [Google Scholar] [CrossRef]
- Stern, K.; Roche, L. Genetics of Forest Ecosystems; Ecological Studies Series 6; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1974. [Google Scholar] [CrossRef]
- Whittaker, R.H. A consideration of climax theory: The climax as a population and pattern. Ecol. Monogr. 1953, 23, 41–78. [Google Scholar] [CrossRef]
- Richards, P.W. The Tropical Rain Forest: An Ecological Study; Cambridge University Press: New York, NY, USA, 1952. [Google Scholar]
- Clements, F.E. Nature and structure of the climax. J. Ecol. 1936, 24, 252–284. [Google Scholar] [CrossRef]
- Frelich, L.E. Forest Dynamics and Disturbance Regimes: Studies from Temperate Evergreen-Deciduous Forests; Cambridge Studies in Ecology Series; Cambridge University Press: Cambridge, UK, 2002; p. 266. [Google Scholar]
- Lewis, J.S.; Farnsworth, M.L.; Burdett, C.L.; Theobald, D.M.; Gray, M.; Miller, R.S. Biotic and abiotic factors predicting the global distribution and population density of an invasive large mammal. Sci. Rep. 2017, 7, 44152. [Google Scholar] [CrossRef]
- Eliot, C.H. The Legend of Order and Chaos. In Philosophy of Ecology; DeLaplante, K., Brown, B., Peacock, K.A., Eds.; Elsevier: Waltham, MA, USA, 2011; pp. 49–107. [Google Scholar]
- Eliot, C.H. Method and metaphysics in Clements’s and Gleason’s ecological explanations. Stud. Hist. Philos. Sci. C 2007, 38, 85–109. [Google Scholar] [CrossRef]
- Hagen, J.B. An Entangled Bank: The Origins of Ecosystem Ecology; Rutgers University Press: New Brunswick, NJ, USA, 1992. [Google Scholar]
- Hagen, J.B. Organism and Environment: Frederic Clements’s Vision of a Unified Physiological Ecology. In The American Development of Biology; Rainger, R., Benson, K.R., Maienschein, J., Eds.; University of Pennsylvania Press: Philadelphia, PA, USA, 1988; pp. 257–280. [Google Scholar]
- Borman, M.M.; Pyke, D.A. Successional Theory and the Desired Plant Community Approach. Rangelands 1994, 16, 82–84. [Google Scholar]
- Bazzaz, F.A. Plants in Changing Environments: Linking Physiological, Population, and Community Ecology; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Rusbult, C. Einstein’s Theory of Relativity is a Theory of Invariance-Constancy. 2007. Available online: https://www.asa3.org/ASA/education/views/invariance.htm (accessed on 27 October 2019).
- Kazansky, A.B. Agential anticipation in the central nervous system. In Anticipation: Learning from the Past; Nadin, M., Ed.; Cognitive Systems Monographs 25; Springer International Publishing: Cham, Switzerland, 2015; pp. 101–112. [Google Scholar]
- Li, B.-L. Fractal geometry applications in description and analysis of patch patterns and patch dynamics. Ecol. Model. 2000, 132, 33–50. [Google Scholar] [CrossRef]
- Gergle, S.E.; Turner, M.G. Learning Landscape Ecology: A Practice Guide to Concepts and Techniques; Springer: New York, NY, USA, 2001. [Google Scholar]
- Angelstam, P.; Manton, M.; Pedersen, S.; Elbakidze, M. Disrupted Trophic Interactions Affect Recruitment of Boreal Deciduous and Coniferous Trees in Northern Europe. Ecol. Appl. 2017, 27, 1108–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edenius, L.; Bergman, M.; Ericsson, G.; Danell, K. The role of moose as a disturbance factor in managed boreal forests. Silva Fenn. 2002, 36, 57–67. [Google Scholar]
- Gleason, H.A. The structure and development of the plant association. Bull. Torrey Bot. Club 1917, 44, 463–481. [Google Scholar] [CrossRef]
- Petrokas, R.; Baliuckas, V. Self-sustaining forest. Appl. Ecol. Environ. Res. 2017, 15, 409–426. [Google Scholar] [CrossRef]
- Wendt, H.; Didier, G.; Combrexelle, S.; Abry, P. Multivariate Hadamard self-similarity: Testing fractal connectivity. Phys. D Nonlinear Phenom. 2017, 356–357, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Combrexelle, S.; Wendt, H.; Didier, G.; Abry, P. Multivariate scale-free dynamics: Testing fractal connectivity. In Proceedings of the 42nd IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), New Orleans, LA, USA, 5–9 March 2017; pp. 3984–3988. [Google Scholar]
- Sun, J.; Southworth, J. Remote sensing-based fractal analysis and scale dependence associated with forest fragmentation in an Amazon tri-national frontier. Remote Sens. 2013, 5, 454–472. [Google Scholar] [CrossRef] [Green Version]
- Elliott, S.D.; Blakesley, D.; Hardwick, K. Restoring Tropical Forests: A Practical Guide; Royal Botanic Gardens: Kew, UK, 2013; p. 344. [Google Scholar]
- Gorshkov, V.G.; Makarieva, A.M. Biotic Regulation Overview. 2001–2019. Available online: https://www.bioticregulation.ru/life/life2.php (accessed on 5 August 2020).
- Chazdon, R.L. Second Growth: The Promise of Tropical Forest Regeneration in an Age of Deforestation; University of Chicago Press: Chicago, IL, USA, 2014. [Google Scholar]
- Erickson, V.; Aubry, C.; Berrang, P.; Blush, T.; Bower, A.; Crane, B.; DeSpain, T.; Gwaze, D.; Hamlin, J.; Horning, M.; et al. Genetic Resource Management and Climate Change: Genetic Options for Adapting National Forests to Climate Change; USDA Forest Service: Washington, DC, USA, 2012; p. 19. [Google Scholar]
- Swenson, N.G.; Anglada-Cordero, P.; Barone, J.A. Deterministic tropical tree community turnover: Evidence from patterns of functional beta diversity along an elevational gradient. Proc. R. Soc. B Biol. Sci. 2011, 278, 877–884. [Google Scholar] [CrossRef] [Green Version]
- Gorshkov, V.G.; Makarieva, A.M.; Gorshkov, V.V. Revising the fundamentals of ecological knowledge: The biota-environment interaction. Ecol. Complex. 2004, 1, 17–36. [Google Scholar] [CrossRef] [Green Version]
- Petrere, M., Jr.; Giordano, L.C.; De Marco, P., Jr. Empirical diversity indices applied to forest communities in different successional stages. Braz. J. Biol. 2004, 64, 841–851. [Google Scholar] [CrossRef] [Green Version]
- Godvod, K.; Brazaitis, G.; Bačkaitis, J.; Kulbokas, G. The development and growth of larch stands in Lithuania. J. For. Sci. 2018, 64, 199–206. [Google Scholar]
- Bohn, U.; Neuhäusl, R.; Gollub, G.; Hettwer, C.; Neuhäuslová, Z.; Raus, T.; Schluter, H.; Weber, H. Karte Der Natürlichen Vegetation Europas/Map of the Natural Vegetation of Europe. Maßstab/Scale 1: 2 500 000; Bundesamt für Naturschutz/Federal Agency for Nature Conservation: Bonn, Germany, 2003. [Google Scholar]
- Smirnova, O.V.; Bobrovsky, M.V.; Khanina, L.G.; Braslavskaya, T.Y.; Starodubtseva, E.A.; Evstigneev, O.I.; Korotkov, V.N.; Smirnov, V.E.; Ivanova, N.V. Nemoral Forests. In European Russian Forests; Smirnova, O.V., Bobrovsky, M.V., Khanina, L.G., Eds.; Plant and Vegetation 15; Springer: Dordrecht, The Netherlands, 2017; p. 461. [Google Scholar] [CrossRef]
- State Forest Service. Lithuanian Statistical Yearbook of Forestry 2017; Lutute: Vilnius, Lithuania, 2017. [Google Scholar]
- Karazija, S. Types of Lithuanian Forest; Mokslas: Vilnius, Lithuania, 1988. (In Lithuanian) [Google Scholar]
- Navasaitis, M.; Ozolinčius, R.; Smaliukas, D.; Balevičienė, J. Dendroflora of Lithuania; Lututė: Kaunas, Lithuania, 2003. (In Lithuanian) [Google Scholar]
- More, D.; White, J. Cassell’s Trees of Britain and Northern Europe; Cassell: London, UK, 2003. [Google Scholar]
- Lithuanian Republic. Republic of Lithuania Order on the Approval of Forestry Rules; Lithuanian Ministry of the Environment: Vilnius, Lithuania, 2010. [Google Scholar]
- Petrokas, R. Forest climax phenomenon: An invariance of scale. Forests 2020, 11, 56. [Google Scholar] [CrossRef] [Green Version]
- Nottale, L. Lecture 19. Scale Relativity. In Scale Invariance and Beyond; Dubrulle, B., Graner, F., Sornette, D., Eds.; Centre de Physique des Houches Series 7; Springer: Berlin/Heidelberg, Germany, 1997; pp. 249–261. [Google Scholar]
- Watt, A.S. Pattern and process in the plant community. J. Ecol. 1947, 35, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Král, K.; Shue, J.; Vrška, T.; Gonzalez-Akre, E.B.; Parker, G.G.; McShea, W.J.; McMahon, S.M. Fine-scale patch mosaic of developmental stages in Northeast American secondary temperate forests: The European perspective. Eur. J. For. Res. 2016, 135, 981–996. [Google Scholar] [CrossRef]
- McCarthy, J.W. Gap dynamics of forest trees: A review with particular attention to Boreal forests. Environ. Rev. 2001, 9, 1–59. [Google Scholar] [CrossRef]
- McGarigal, K.; Cushman, S.A. The Gradient Concept of Landscape Structure. In Issues and Perspectives in Landscape Ecology; Wiens, J., Moss, M., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 112–119. [Google Scholar]
- Bugmann, H. A review of forest gap models. Clim. Chang. 2001, 51, 259–305. [Google Scholar] [CrossRef]
- Whitmore, T.C. On Pattern and Process in Forests. In The Plant Community as a Working Mechanism; Newman, E.I., Ed.; Blackwell Scientific Publications: Oxford, UK, 1982; pp. 45–59. [Google Scholar]
- Chazdon, R.L.; Chao, A.; Colwell, R.K.; Lin, S.-Y.; Norden, N.; Letcher, S.G.; Clark, D.B.; Finegan, B.; Arroyo, J.P. A novel statistical method for classifying habitat generalists and specialists. Ecology 2011, 92, 1332–1343. [Google Scholar] [CrossRef]
- Clark, D.A.; Clark, D.B. Life history diversity of canopy and emergent trees in a neotropical forest. Ecol. Monogr. 1992, 62, 315–344. [Google Scholar] [CrossRef]
- Whitmore, T.C. Canopy gaps and the two major groups of forest trees. Ecology 1989, 70, 536–538. [Google Scholar] [CrossRef]
- Yamamoto, S. Gap regeneration of major tree species in different forest types of Japan. Vegetatio 1996, 127, 203–213. [Google Scholar] [CrossRef]
- Petrokas, R.; Pliūra, A. Persistence of progenies of wild cherry (Prunus avium L.) at northern limit of natural distribution range in transfer to Lithuania. Balt. For. 2014, 20, 58–69. [Google Scholar]
- Gabrilavičius, R.; Petrokas, R.; Danusevičius, J. Rare Tree Species in the Lithuanian Forests; Baltic Printing House: Klaipėda, Lithuania, 2013; p. 191. (In Lithuanian) [Google Scholar]
- Petrokas, R. Height growth and its relation to the branching habits of wych elm (Ulmus glabra Hudson) in Lithuania. Balt. For. 2011, 17, 83–90. [Google Scholar]
- Petrokas, R. Phenotypic Variability of Wild Apple and Wild Pear. Ph.D. Thesis, Lithuanian Forest Research Institute, Lithuanian University of Agriculture, Kaunas, Lithuania, 2006. [Google Scholar]
- Baliuckas, V. Life History Traits and Broadleaved Tree Genetics. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2002. [Google Scholar]
- Lienard, J.; Florescu, I.; Strigul, N. An appraisal of the classic forest succession paradigm with the shade tolerance index. PLoS ONE 2015, 10, e0117138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gravel, D.; Canham, C.D.; Beaudet, M.; Messier, C. Shade tolerance, canopy gaps and mechanisms of coexistence of forest trees. Oikos 2010, 119, 475–484. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.R. Concepts, Theories and Models of Succession in the Boreal Forest of Central Canada. Ph.D. Thesis, Lakehead University, Thunder Bay, ON, Canada, 2009. [Google Scholar]
- Melechow, J.S. Zur Frage der natürlichen Verjüngung der Fichte auf Brandflächen. Forstwiss. Centralbl. 1934, 78, 47–61. [Google Scholar] [CrossRef]
- Jõgiste, K.; Frelich, L.E.; Laarmann, D.; Vodde, F.; Baders, E.; Donis, J.; Jansons, A.; Kangur, A.; Korjus, H.; Köster, K.; et al. Imprints of management history on hemiboreal forest ecosystems in the Baltic States. Ecosphere 2018, 9, e02503. [Google Scholar] [CrossRef] [Green Version]
- Klopčič, M.; Simončič, T.; Bončina, A. Comparison of regeneration and recruitment of shade-tolerant and light-demanding tree species in mixed uneven-aged forests: Experiences from the Dinaric region. Forestry 2015, 88, 552–563. [Google Scholar] [CrossRef] [Green Version]
- Lewontin, R.C. Selection for Colonizing Ability. In The Genetics of Colonizing Species; Baker, H.G., Stebbins, G.L., Eds.; Academic Press: New York, NY, USA; London, UK, 1965; pp. 77–94. [Google Scholar]
- Lewontin, R.C. The interaction of selection and linkage II. Optimum models. Genetics 1964, 50, 757–782. [Google Scholar]
- Richards, P.W. The secondary succession in the tropical rain forest. Sci. Prog. 1955, 43, 49–53. [Google Scholar]
- Delcourt, H.R.; Delcourt, P.A. Quaternary Ecology: A Paleoecological Perspective; Springer Science & Business Media: New York, NY, USA, 1991; pp. 27–28. [Google Scholar]
- Braun, E. The unforeseen challenge: From genotype-to-phenotype in cell populations. Rep. Prog. Phys. 2015, 78, 036602. Available online: http://iopscience.iop.org/0034-4885/78/3/036602 (accessed on 1 May 2020). [CrossRef]
- Stern, S.; Fridmann-Sirkis, Y.; Braun, E.; Soen, Y. Epigenetically heritable alteration of fly development in response to toxic challenge. Cell Rep. 2012, 1, 528–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, E.; David, L.; Gissis, S.B.; Jablonka, E. The Role of Cellular Plasticity in the Evolution of Regulatory Novelty. In Transformations of Lamarckism: From Subtle Fluids to Molecular Biology; Gissis, S.B., Jablonka, E., Eds.; MIT Press: Cambridge, MA, USA, 2011; pp. 181–191. [Google Scholar]
- Lamm, E.; Jablonka, E. The nurture of nature: Hereditary plasticity in evolution. Philos. Psychol. 2008, 21, 305–319. [Google Scholar] [CrossRef]
- Stancioiu, P.T.; O’Hara, K.L. Regeneration growth in different light environments of mixed species, multiaged, mountainous forests of Romania. Eur. J. For. Res. 2006, 125, 151–162. [Google Scholar] [CrossRef]
- Frank, S.A.; Bascompte, J. Invariance in ecological pattern. F1000Research 2019, 8, 2093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simard, S.W.; Martin, K.; Vyse, A.; Larson, B. Meta-Networks of Fungi, Fauna and Flora as Agents of Complex Adaptive Systems. In Managing World Forests as Complex Adaptive Systems; Messier, C., Puettmann, K.J., Coates, K.D., Eds.; Routledge: London, UK, 2013; pp. 133–164. [Google Scholar]
- Goetze, D.; Karlowski, U.; Porembski, S.; Tockner, K.; Watve, A.; Riede, K. Spatial and Temporal Dimensions of Biodiversity Dynamics. In Biodiversity: Structure and Function; Barthlott, W., Linsenmair, K.E., Porembski, S., Eds.; Encyclopedia of Life Support Systems (EOLSS): Oxford, UK, 2009; pp. 166–208. [Google Scholar]
- Lindenmayer, D.B.; Burton, P.J.; Franklin, J.F. Salvage Logging and Its Ecological Consequences; Island Press: Washington, DC, USA, 2008; 227p. [Google Scholar]
- Trakai Historical National Park. Law on the Amendment of the Forest Law of the Republic of Lithuania. 2011–2009. Available online: http://www.seniejitrakai.lt/law-on-the-amendment/ (accessed on 5 August 2020).
- Roberge, J.-M.; Angelstam, P. Indicator Species among Resident Forest Birds—A Cross-Regional Evaluation in Northern Europe. Biol. Conserv. 2006, 130, 134–147. [Google Scholar] [CrossRef]
- Elbakidze, M.; Ražauskaitė, R.; Manton, M.; Angelstam, P.; Mozgeris, G.; Brūmelis, G.; Brazaitis, G.; Vogt, P. The Role of Forest Certification for Biodiversity Conservation: Lithuania as a Case Study. Eur. J. For. Res. 2016, 135, 361–376. [Google Scholar] [CrossRef]
- Houston Durrant, T.; de Rigo, D.; Caudullo, G. Fagus sylvatica and Other Beeches in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publ. Off. EU: Luxembourg, 2016; p. e012b90+. [Google Scholar]
- Frelich, L.E.; Jõgiste, K.; Stanturf, J.A.; Parro, K.; Baders, E. Natural Disturbances and Forest Management: Interacting Patterns on the Landscape. In Ecosystem Services from Forest Landscapes; Springer International Publishing: Cham, Switzerland, 2018; pp. 221–248. [Google Scholar]
| Tree Species | Life History Traits | ||||||
|---|---|---|---|---|---|---|---|
| Dominant Stand Proportion [40] | Soil Moisture A [41,42] | Soil Fertility B [41,42] | Shade Tolerance | Hardiness C [43] | Life Expectancy [42] (Harvesting Age) [44] | Successional Strategy | |
| Dominant Forest Tree Species | |||||||
| Scots pine (Pinus sylvestris L.) | 34.6% | 1–3 and 5 | 1–3 and 5 | Intolerant | 9 | 300–400 (110) | Disturbance generalist |
| Norway spruce (Picea abies L. Karst) | 20.9% | 3–4 | 3–4 | Intermediate | 7 | 200–300 (71) | Succession generalist |
| Silver birch (Betula pendula Roth) | 22.0% | 2–5 | 2–4 | Intolerant | 9–10 | 150 (61) | Disturbance generalist |
| Black alder (Alnus glutinosa L. Gaertn) | 7.6% | 4–5 | 3–4 | Intermediate | 7 | 180–200 (61) | Disturbance generalist |
| Grey alder (Alnus incana L. Moench) | 5.9% | 2–5 | 3–4 | Intermediate | 9 | 50–70 (31) | Disturbance generalist |
| Eurasian aspen (Populus tremula) | 4.6% | 3–4 | 3–4 | Intolerant | 9 | 80–100 (41) | Disturbance generalist |
| English oak (Quercus robur L.) | 2.2% | 3–4 | 3–4 | Intolerant | 6–7 | 500–600 (121) | Disturbance specialist |
| European ash (Fraxinus excelsior L.) | 0.9% | 3–5 | 4–5 | Intermediate | 7–8 | > 300 (101) | Succession specialist |
| Other Secondary Native Forest Species | |||||||
| Small-leaved lime (Tilia cordata Mill.) | 0.4% | 3 | 3–4 | Intermediate | 7 | 500–600 (61) | Succession specialist |
| Downy birch (Betula pubescens Ehrh) | 0.4% | 3–5 | 2–5 | Intolerant | 9 | 100 D | Disturbance generalist |
| European hornbeam (Carpinus betulus L.) | 0.2% | 3 | 3–4 | Tolerant | 5 | 200–300 (61) | Disturbance generalist |
| Norway maple (Acer platanoides L.) | 0.2% | 3–4 | 3–5 | Tolerant | 8 | 150–300 (101) | Disturbance specialist |
| White willow (Salix alba L.) | <0.2% | 4 | 4–5 | Intolerant | 8 | >100 (31) | Disturbance generalist |
| Bird cherry (Prunus padus L.) | <0.2% | 4–5 | 3–5 | Intermediate | 9 | 150 D | Disturbance specialist |
| Crack willow (Salix fragilis L) | <0.2% | 4 | 4–5 | Intolerant | 8 | 75 (31) | Disturbance generalist |
| Field elm (Ulmus minor Mill.) | <0.2% | 2–4 | 4 | Intermediate | 5 | 300 (101) | Succession specialist |
| European white elm (Ulmus laevis Pall.) | <0.2% | 3–4 | 3–4 | Tolerant | 6–7 | 250–300 (101) | Succession specialist |
| Wych elm (Ulmus. glabra Huds.) | <0.2% | 3–4 | 4–5 | Tolerant | 6 | 300 (101) | Succession specialist |
| Wild apple (Malus sylvestris L. Mill.) | <0.2% | 4–5 | 3–5 | Intolerant | 8 | 300 D | Disturbance specialist |
| Wild pear (Pyrus pyraster L. Burgsd.) | <0.2% | 3–4 | 3–4 | Intermediate | 6 | 200–300 D | Disturbance specialist |
| Introduced Species | |||||||
| European beech (Fagus sylvatica L.) | <0.2% | 3 [37] | 3–4 | Tolerant | 5 | 500 (101) | Succession generalist |
| Sessile oak (Quercus petraea Matt. Liebl.) | <0.2% | 3 | 2–3 | Intermediate | 6–7 | 500–600 D | Disturbance specialist |
| Large-leaved lime (Tilia platyphyllos Scop.) | <0.2% | 3–4 | 4–5 | Intermediate | 7 | 500–600 D | Succession specialist |
| Wild cherry (Prunus avium L.) | <0.02% | 3–4 | 3–4 | Tolerant | 8 | 100 D | Disturbance generalist |
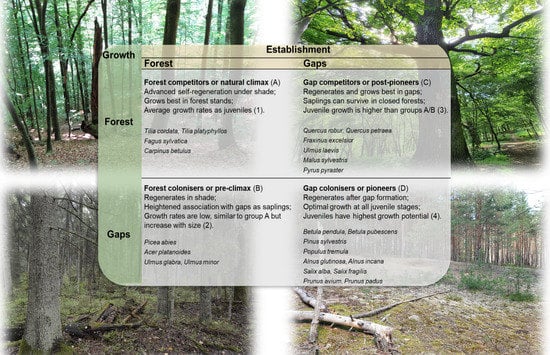
| Growth | Establishment | |
|---|---|---|
| Forest | Gaps | |
| Forest | Forest Competitors or Natural Climax (A) Advanced self-regeneration under shade and grows best in forest stands; average growth rates, especially as juveniles (1). | Gap Competitors or Post-pioneers (C) Regenerates and grows best in gaps, saplings can survive in closed forests; increased juvenile growth potential over groups A or B (3). |
| Tilia cordata Tilia platyphyllos Fagus sylvatica Carpinus betulus | Quercus robur Quercus petraea Fraxinus excelsior Ulmus laevis Malus sylvestris Pyrus pyraster | |
| Gaps | Forest Colonizers or Pre-climax (B) Regenerates in shade but shows heightened association with gaps as saplings; growth rates are as low as group A but increase with size (2). | Gap Colonizers or Pioneers (D) Regenerates after gap formation and achieves optimal growth at all juvenile stages; juveniles have the highest growth potential (4). |
| Picea abies Acer platanoides Ulmus glabra Ulmus minor | Betula pendula Betula pubescens Pinus sylvestris Populus tremula Alnus glutinosa Alnus incana Salix alba Salix fragilis Prunus avium Prunus padus | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrokas, R.; Baliuckas, V.; Manton, M. Successional Categorization of European Hemi-boreal Forest Tree Species. Plants 2020, 9, 1381. https://doi.org/10.3390/plants9101381
Petrokas R, Baliuckas V, Manton M. Successional Categorization of European Hemi-boreal Forest Tree Species. Plants. 2020; 9(10):1381. https://doi.org/10.3390/plants9101381
Chicago/Turabian StylePetrokas, Raimundas, Virgilijus Baliuckas, and Michael Manton. 2020. "Successional Categorization of European Hemi-boreal Forest Tree Species" Plants 9, no. 10: 1381. https://doi.org/10.3390/plants9101381