Auxin Homeostasis and Distribution of the Auxin Efflux Carrier PIN2 Require Vacuolar NHX-Type Cation/H+ Antiporter Activity
Abstract
:1. Introduction
2. Results
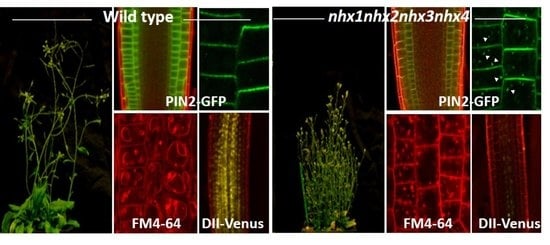
2.1. The Morphological Phenotype of nhx1nxh2nhx3nhx4 Knockout Mutants
2.2. The nhx1nhx2nhx3nhx4 Knockout Mutant Exhibited Altered Responses to Auxin
2.3. Decreased PIN2 Abundance on the PM of nhx1nhx2nhx3nhx4 Mutant
2.4. Intracellular Trafficking to the Vacuole is Affected in the nhx1nhx2nhx3nhx4 Mutant
2.5. Auxin Accumulated in the Root Tip Cells of nhx1nhx2nhx3nhx4 Mutant
3. Discussion
4. Materials and Methods
4.1. Arabidopsis Seeds, Agrobacteria Strains, Growth Conditions and Treatments
4.2. Assays for Root Elongation and Root Gravitropism
4.3. Fluorescence Microscopy
4.4. IAA Content Measurement
4.5. RNA Extraction and qPCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blumwald, E.; Rea, P.A.; Poole, R.J. Preparation of Tonoplast Vesicles: Applications to H+-Coupled Secondary Transport in Plant Vacuoles, In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; pp. 115–123. [Google Scholar]
- Orlowski, J.; Grinstein, S. Emerging roles of alkali cation/proton exchangers in organellar homeostasis. Curr. Opin. Cell Biol. 2007, 19, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Martinoia, E.; Maeshima, M.; Neuhaus, H.E. Vacuolar transporters and their essential role in plant metabolism. J. Exp. Bot. 2007, 58, 83–102. [Google Scholar] [CrossRef]
- Amtmann, A.; Leigh, R. Ion homeostasis. In Abiotic Stress Adaptation in Plants; Springer: Berlin/Heidelberg, Germany, 2009; pp. 245–262. [Google Scholar]
- Bassil, E.; Coku, A.; Blumwald, E. Cellular ion homeostasis: Emerging roles of intracellular NHX Na+/H+ antiporters in plant growth and development. J. Exp. Bot. 2012, 63, 5727–5740. [Google Scholar] [CrossRef] [Green Version]
- Bassil, E.; Tajima, H.; Liang, Y.C.; Ohto, M.; Ushijima, K.; Nakano, R.; Esumi, T.; Coku, A.; Belmonte, M.; Blumwald, E. The Arabidopsis Na+/H+ Antiporters NHX1 and NHX2 Control Vacuolar pH and K+ Homeostasis to Regulate Growth, Flower Development, and Reproduction. Plant Cell 2011, 23, 3482–3497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCubbin, T.; Bassil, E.; Zhang, S.; Blumwald, E. Vacuolar Na+/H+ NHX-Type Antiporters Are Required for Cellular K+ Homeostasis, Microtubule Organization and Directional Root Growth. Plants 2014, 3, 409–426. [Google Scholar] [CrossRef] [Green Version]
- Bassil, E.; Ohto, M.A.; Esumi, T.; Tajima, H.; Zhu, Z.; Cagnac, O.; Belmonte, M.; Peleg, Z.; Yamaguchi, T.; Blumwald, E. The Arabidopsis Intracellular Na+/H+ Antiporters NHX5 and NHX6 Are Endosome Associated and Necessary for Plant Growth and Development. Plant Cell 2011, 23, 224–239. [Google Scholar] [CrossRef] [Green Version]
- Martinière, A.; Bassil, E.; Jublanc, E.; Alcon, C.; Reguera, M.; Sentenac, H.; Blumwald, E.; Paris, N. In Vivo Intracellular pH Measurements in Tobacco and Arabidopsis Reveal an Unexpected pH Gradient in the Endomembrane System. Plant Cell 2013, 25, 4028–4043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reguera, M.; Bassil, E.; Tajima, H.; Wimmer, M.; Chanoca, A.; Otegui, M.S.; Paris, N.; Blumwald, E. pH Regulation by NHX-Type Antiporters Is Required for Receptor-Mediated Protein Trafficking to the Vacuole in Arabidopsis. Plant Cell 2015, 27, 1200–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragwidge, J.M.; Ford, B.A.; Ashnest, J.R.; Das, P.; Gendall, A.R. Two Endosomal NHX-Type Na+/H+ Antiporters are Involved in Auxin-Mediated Development in Arabidopsis thaliana. Plant Cell Physiol. 2018, 59, 1660–1669. [Google Scholar] [CrossRef]
- Wu, X.X.; Ebine, K.; Ueda, T.; Qiu, Q.S. AtNHX5 and AtNHX6 Are Required for the Subcellular Localization of the SNARE Complex That Mediates the Trafficking of Seed Storage Proteins in Arabidopsis. PLoS ONE 2016, 11, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.Z.; Quintero, F.J.; Pardo, J.M.; Zhu, J.K. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 2002, 14, 465–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, R.; Chen, Q.J.; Chai, M.F.; Lu, P.L.; Su, Z.; Qin, Z.X.; Chen, J.; Wang, X.C. AtNHX8, a member of the monovalent cation: Proton antiporter-1 family in Arabidopsis thaliana, encodes a putative Li+/H+ antiporter. Plant J. 2007, 49, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Barragán, V.; Leidi, E.O.; Andrés, Z.; Rubio, L.; De Luca, A.; Fernández, J.A.; Cubero, B.; Pardo, J.M. Ion Exchangers NHX1 and NHX2 Mediate Active Potassium Uptake into Vacuoles to Regulate Cell Turgor and Stomatal Function in Arabidopsis. Plant Cell 2012, 24, 1127–1142. [Google Scholar] [CrossRef] [Green Version]
- Bassil, E.; Zhang, S.; Gong, H.; Tajima, H.; Blumwald, E. Cation specificity of vacuolar NHX-type cation/H+ antiporters. Plant Physiol. 2019, 179, 616–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigas, S.; Debrosses, G.; Haralampidis, K.; Vicente-Agullo, F.; Feldmann, K.A.; Grabov, A.; Dolan, L.; Hatzopoulos, P. TRH1 encodes a potassium transporter required for tip growth in Arabidopsis root hairs. Plant Cell 2001, 13, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Rigas, S.; Ditengou, F.A.; Ljung, K.; Daras, G.; Tietz, O.; Palme, K.; Hatzopoulos, P. Root gravitropism and root hair development constitute coupled developmental responses regulated by auxin homeostasis in the Arabidopsis root apex. New Phytol. 2013, 197, 1130–1141. [Google Scholar] [CrossRef]
- Elumalai, R.P.; Nagpal, P.; Reed, J.W. A mutation in the Arabidopsis KT2/KUP2 potassium transporter gene affects shoot cell expansion. Plant Cell 2002, 14, 119–131. [Google Scholar] [CrossRef] [Green Version]
- Desbrosses, G.; Josefsson, C.; Rigas, S.; Hatzopoulos, P.; Dolan, L. AKT1 and TRH1 are required during root hair elongation in Arabidopsis. J. Exp. Bot. 2003, 54, 781–788. [Google Scholar] [CrossRef]
- Vicente-Agullo, F.; Rigas, S.; Desbrosses, G.; Dolan, L.; Hatzopoulos, P.; Grabov, A. Potassium carrier TRH1 is required for auxin transport in Arabidopsis roots. Plant J. 2004, 40, 523–535. [Google Scholar] [CrossRef]
- Osakabe, Y.; Arinaga, N.; Umezawa, T.; Katsura, S.; Nagamachi, K.; Tanaka, H.; Ohiraki, H.; Yamada, K.; Seo, S.-U.; Abo, M. Osmotic stress responses and plant growth controlled by potassium transporters in Arabidopsis. Plant Cell 2013, 25, 609–624. [Google Scholar] [CrossRef] [Green Version]
- Daras, G.; Rigas, S.; Tsitsekian, D.; Iacovides, T.A.; Hatzopoulos, P. Potassium transporter TRH1 subunits assemble regulating root-hair elongation autonomously from the cell fate determination pathway. Plant Sci. 2015, 231, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Ueda, J.; Komaki, M.K.; Bell, C.J.; Shimura, Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 1991, 3, 677–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashotte, A.M.; Poupart, J.; Waddell, C.S.; Muday, G.K. Transport of the two natural auxins, indole-3-butyric acid and indole-3-acetic acid, in Arabidopsis. Plant Physiol. 2003, 133, 761–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, E.; Davies, P. Evidence for three different systems of movement of indoleacetic acid in intact roots of Phaseolus coccineus. Physiol. Plant. 1975, 33, 290–294. [Google Scholar] [CrossRef]
- Tsurumi, S.; Ohwaki, Y. Transport of 14C-lableled indoleacetic acid in Vicia root segments. Plant Cell Physiol. 1978, 19, 1195–1206. [Google Scholar] [CrossRef]
- Rashotte, A.M.; Brady, S.R.; Reed, R.C.; Ante, S.J.; Muday, G.K. Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol. 2000, 122, 481–490. [Google Scholar] [CrossRef] [Green Version]
- Křeček, P.; Skůpa, P.; Libus, J.; Naramoto, S.; Tejos, R.; Friml, J.; Zažímalová, E. The PIN-FORMED (PIN) protein family of auxin transporters. Genome Biol. 2009, 10, 249. [Google Scholar] [CrossRef] [Green Version]
- Adamowski, M.; Friml, J. PIN-Dependent Auxin Transport: Action, Regulation, and Evolution. Plant Cell 2015, 27, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Zazímalová, E.; Murphy, A.S.; Yang, H.; Hoyerová, K.; Hosek, P. Auxin transporters—Why so many? Cold Spring Harb. Perspect. Biol. 2010, 2, a001552. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, M.E.; Thompson, B. Meristem identity and phyllotaxis in inflorescence development. Front. Plant Sci. 2014, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Delbarre, A.; Muller, P.; Imhoff, V.; Guern, J. Comparison of mechanisms controlling uptake and accumulation of 2,4-dichlorophenoxy acetic acid, naphthalene-1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta 1996, 198, 532–541. [Google Scholar] [CrossRef]
- Marchant, A.; Kargul, J.; May, S.T.; Muller, P.; Delbarre, A.; Perrot-Rechenmann, C.; Bennett, M.J. AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J. 1999, 18, 2066–2073. [Google Scholar] [CrossRef]
- Chen, R.J.; Hilson, P.; Sedbrook, J.; Rosen, E.; Caspar, T.; Masson, P.H. The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc. Natl. Acad. Sci. USA 1998, 95, 15112–15117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, A.; Guan, C.H.; Galweiler, L.; Tanzler, P.; Huijser, P.; Marchant, A.; Parry, G.; Bennett, M.; Wisman, E.; Palme, K. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 1998, 17, 6903–6911. [Google Scholar] [CrossRef] [PubMed]
- Luschnig, C.; Gaxiola, R.A.; Grisafi, P.; Fink, G.R. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 1998, 12, 2175–2187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abas, L.; Benjamins, R.; Malenica, N.; Paciorek, T.; Wirniewska, J.; Moulinier-Anzola, J.C.; Sieberer, T.; Friml, J.; Luschnig, C. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 2006, 8, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Kleine-Vehn, J.; Leitner, J.; Zwiewka, M.; Sauer, M.; Abas, L.; Luschnig, C.; Friml, J. Differential degradation of PIN2 auxin efflux carrier by retromer-dependent vacuolar targeting. Proc. Natl. Acad. Sci. USA 2008, 105, 17812–17817. [Google Scholar] [CrossRef] [Green Version]
- Dettmer, J.; Hong-Hermesdorf, A.; Stierhof, Y.D.; Schumacher, K. Vacuolar H+-ATPase activity is required for Endocytic and secretory trafficking in Arabidopsis. Plant Cell 2006, 18, 715–730. [Google Scholar] [CrossRef] [Green Version]
- Bolte, S.; Talbot, C.; Boutte, Y.; Catrice, O.; Read, N.D.; Satiat-Jeunemaitre, B. FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. J. Microsc. 2004, 214, 159–173. [Google Scholar] [CrossRef]
- Kleine-Vehn, J.; Langowski, L.; Wisniewska, J.; Dhonukshe, P.; Brewer, P.B.; Friml, J. Cellular and Molecular Requirements for Polar PIN Targeting and Transcytosis in Plants. Mol. Plant 2008, 1, 1056–1066. [Google Scholar] [CrossRef]
- Brunoud, G.; Wells, D.M.; Oliva, M.; Larrieu, A.; Mirabet, V.; Burrow, A.H.; Beeckman, T.; Kepinski, S.; Traas, J.; Bennett, M.J.; et al. A novel sensor to map auxin response and distribution at high spatio-temporal resolution. Nature 2012, 482, 103. [Google Scholar] [CrossRef] [PubMed]
- Remy, E.; Baster, P.; Friml, J.; Duque, P. ZIFL1.1 transporter modulates polar auxin transport by stabilizing membrane abundance of multiple PINs inArabidopsisroot tip. Plant Signal. Behav. 2013, 8, e25688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remy, E.; Cabrito, T.R.; Baster, P.; Batista, R.A.; Teixeira, M.C.; Friml, J.; Sá-Correia, I.; Duque, P. A major facilitator superfamily transporter plays a dual role in polar auxin transport and drought stress tolerance in Arabidopsis. Plant Cell 2013, 25, 901–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L. Na+, K+/H+ antiporters regulate the pH of endoplasmic reticulum and auxin-mediated development. Plant Cell Environ. 2018, 41, 850–864. [Google Scholar] [CrossRef]
- Yang, T.; Zhao, L.; Hu, H.; Li, W.; Novák, O.; Strnad, M.; Simon, S.; Friml, J.; Shen, J.; Jiang, L.; et al. The Potassium Transporter OsHAK5 Alters Rice Architecture via ATP-Dependent Transmembrane Auxin Fluxes. Plant Commun. 2020, 1, 100052. [Google Scholar] [CrossRef]
- Geldner, N.; Friml, J.; Stierhof, Y.-D.; Jürgens, G.; Palme, K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 2001, 413, 425–428. [Google Scholar] [CrossRef]
- Dhonukshe, P.; Aniento, F.; Hwang, I.; Robinson, D.G.; Mravec, J.; Stierhof, Y.-D.; Friml, J. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Current Biol. 2007, 17, 520–527. [Google Scholar] [CrossRef]
- Kleine-Vehn, J.; Dhonukshe, P.; Sauer, M.; Brewer, P.B.; Wiśniewska, J.; Paciorek, T.; Benková, E.; Friml, J. ARF GEF-dependent transcytosis and polar delivery of PIN auxin carriers in Arabidopsis. Current Biol. 2008, 18, 526–531. [Google Scholar] [CrossRef]
- Li, K.; Kamiya, T.; Fujiwara, T. Differential Roles of PIN1 and PIN2 in Root Meristem Maintenance Under Low-B Conditions in Arabidopsis thaliana. Plant Cell Physiol. 2015, 56, 1205–1214. [Google Scholar] [CrossRef] [Green Version]
- Birnbaum, K.; Shasha, D.E.; Wang, J.Y.; Jung, J.W.; Lambert, G.M.; Galbraith, D.W.; Benfey, P.N. A Gene Expression Map of the Arabidopsis Root. Science 2003, 302, 1956. [Google Scholar] [CrossRef] [Green Version]
- Nawy, T.; Lee, J.-Y.; Colinas, J.; Wang, J.Y.; Thongrod, S.C.; Malamy, J.E.; Birnbaum, K.; Benfey, P.N. Transcriptional Profile of the Arabidopsis Root Quiescent Center. Plant Cell 2005, 17, 1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benkova, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertova, D.; Jurgens, G.; Friml, J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 2003, 115, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spalding, E.P.; Hirsch, R.E.; Lewis, D.R.; Qi, Z.; Sussman, M.R.; Lewis, B.D. Potassium uptake supporting plant growth in the absence of AKT1 channel activity—Inhibition by ammonium and stimulation by sodium. J. Gen. Physiol. 1999, 113, 909–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, M.; Greenham, K.; Prigge, M.J.; Jensen, P.J.; Estelle, M. The TRANSPORT INHIBITOR RESPONSE2 Gene Is Required for Auxin Synthesis and Diverse Aspects of Plant Development. Plant Physiol. 2009, 151, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, G.; Coneva, V.; Casaretto, J.A.; Ying, S.; Mahmood, K.; Liu, F.; Nambara, E.; Bi, Y.M.; Rothstein, S.J. OsPIN5b modulates rice (Oryza sativa) plant architecture and yield by changing auxin homeostasis, transport and distribution. Plant J. 2015, 83, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Tajima, H.; Nambara, E.; Blumwald, E.; Bassil, E. Auxin Homeostasis and Distribution of the Auxin Efflux Carrier PIN2 Require Vacuolar NHX-Type Cation/H+ Antiporter Activity. Plants 2020, 9, 1311. https://doi.org/10.3390/plants9101311
Zhang S, Tajima H, Nambara E, Blumwald E, Bassil E. Auxin Homeostasis and Distribution of the Auxin Efflux Carrier PIN2 Require Vacuolar NHX-Type Cation/H+ Antiporter Activity. Plants. 2020; 9(10):1311. https://doi.org/10.3390/plants9101311
Chicago/Turabian StyleZhang, Shiqi, Hiromi Tajima, Eiji Nambara, Eduardo Blumwald, and Elias Bassil. 2020. "Auxin Homeostasis and Distribution of the Auxin Efflux Carrier PIN2 Require Vacuolar NHX-Type Cation/H+ Antiporter Activity" Plants 9, no. 10: 1311. https://doi.org/10.3390/plants9101311