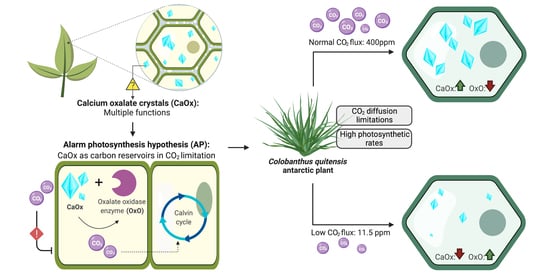
Decomposition of Calcium Oxalate Crystals in Colobanthus quitensis under CO2 Limiting Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Calcium Oxalate Crystal Decomposition
2.2. Chlorophyll Fluorescence and Oxalate Oxidase Activity Measurements
2.3. Calcium Oxalate Crystal Decomposition under Non-Photorespiratory Conditions
3. Materials and Methods
3.1. Plant Material and Growth Conditions
3.2. Experimental Design and Sample Collection
3.3. Chlorophyll Fluorescence
3.4. Measurements of Crystal Degradation
3.5. Oxalate Oxidase Activity Determination
3.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prasad, R.; Shivay, Y.S. Oxalic acid/oxalates in plants: From self-defence to phytoremediation. Curr. Sci. 2017, 112, 1665–1667. [Google Scholar] [CrossRef]
- Raman, V.; Horner, H.T.; Khan, I.A. New and unusual forms of calcium oxalate raphide crystals in the plant kingdom. J. Plant Res. 2014, 127, 721–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, V. Calcium oxalate in plants. Trends Plant Sci. 2001, 6, 331. [Google Scholar] [CrossRef]
- Webb, M.A. Cell-mediated crystallization of calcium oxalate in plants. Plant Cell 1999, 11, 751–761. [Google Scholar] [CrossRef] [Green Version]
- Dickison, W.C. Integrative Plant Anatomy; Academic Press: Cambridge, MA, USA, 2000; ISBN 978-0-12-215170-5. [Google Scholar]
- Franceschi, V.R.; Nakata, P.A. Calcium Oxalate in Plants: Formation and Function. Annu. Rev. Plant Biol. 2005, 56, 41–71. [Google Scholar] [CrossRef] [PubMed]
- Nakata, P.A. Advances in our understanding of calcium oxalate crystal formation and function in plants. Plant Sci. 2003, 164, 901–909. [Google Scholar] [CrossRef]
- Karabourniotis, G.; Horner, H.T.; Bresta, P.; Nikolopoulos, D.; Liakopoulos, G. New insights into the functions of carbon–calcium inclusions in plants. New Phytol. 2020, nph.16763. [Google Scholar] [CrossRef]
- Horner, H.; Wagner, B. Calcium oxalate formation in higher plants. In Calcium Oxalate in Biological Systems; Khan, S., Ed.; CRC Press: Boca Raton, FL, USA, 1995; pp. 53–72. [Google Scholar]
- Tooulakou, G.; Giannopoulos, A.; Nikolopoulos, D.; Bresta, P.; Dotsika, E.; Orkoula, M.G.; Kontoyiannis, C.G.; Fasseas, C.; Liakopoulos, G.; Klapa, M.I.; et al. “Alarm photosynthesis”: Calcium oxalate crystals as an internal CO2 source in plants. Plant Physiol. 2016, 171, 2577–2585. [Google Scholar] [CrossRef] [Green Version]
- Gaberščik, A.; Grašič, M.; Vogel-Mikuš, K.; Germ, M.; Golob, A. Water shortage strongly alters formation of calcium oxalate druse crystals and leaf traits in Fagopyrum esculentum. Plants 2020, 9, 917. [Google Scholar] [CrossRef]
- Tooulakou, G.; Giannopoulos, A.; Nikolopoulos, D.; Bresta, P.; Dotsika, E.; Orkoula, M.G.; Kontoyannis, C.G.; Fasseas, C.; Liakopoulos, G.; Klapa, M.I.; et al. Reevaluation of the plant “gemstones”: Calcium oxalate crystals sustain photosynthesis under drought conditions. Plant Signal. Behav. 2016, 11, e1215793. [Google Scholar] [CrossRef]
- Paiva, E.A.S. Are calcium oxalate crystals a dynamic calcium store in plants? New Phytol. 2019, 223, 1707–1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuentes-Lillo, E.; Cuba-Díaz, M.; Rifo, S. Morpho-physiological response of Colobanthus quitensis and Juncus bufonius under different simulations of climate change. Polar Sci. 2017, 11, 11–18. [Google Scholar] [CrossRef]
- Convey, P.; Gibson, J.A.E.; Hillenbrand, C.D.; Hodgson, D.A.; Pugh, P.J.A.; Smellie, J.L.; Stevens, M.I. Antarctic terrestrial life—Challenging the history of the frozen continent? Biol. Rev. 2008, 83, 103–117. [Google Scholar] [CrossRef]
- Cavieres, L.A.; Sáez, P.; Sanhueza, C.; Sierra-Almeida, A.; Rabert, C.; Corcuera, L.J.; Alberdi, M.; Bravo, L.A. Ecophysiological traits of Antarctic vascular plants: Their importance in the responses to climate change. Plant Ecol. 2016, 217, 343–358. [Google Scholar] [CrossRef]
- Prychid, C.J.; Rudall, P.J. Calcium oxalate crystals in monocotyledons: A review of their structure and systematics. Ann. Bot. 1999. [Google Scholar] [CrossRef]
- Brönnimann, D.; Ismail-Meyer, K.; Rentzel, P.; Pümpin, C.; Lisá, L. Excrements of Herbivores. In Archaeological Soil and Sediment Micromorphology; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 55–65. [Google Scholar]
- Cuba-Díaz, M.; Cerda, G.; Rivera, C.; Gómez, A. Genome size comparison in Colobanthus quitensis populations show differences in species ploidy. Polar Biol. 2017, 40, 1475–1480. [Google Scholar] [CrossRef]
- Arthofer, W.; Bertini, L.; Caruso, C.; Cicconardi, F.; Delph, L.F.; Fields, P.D.; Ikeda, M.; Minegishi, Y.; Proietti, S.; Ritthammer, H.; et al. Genomic Resources Notes accepted 1 February 2015–31 March 2015. Mol. Ecol. Resour. 2015, 15, 1014–1015. [Google Scholar] [CrossRef]
- Cho, S.M.; Lee, H.; Jo, H.; Lee, H.; Kang, Y.; Park, H.; Lee, J. Comparative transcriptome analysis of field- and chamber-grown samples of Colobanthus quitensis (Kunth) Bartl, an Antarctic flowering plant. Sci. Rep. 2018, 8, 11049. [Google Scholar] [CrossRef] [Green Version]
- Sáez, P.L.; Bravo, L.A.; Cavieres, L.A.; Vallejos, V.; Sanhueza, C.; Font-Carrascosa, M.; Gil-Pelegrín, E.; Javier Peguero-Pina, J.; Galmés, J. Photosynthetic limitations in two Antarctic vascular plants: Importance of leaf anatomical traits and Rubisco kinetic parameters. J. Exp. Bot. 2017, 68, 2871–2883. [Google Scholar] [CrossRef] [Green Version]
- Sáez, P.L.; Galmés, J.; Ramírez, C.F.; Poblete, L.; Rivera, B.K.; Cavieres, L.A.; Clemente-Moreno, M.J.; Flexas, J.; Bravo, L.A. Mesophyll conductance to CO2 is the most significant limitation to photosynthesis at different temperatures and water availabilities in Antarctic vascular species. Environ. Exp. Bot. 2018, 156, 279–287. [Google Scholar] [CrossRef]
- Pérez-Torres, E.; Bascuñán, L.; Sierra, A.; Bravo, L.A.; Corcuera, L.J. Robustness of activity of Calvin cycle enzymes after high light and low temperature conditions in Antarctic vascular plants. Polar Biol. 2006, 29, 909–916. [Google Scholar] [CrossRef]
- Giannopoulos, A.; Bresta, P.; Nikolopoulos, D.; Liakopoulos, G.; Fasseas, C.; Karabourniotis, G. Changes in the properties of calcium-carbon inclusions during leaf development and their possible relationship with leaf functional maturation in three inclusion-bearing species. Protoplasma 2019, 256, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Tooulakou, G.; Nikolopoulos, D.; Dotsika, E.; Orkoula, M.G.; Kontoyannis, C.G.; Liakopoulos, G.; Klapa, M.I.; Karabourniotis, G. Changes in size and composition of pigweed (Amaranthus hybridus L.) calcium oxalate crystals under CO2 starvation conditions. Physiol. Plant 2019, 166, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Seal, S.N.; Sen, S.P. The photosynthetic production of oxalic acid in Oxalis corniculata. Plant Cell Physiol. 1970, 11, 119–128. [Google Scholar] [CrossRef]
- Casanova-Katny, M.A.; Bravo, L.A.; Molina-Montenegro, M.; Corcuera, L.J.; Cavieres, L.A. Photosynthetic performance of Colobanthus quitensis (Kunth) Bartl. (Caryophyllaceae) in a high-elevation site of the Andes of central Chile. Rev. Chil. Hist. Nat. 2006, 79, 41–53. [Google Scholar] [CrossRef]
- Gerhart, L.M.; Ward, J.K. Plant responses to low [CO2] of the past. New Phytol. 2010, 188, 674–695. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, K.; Yoshida, K. Interaction between photosynthesis and respiration in illuminated leaves. Mitochondrion 2008, 8, 87–99. [Google Scholar] [CrossRef]
- Pérez-Torres, E.; Bravo, L.A.; Corcuera, L.J.; Johnson, G.N. Is electron transport to oxygen an important mechanism in photoprotection? Contrasting responses from Antarctic vascular plants. Physiol. Plant 2007, 130, 185–194. [Google Scholar] [CrossRef]
- Ort, D.R.; Baker, N.R. A photoprotective role for O2 as an alternative electron sink in photosynthesis? Curr. Opin. Plant Biol. 2002, 5, 193–198. [Google Scholar] [CrossRef]
- Fernández-Marín, B.; Gulías, J.; Figueroa, C.M.; Iñiguez, C.; Clemente-Moreno, M.J.; Nunes-Nesi, A.; Fernie, A.R.; Cavieres, L.A.; Bravo, L.A.; García-Plazaola, J.I.; et al. How do vascular plants perform photosynthesis in extreme environments? An integrative ecophysiological and biochemical story. Plant J. 2020, 101, 979–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenhut, M.; Bräutigam, A.; Timm, S.; Florian, A.; Tohge, T.; Fernie, A.R.; Bauwe, H.; Weber, A.P.M. Photorespiration Is Crucial for Dynamic Response of Photosynthetic Metabolism and Stomatal Movement to Altered CO2 Availability. Mol. Plant 2017, 10, 47–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeinalov, Y. Mechanisms of Photosynthetic Oxygen Evolution and Fundamental Hypotheses of Photosynthesis. In Handbook of Photosynthesis, 2nd ed.; Pessarakli, M., Ed.; Taylor & Francis Group, LLC.: Boca Raton, FL, USA, 2005; pp. 23–25. ISBN 9781420027877. [Google Scholar]
- Sáez, P.L.; Cavieres, L.A.; Galmés, J.; Gil-Pelegrin, E.; Peguero-Pina, J.J.; Sancho-Knapik, D.; Vivas, M.; Sanhueza, C.; Ramirez, C.F.; Riviera, B.K.; et al. In situ warming in the Antarctic: Effects on growth and photosynthesis in Antarctic vascular plants. New Phytol. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- JASP Team. JASP, version 0.13.1; Computer software. 2020.




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Espinoza, O.; González-Ramírez, D.; Bresta, P.; Karabourniotis, G.; Bravo, L.A. Decomposition of Calcium Oxalate Crystals in Colobanthus quitensis under CO2 Limiting Conditions. Plants 2020, 9, 1307. https://doi.org/10.3390/plants9101307
Gómez-Espinoza O, González-Ramírez D, Bresta P, Karabourniotis G, Bravo LA. Decomposition of Calcium Oxalate Crystals in Colobanthus quitensis under CO2 Limiting Conditions. Plants. 2020; 9(10):1307. https://doi.org/10.3390/plants9101307
Chicago/Turabian StyleGómez-Espinoza, Olman, Daniel González-Ramírez, Panagiota Bresta, George Karabourniotis, and León A. Bravo. 2020. "Decomposition of Calcium Oxalate Crystals in Colobanthus quitensis under CO2 Limiting Conditions" Plants 9, no. 10: 1307. https://doi.org/10.3390/plants9101307