The Essential Oil of Thymbra capitata and its Application as A Biocide on Stone and Derived Surfaces
Abstract
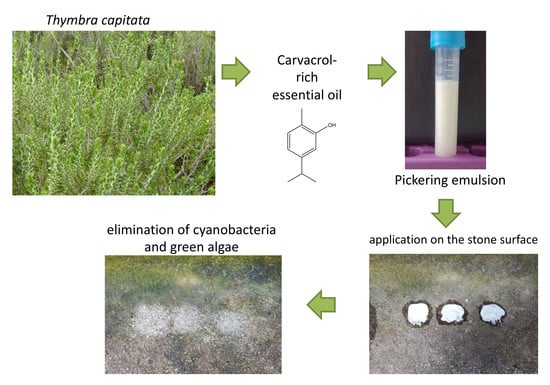
:1. Introduction
2. Results and Discussion
2.1. Essential Oil
2.2. Pickering Emulsion
2.3. Application
3. Materials and Methods
3.1. Plant Material
3.2. Essential Oil Distillation
3.3. Qualitative and Quantitative Analyses of Essential Oil (GS and GC/MS)
3.4. Pickering Emulsion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barros, L.; Heleno, S.A.; Carvalho, A.M.; Ferreira, I.C.F.R. Lamiaceae often used in Portuguese folk medicine as a source of powerful antioxidants: Vitamins and phenolic. Food Sci. Technol. 2010, 43, 544–550. [Google Scholar] [CrossRef]
- Zarzuelo, A.; Crespo, E. The medicinal and non-medicinal uses of Thyme. In Thyme: The Genus Thymus; Stahl-Bishup, E., Sáez, F., Eds.; Taylor & Francis: London, UK, 2002; Volume 17, pp. 263–292. [Google Scholar]
- Mulas, M. Traditional uses of Labiatae in the Mediterranean area. Acta Hort. 2006, 723, 25–32. [Google Scholar] [CrossRef]
- Könemann, G. Botanica: The Illustrated A-Z of Over 10,000 Garden Plants and How to Cultivate Them; Gordon Cheers Publication: Hong Kong, China, 1999; p. 885. [Google Scholar]
- Morales, R. The history, botany and taxonomy of the genus Thymus. In Thyme: The Genus Thymus; Stahl-Bishup, E., Sáez, F., Eds.; Taylor & Francis: London, UK, 2002; Volume 17, pp. 1–43. [Google Scholar]
- Lawrence, B.M.; Tucker, A.O. The genus Thymus as a source of commercial products. In Thyme: The Genus Thymus; Stahl-Bishup, E., Sáez, F., Eds.; Taylor & Francis: London, UK, 2002; Volume 17, pp. 252–262. [Google Scholar]
- Vila, R. Flavonoids and further polyphenols in the genus Thymus. In Thyme: The Genus Thymus; Stahl-Bishup, E., Sáez, F., Eds.; Taylor & Francis: London, UK, 2002; Volume 17, pp. 144–176. [Google Scholar]
- Bagamboula, C.F.; Uyttendaele, M.; Debevere, J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 2004, 21, 33–42. [Google Scholar] [CrossRef]
- Baydar, H.; Sagdiç, O.; Özcan, G.; Karadogan, T. Antibacterial activity and composition of essential oils from Origanum, Thymbra, and Satureja, species with commercial importance in Turkey. Food Control 2004, 15, 169–172. [Google Scholar] [CrossRef]
- Lee, S.J.; Umano, K.; Shibamoto, T.; Lee, K.G. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 2005, 91, 131–137. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Simin, N.; Anackov, G. Characterization of the volatile composition of essential oils of some Lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food Chem. 2006, 54, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Rasooli, I.; Rezaei, M.B.; Allameh, A. Ultrastructural studies on antimicrobial of thyme essential oils on Listeria monocytogenes. Int. J. Infect. Dis. 2006, 10, 236–241. [Google Scholar] [CrossRef]
- Omidbeygi, M.; Barzegar, M.; Hamidi, Z.; Naghdibadi, H. Antifungal activity of thyme, summer savory and clove essential oils against Aspergillum flavus in liquid medium and tomato paste. Food Control 2007, 18, 1518–1523. [Google Scholar] [CrossRef]
- Solomakos, N.; Govaris, A.; Koidis, P.; Botsoglou, N. The antimicrobial effect of thyme essential oil, nisin and their combination against Escherichia coli O157:H7 in minced beef during refrigerated storage. Meat Sci. 2008, 80, 159–166. [Google Scholar] [CrossRef]
- Nedorostova, L.; Kloucek, P.; Kokoska, L.; Stolcova, M.; Pulkrabek, J. Antimicrobial properties of selected essential oils in vapour phase against foodborne bacteria. Food Control 2009, 20, 157–160. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernandez-Lopez, J.; Perez-Alvarez, J.A. Effect of adding citrus waste water, thyme and oregano essential oil, on the chemical, physical and sensory characteristic of a bologna sausage. Int. Food Sci. Emerg. Tech. 2009, 10, 655–660. [Google Scholar] [CrossRef]
- Poulose, A.J.; Croteau, R. Biosynthesis of aromatic monoterpenes: Conversion of γ-terpinene to p-cymene and thymol in Thymus vulgaris L. Arch. Biochem. Biophys. 1978, 187, 307–314. [Google Scholar] [CrossRef]
- Poulose, A.J.; Croteau, R. γ-Terpinene synthetase: A key enzyme in the biosynthesis of aromatic monoterpenes. Arch. Biochem. Biophys. 1978, 191, 400–411. [Google Scholar] [CrossRef]
- Stahl-Biskup, E. Essential oil chemistry of the genus Thymus—A global view. In Thyme: The Genus Thymus; Stahl-Bishup, E., Sáez, F., Eds.; Taylor & Francis: London, UK, 2002; Volume 17, pp. 75–124. [Google Scholar]
- Stahl-Biskup, E. Thyme in Handbook of Herbs and Spices; Peter, K.V., Ed.; Woodhead Publish: Cambridge, UK, 2004; Volume 2, pp. 297–320. [Google Scholar]
- Sturtevant, E.L. Sturtevant’s Edible Plants of the World; Hedrick, U.P., Ed.; Dover Pubns: New York, NY, USA, 1972. [Google Scholar]
- Facciola, S. Cornucopia: A Source Book of Edible Plants; Kampong Publications: Vista, CA, USA, 1990. [Google Scholar]
- Griffiths, M. The New RHS Dictionary of Gardening; Macmillan Press Ltd.: London, UK, 1992. [Google Scholar]
- Faleiro, L.; Miguel, G.; Gomes, S.; Costa, L.; Venancio, F.; Teixeira, A.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Antibacterial and antioxidant activities of essential oils isolated from Thymbra capitata (L.) Cav. and Origanum vulgare L. J. Agric. Food Chem. 2005, 53, 8162–8168. [Google Scholar] [CrossRef]
- Blanco, J.; Ruiz, T.; Pérez-Alonso, M.J.; Vázquez, F.M.; Cases, M.A.; Gervasini, C. Chemical composition and antioxidant activity of the essential oil of Thymbra capitata (L.) Cav. in Spain. Act. Bot. Gall. 2010, 157, 55–63. [Google Scholar]
- Mohamed, S.B.; Eddine, A.D. Antibacterial activity of essential oils of some Algerian aromatic plants against multidrug resistant bacteria. J. Essent. Oil Bear. Plants 2010, 13, 362–370. [Google Scholar] [CrossRef]
- Albano, S.M.; Miguel, M.G. Biological activities of extracts of plants grown in Portugal. Ind. Crop. Prod. 2011, 33, 338–343. [Google Scholar] [CrossRef]
- El Ajjouri, M.; Satrani, B.; Ghanmi, M.; Aafi, A.; Farah, A.; Rahouti, M.; Amarti, F.; Aberchane, M. Activité antifongique des huiles essentielles de Thymus bleicherianus Pomel et Thymus capitatus (L.) Hoffm. & Link contre les champignons de pourriture du bois d’oeuvre. Biotechnol. Agron. Soc. Environ. 2008, 12, 345–351. [Google Scholar]
- Casiglia, S.; Bruno, M.; Scandolera, E.; Senatore, F.; Senatore, F. Influence of harvesting time on composition of the essential oil of Thymus capitatus (L.) Hoffmanns. & Link. growing wild in northern Sicily and its activity on microorganisms affecting historical art crafts. Arabian J. Chem. 2015, in press. [Google Scholar] [CrossRef]
- Cosentino, S.; Tuberoso, C.I.G.; Pisano, B.; Satta, M.; Mascia, V.; Arzedi, E.; Palmas, F. In-vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett. Appl. Microbiol. 1999, 29, 130–135. [Google Scholar] [CrossRef]
- Arras, G.; Usai, M. Fungitoxic activity of 12 EOs against four postharvest citrus pathogens: Chemical analysis of Thymus capitatus oil and its effect in subatmospheric pressure conditions. J. Food Prot. 2001, 64, 1025–1029. [Google Scholar] [CrossRef]
- Miceli, A.; Negro, C.; Tommasi, L. Essential oil variability in Thymbra capitata (L.) Cav. growing wild in Southern Apulia (Italy). Biochem. System. Ecol. 2006, 34, 528–535. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Salgueiro, L.; Miguel, M.G.; Faleiro, M.L. Portuguese Thymbra and Thymus species volatiles: Chemical composition and biological activities. Curr. Pharm. Des. 2008, 14, 3120–3140. [Google Scholar] [CrossRef]
- Napoli, E.M.; Curcuruto, G.; Ruberto, G. Screening of the essential oil composition of wild Sicilian thyme. Biochem. System. Ecol. 2010, 38, 816–822. [Google Scholar] [CrossRef]
- Tawaha, K.A.; Hudaib, M.M. Chemical composition of the essential oil from flowers, flower buds and leaves of Thymus capitatus Hoffmanns. & Link from Jordan. J. Essent. Oil-Bear. Plants 2012, 15, 988–996. [Google Scholar]
- Yvon, Y.; Raoelison, E.G.; Razafindrazaka, R.; Randriantsoa, A.; Romdhane, M.; Chabir, N.; Mkaddem, M.G.; Bouajila, J. Relation between chemical composition or antioxidant activity and antihypertensive activity for six essential oils. J. Food Sci. 2012, 77, H184–H191. [Google Scholar] [CrossRef]
- Ballester-Costa, C.; Sendra, E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical composition and in vitro antibacterial properties of essentialoils of four Thymus species from organic growth. Ind. Crops Prod. 2013, 50, 304–311. [Google Scholar] [CrossRef]
- Chedia, A.; Ghazghazi, H.; Dallali, S.; Houssine, S.; Brahim, H.; Abderazzak, M. Comparison of chemical composition, antioxidant and antimicrobial activities of Thymus capitatus L. essential oils from two Tunisian localities (Sousse and Bizerte). J. Agron. Plant Prod. 2013, 4, 1772–1781. [Google Scholar]
- Saoud, I.; Hamrouni, L.; Gargouri, S.; Amri, I.; Hanana, M.; Fezzani, T.; Bouzid, S.; Jamoussi, B. Chemical composition, weed killer and antifungal activities of Tunisian thyme (Thymus capitatus Hoff. et Link.) essential oils. Acta Aliment. 2013, 42, 417–427. [Google Scholar] [CrossRef]
- Hortigón-Vinagre, M.P.; Blanco, J.; Ruiz, T.; Henao, F. Thymbra capitata essential oil prevents cell death induced by 4-hydroxy-2-nonenal in neonatal rat cardiac myocytes. Planta Med. 2014, 80, 1284–1290. [Google Scholar] [CrossRef]
- Bouayad Alam, S.; Gaouar Benyelles, N.; El Amine Dib, M.; Djabou, N.; Tabti, L.; Paolini, J.; Muselli, A.; Costa, J. Antifungal activity of essential oils of three aromatic plants from western Algeria against five fungal pathogens of tomato (Lycopersicon esculentum Mill). J. Appl. Bot. Food Qual. 2014, 87, 56–61. [Google Scholar]
- Ben Arfa, A.; Combes, S.; Preziosi-Belloy, L.; Gontard, N.; Chalier, P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006, 43, 149–154. [Google Scholar] [CrossRef]
- Caneva, G.; Nugari, M.P.; Salvadori, O. La Biologia Vegetale per I Beni Culturali; Nardini: Bassano del Grappa, Italy, 2007; Volume 1. (In Italian) [Google Scholar]
- Eckhardt, F.E.W. Solubilization, transport, and deposition of mineral cations by microorganisms-Efficient rock weathering agents. In The Chemistry of Weathering; Springer: New York, NY, USA, 1985; pp. 161–173. [Google Scholar]
- Stupar, M.; Grbić, M.L.; Džamić, A.; Unković, N.; Ristić, M.; Jelikić, A.; Vukojević, J. Antifungal activity of selected essential oils and biocide benzalkonium chloride against the fungi isolated from cultural heritage objects. S. Afr. J. Bot. 2014, 93, 118–124. [Google Scholar] [CrossRef]
- Mansour, M.M. Proactive investigation using bioagents and fungicide for preservation of Egyptian stone sarcophagus. J. Appl. Sci. Res. 2013, 9, 1917–1930. [Google Scholar]
- Rakotonirainy, M.S.; Lavèdrine, B. Screening for antifungal activity of essential oils and related compounds to control the biocontamination in libraries and archives storage areas. Int. Biodeter. Biodegrad. 2005, 55, 141–147. [Google Scholar] [CrossRef]
- Casiglia, S.; Bruno, M.; Senatore, F. Volatile constituents of Dianthus rupicola Biv. from Sicily: Activity against microorganisms affecting cellulosic objects. Nat. Prod. Res. 2014, 28, 1739–1746. [Google Scholar] [CrossRef]
- Casiglia, S.; Bruno, M.; Senatore, F. Activity against microorganisms affecting cellulosic objects of the volatile constituents of Leonotis nepetaefolia from Nicaragua. Nat. Prod. Commun. 2014, 9, 1637–1639. [Google Scholar] [CrossRef]
- Casiglia, S.; Ben Jemia, M.; Riccobono, L.; Bruno, M.; Scandolera, E.; Senatore, F. Chemical composition of the essential oil of Moluccella spinosa L. (Lamiaceae) collected wild in Sicily and its activity on microorganisms affecting historical textiles. Nat. Prod. Res. 2015, 29, 1201–1206. [Google Scholar] [CrossRef]
- Devreux, G.; Santamaria, U.; Morresi, F.; Rodolfo, A.; Barbabietola, N.; Fratini, F.; Rita Reale, R. Fitoconservazione. Trattamenti alternativi sulle opere in materiale lapideo nei Giardini Vaticani. In Proceedings of the 13th Congresso Nazionale IGIIC -Lo Stato dell’Arte, Turin, Italy, 22–24 October 2015; pp. 199–206. (In Italian). [Google Scholar]
- Levinskaité, L.; Paškevičius, A. Fungi in water-damaged buildings of Vilnius Old City and their susceptibility towards disinfectants and essential oils. Indoor Built Environ. 2013, 22, 766–775. [Google Scholar] [CrossRef]
- Merino, N.; Berdejo, D.; Bento, R.; Salman, H.; Lanz, M.; Maggi, F.; Sánchez-Gómez, S.; García-Gonzalo, D.; Pagán, R. Antimicrobial efficacy of Thymbra capitata (L.) Cav. essential oil loaded in self-assembled zein nanoparticles in combination with heat. Ind. Crops Prod. 2019, 133, 98–104. [Google Scholar] [CrossRef]
- Mondello, F.; Girolamo, A.; Cassone, A. Oli essenziali: Ruolo e prospettive d’uso nelle infezioni fungine. In Proceedings of the 1st Convegno Nazionale Sostanze Naturali, Roma, Italy, 23–25 March 2009; pp. 110–119. (In Italian). [Google Scholar]
- Mironescu, M.; Georgescu, C. Activity of some essential oils against common spoilage fungi of buildings. Acta Univ. Cibiniensis Ser. E Food Technol. 2010, 14, 41–46. [Google Scholar]
- Fidanza, M.R.; Caneva, G. Natural biocides for the conservation of stone cultural heritage: A review. J. Cult. Heritage 2019, 38, 271–286. [Google Scholar] [CrossRef]
- Zembyla, M.; Murray, B.S.; Sarkar, A. Water-in-oil Pickering emulsions stabilized by water-insoluble polyphenol crystals. Langmuir 2018, 34, 10001–10011. [Google Scholar] [CrossRef]
- Cavallaro, G.; Milioto, S.; Nigamatzyanova, L.; Akhatova, F.; Fakhrullin, R.; Lazzara, G. Pickering emulsion gels based on halloysite nanotubes and ionic biopolymers: Properties and cleaning action on marble surface. ACS Appl. Nano Mater. 2019, 2, 3169–3176. [Google Scholar] [CrossRef]
- Owoseni, O.; Nyankson, E.; Zhang, Y.; Adams, S.J.; He, J.; McPherson, G.L.; Bose, A.; Gupta, R.B.; John, V.T. Release of surfactant cargo from interfacially-active halloysite clay nanotubes for oil spill remediation. Langmuir 2014, 30, 13533–13541. [Google Scholar] [CrossRef]
- von Klitzing, R.; Stehl, D.; Pogrzeba, T.; Schomäcker, R.; Minullina, R.; Panchal, A.; Konnova, S.; Fakhrullin, R.; Koetz, J.; Möhwald, H.; Lvov, Y. Halloysites stabilized emulsions for hydroformylation of long chain olefins. Adv. Mater. Interfaces 2017, 4, 1600435. [Google Scholar] [CrossRef]
- Lam, S.; Velikov, K.P.; Velev, O.D. Pickering stabilization of foams and emulsions with particles of biological origin. Curr. Opin. Colloid Interface Sci. 2014, 19, 490–500. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Y.; Huang, Q. Recent advances on food-grade particles stabilized Pickering emulsions: Fabrication, characterization and research trends. Trends Food Sci. Technol. 2016, 55, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Pickering, S.U. CXCVI.-Emulsions. J. Chem. Soc. Dalton Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef]
- Dickinson, E. Use of nanoparticles and microparticles in the formation and stabilization of food emulsions. Trends Food Sci. Technol. 2012, 24, 4–12. [Google Scholar] [CrossRef]
- Sarkar, A.; Murray, B.; Holmes, M.; Ettelaie, R.; Abdalla, A.; Yang, X. In vitro digestion of Pickering emulsions stabilized by soft whey protein microgel particles: Influence of thermal treatment. Soft Matter 2016, 12, 3558–3569. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Pariri, F. Halloysite nanotubes for cleaning, consolidation and protection. Chem. Rec. 2018, 18, 940–949. [Google Scholar] [CrossRef]
- Lo Dico, G.; Semilia, F.; Milioto, S.; Parisi, F.; Cavallaro, G.; Inguì, G.; Makaremi, M.; Pasbakhsh, P.; Lazzara, G. Microemulsion encapsulated into halloysite nanotubes and their applications for cleaning of a marble surface. Appl. Sci. 2018, 8, 1455. [Google Scholar] [CrossRef]
- De Lisi, R.; Gradzielski, M.; Lazzara, G.; Milioto, S.; Muratore, M.; Prévost, S. Aqueous laponite clay dispersions in the presence of poly(ethylene oxide) or poly(propylene oxide) oligomers and their triblock copolymers. J. Phys. Chem. B 2008, 112, 9328–9336. [Google Scholar] [CrossRef]
- De Martino, L.; Bruno, M.; Formisano, C.; De Feo, V.; Napolitano, F.; Rosselli, S.; Senatore, F. Chemical composition and antimicrobial activity of the essential oils from two species of thymus growing wild in Southern Italy. Molecules 2009, 14, 4614–4624. [Google Scholar] [CrossRef]




| No | Component a | RI b | RI Lit. c | % d | ID e |
|---|---|---|---|---|---|
| 1 | α-thujene | 921 | 924 | 1.4 ± 0.3 | RI,MS |
| 2 | α-pinene | 929 | 932 | 1.0 ± 0.2 | Std,RI,MS |
| 3 | camphene | 936 | 946 | 0.5 ± 0.1 | Std,RI,MS |
| 4 | sabinene | 966 | 969 | 0.1 ± 0.0 | Std,RI,MS |
| 5 | β-pinene | 971 | 974 | 0.1 ± 0.0 | Std,RI,MS |
| 6 | 1-octen-3-ol | 973 | 974 | 0.4 ± 0.1 | Std,RI,MS |
| 7 | myrcene | 985 | 988 | 1.1 ± 0.2 | Std,RI,MS |
| 8 | 3-octanol | 994 | 988 | 0.2 ± 0.0 | RI,MS |
| 9 | α-phellandrene | 1001 | 1002 | 0.2 ± 0.0 | Std,RI,MS |
| 10 | δ-3-carene | 1005 | 1008 | Tr f | Std,RI,MS |
| 11 | α-terpinene | 1012 | 1014 | 1.4 ± 0.3 | Std,RI,MS |
| 12 | p-cymene | 1019 | 1020 | 4.3 ± 0.7 | Std,RI,MS |
| 13 | limonene | 1023 | 1024 | 0.7 ± 0.2 | Std,RI,MS |
| 14 | γ-terpinene | 1052 | 1054 | 6.9 ± 1.3 | Std,RI,MS |
| 15 | cis-sabinene hydrate | 1062 | 1065 | 0.4 ± 0.1 | RI,MS |
| 16 | terpinolene | 1082 | 1086 | 0.1 ± 0.0 | Std,RI,MS |
| 17 | trans-sabinene hydrate | 1094 | 1098 | 0.1 ± 0.0 | RI,MS |
| 18 | linalool | 1098 | 1095 | 2.6 ± 0.5 | Std,RI,MS |
| 19 | borneol | 1161 | 1165 | 1.4 ± 0.3 | Std,RI,MS |
| 20 | terpinen-4-ol | 1171 | 1174 | 0.4 ± 0.1 | Std,RI,MS |
| 21 | α -terpineol | 1186 | 1186 | 0.1 ± 0.0 | Std,RI,MS |
| 22 | carvone | 1238 | 1239 | 0.2 ± 0.0 | Std,RI,MS |
| 23 | thymol | 1293 | 1289 | 0.2 ± 0.1 | Std,RI,MS |
| 24 | carvacrol | 1301 | 1298 | 73.2 ± 3.1 | Std,RI,MS |
| 25 | carvacrol acetate | 1368 | 1370 | 0.4 ± 0.2 | RI,MS |
| 26 | (E)-caryophyllene | 1414 | 1417 | 2.6 ± 0.4 | Std,RI,MS |
| 27 | α-humulene | 1447 | 1452 | Tr | Std,RI,MS |
| 28 | caryophyllene oxide | 1573 | 1583 | 0.1 ± 0.0 | Std,RI,MS |
| Total identified (%) | 99.9 ± 0.1 | ||||
| Grouped compounds (%) | |||||
| Monoterpene hydrocarbons | 17.8 | ||||
| Oxygenated monoterpenes | 78.9 | ||||
| Sesquiterpene hydrocarbons | 2.6 | ||||
| Oxygenated sesquiterpenes | Tr | ||||
| Aliphatics | 0.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagliano Candela, R.; Maggi, F.; Lazzara, G.; Rosselli, S.; Bruno, M. The Essential Oil of Thymbra capitata and its Application as A Biocide on Stone and Derived Surfaces. Plants 2019, 8, 300. https://doi.org/10.3390/plants8090300
Gagliano Candela R, Maggi F, Lazzara G, Rosselli S, Bruno M. The Essential Oil of Thymbra capitata and its Application as A Biocide on Stone and Derived Surfaces. Plants. 2019; 8(9):300. https://doi.org/10.3390/plants8090300
Chicago/Turabian StyleGagliano Candela, Rossella, Filippo Maggi, Giuseppe Lazzara, Sergio Rosselli, and Maurizio Bruno. 2019. "The Essential Oil of Thymbra capitata and its Application as A Biocide on Stone and Derived Surfaces" Plants 8, no. 9: 300. https://doi.org/10.3390/plants8090300