Organic Certification is Not Enough: The Case of the Methoxydecane Frankincense
Abstract
:1. Introduction
2. Results
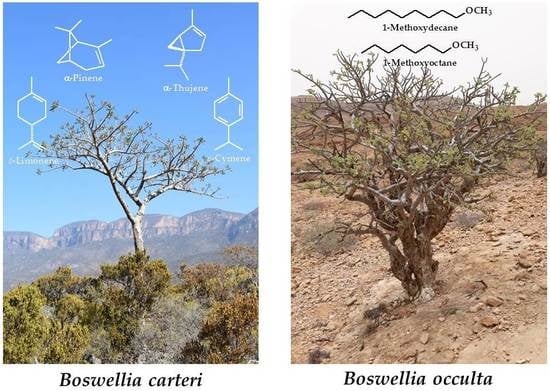
2.1. Botanical Identification of the Methoxydecane Tree
2.2. Chemical Composition of Resin Samples
2.3. Commercial Essential Oil Results
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Sample Identification
4.3. Hydrodistillation
4.4. Gas Chromatographic–Mass Spectral Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eslamieh, J. Cultivation of Boswellia, 2nd ed.; A Book’s Mind: Phoenix, AZ, USA, 2017; ISBN 978-0-9828751-1-7. [Google Scholar]
- Langenheim, J.H. Plant Resins: Chemistry, Evolution, Ecology, and Ethnobotany; Timber Press, Incorporated: Portland, OR, USA, 2003; ISBN 978-0-88192-574-6. [Google Scholar]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [PubMed]
- Hull, B.Z. Frankincense, myrrh, and spices: The oldest global supply chain? J. Macromark. 2008, 28, 275–288. [Google Scholar]
- Dannaway, F.R. Strange fires, weird smokes and psychoactive combustibles: Entheogens and incense in ancient traditions. J. Psychoact. Drugs 2010, 42, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Thulin, M.; Warfa, A.M. The frankincense trees (Boswellia spp., Burseraceae) of northern Somalia and southern Arabia. Kew Bull. 1987, 42, 487–500. [Google Scholar] [CrossRef]
- Woolley, C.L.; Suhail, M.M.; Smith, B.L.; Boren, K.E.; Taylor, L.C.; Schreuder, M.F.; Chai, J.K.; Casabianca, H.; Haq, S.; Lin, H.-K.; et al. Chemical differentiation of Boswellia sacra and Boswellia carterii essential oils by gas chromatography and chiral gas chromatography–mass spectrometry. J. Chromatogr. A 2012, 1261, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Başer, K.H.C.; Demirci, B.; Dekebo, A.; Dagne, E. Essential oils of some Boswellia spp., myrrh and opopanax. Flavour Fragr. J. 2003, 18, 153–156. [Google Scholar] [CrossRef]
- Hamm, S.; Bleton, J.; Connan, J.; Tchapla, A. A chemical investigation by headspace SPME and GC–MS of volatile and semi-volatile terpenes in various olibanum samples. Phytochemistry 2005, 66, 1499–1514. [Google Scholar] [CrossRef]
- Camarda, L.; Dayton, T.; Di Stefano, V.; Pitonzo, R.; Schillaci, D. Chemical composition and antimicrobial activity of some oleogum resin essential oils from Boswellia spp. (Burseraceae). Ann. Chim. 2007, 97, 837–844. [Google Scholar] [CrossRef]
- Van Vuuren, S.F.; Kamatou, G.P.P.; Viljoen, A.M. Volatile composition and antimicrobial activity of twenty commercial frankincense essential oil samples. S. Afr. J. Bot. 2010, 76, 686–691. [Google Scholar] [CrossRef]
- Thulin, M. Flora of Somalia, Volume 2: Angiospermae; Royal Botanic Gardens, Kew: Kew, UK, 1999; ISBN 978-1-900347-77-8.
- PDRC. Somali Customary Law and Traditional Economy: Cross Sectional, Pastoral, Frankincense, and Marine Norms; Puntland Development Research Centre: Garowe, Puntland, 2003. [Google Scholar]
- Rijkers, T.; Ogbazghi, W.; Wessel, M.; Bongers, F. The effect of tapping for frankincense on sexual reproduction in Boswellia papyrifera. J. Appl. Ecol. 2006, 43, 1188–1195. [Google Scholar] [CrossRef]
- Eshete, A.; Sterck, F.J.; Bongers, F. Frankincense production is determined by tree size and tapping frequency and intensity. For. Ecol. Manag. 2012, 274, 136–142. [Google Scholar] [CrossRef]
- Al-Aamri, M. Sustainable Harvesting of Frankincense Trees in Oman; LAP Lambert Academic Publishing: Saarbrücken, Germany, 2015; ISBN 978-3-659-74581-2. [Google Scholar]
- DeCarlo, A.; Johnson, S.; Aromatic Plant Research Center, Lehi, UT, USA; Ceroni, M.; Academy for Systems Change, Burlington, VT, USA. Unpublished work. 2017.
- DeCarlo, A.; Ali, S. Sustainable Sourcing of Phytochemicals as a Development Tool: The Case of Somaliland’s Frankincense Industry; Institute for Environmental Diplomacy and Security, University of Vermont: Burlington, VT, USA, 2014. [Google Scholar]
- Viana, V.; Ervin, J.; Donovan, R.; Elliott, C.; Gholz, H. (Eds.) Certification of Forest Products: Issues and Perspectives; Island Press: Washington, DC, USA, 1996; ISBN 978-1-55963-494-6. [Google Scholar]
- Shanley, P.; Pierce, A.R.; Laird, S.A.; Guillen, A. (Eds.) Tapping the Green Market: Certification and Management of Non-timber Forest Products; Earthscan Publications: London, UK, 2002; ISBN 978-1-85383-810-1. [Google Scholar]
- Walter, S. Certification and Benefit-Sharing Mechanisms in the Field of Non-Wood Forest Products—An Overview; Medicinal Plant Conservation 8; Newsletter of the IUCN Species Survival Commission; IUCN: Bonn, Germany, 2002. [Google Scholar]
- Vantomme, P.; Walter, S. Opportunities and Challenges for Non-wood Forest Products Certification; FAO: Rome, Italy, 2003. [Google Scholar]
- USDA Organic Integrity Database. Available online: https://organic.ams.usda.gov/Integrity/Default.aspx (accessed on 17 February 2019).
- Li, S.; Han, Q.; Qiao, C.; Song, J.; Lung Cheng, C.; Xu, H. Chemical markers for the quality control of herbal medicines: An overview. Chin. Med 2008, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- DeCarlo, A.; Johnson, S.; Poudel, A.; Satyal, P.; Bangerter, L.; Setzer, W.N. Chemical variation in essential oils from the oleo-gum resin of Boswellia carteri: A preliminary investigation. Chem. Biodivers. 2018, 15, e1800047. [Google Scholar] [CrossRef] [PubMed]
- Satyal, P.; Pappas, R. First reporting on the chemistry and biological activity of a novel Boswellia chemotype: The methoxy alkane frankincense. Glob. J. Sci. Front. Res. B Chem. 2016, 16, 1–9. [Google Scholar]
- Singh, B.; Kumar, R.; Bhandari, S.; Pathania, S.; Lal, B. Volatile constituents of natural Boswellia serrata oleo-gum-resin and commercial samples. Flavour Fragr. J. 2007, 22, 145–147. [Google Scholar] [CrossRef]
- Al-Harrasi, A.; Al-Saidi, S. Phytochemical analysis of the essential oil from botanically certified oleogum resin of Boswellia sacra (Omani luban). Molecules 2008, 13, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Martina, W.; Norbert, A.; Axel, T.; Jürgen, S.; Lindequist, U.; Ludger, W. Chemical composition and biological activities of essential oils from the oleogum resins of three endemic Soqotraen Boswellia species. Rec. Nat. Prod. 2008, 2, 6–12. [Google Scholar]
- Suhail, M.M.; Wu, W.; Cao, A.; Mondalek, F.G.; Fung, K.-M.; Shih, P.-T.; Fang, Y.-T.; Woolley, C.; Young, G.; Lin, H.-K. Boswellia sacra essential oil induces tumor cell-specific apoptosis and suppresses tumor aggressiveness in cultured human breast cancer cells. BMC Complement. Altern. Med. 2011, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Mothana, R.; Hasson, S.; Schultze, W.; Mowitz, A.; Lindequist, U. Phytochemical composition and in vitro antimicrobial and antioxidant activities of essential oils of three endemic Soqotraen Boswellia species. Food Chem. 2011, 126, 1149–1154. [Google Scholar] [CrossRef]
- Bekana, D.; Kebede, T.; Assefa, M.; Kassa, H. Comparative phytochemical analyses of resins of Boswellia species (B. papyrifera (Del.) Hochst., B. neglecta S. Moore, and B. rivae Engl.) from northwestern, southern, and southeastern Ethiopia. ISRN Anal. Chem. 2014, 2014, 374678. [Google Scholar] [CrossRef]
- Reddy, P.A.; Reddy, B.N.; Ratnam, K.V.; Bhakshu, M.; Reddy, L.V. Chemical profile, antioxidant and antimicrobial activity of essential oils From Boswellia ovalifoliolata Bal. Et Henry. Int. J. Pharmaceut. Clin. Res. 2015, 7, 96–101. [Google Scholar]
- Niebler, J.; Buettner, A. Frankincense revisited, part I: Comparative analysis of volatiles in commercially relevant Boswellia species. Chem. Biodivers. 2016, 13, 613–629. [Google Scholar] [CrossRef]
- Benelli, G.; Rajeswary, M.; Vijayan, P.; Senthilmurugan, S.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Govindarajan, M. Boswellia ovalifoliolata (Burseraceae) essential oil as an eco-friendly larvicide? Toxicity against six mosquito vectors of public health importance, non-target mosquito fishes, backswimmers, and water bugs. Environ. Sci. Pollut. Res. 2018, 25, 10264–10271. [Google Scholar] [CrossRef]
- Gupta, M.; Rout, P.K.; Misra, L.N.; Gupta, P.; Singh, N.; Darokar, M.P.; Saikia, D.; Singh, S.C.; Bhakuni, R.S. Chemical composition and bioactivity of Boswellia serrata Roxb. essential oil in relation to geographical variation. Plant Biosyst. 2017, 151, 623–629. [Google Scholar] [CrossRef]
- Maděra, P.; Paschová, Z.; Ansorgová, A.; Vrškový, B.; Lvončík, S.; Habrová, H. Volatile compounds in oleo-gum resin of Socotran species of Burseraceae. Acta Univ. Agric. Silvic. Mendel. Brun. AUASFV 2017, 65, 73–90. [Google Scholar] [CrossRef]
- Thulin, M.; DeCarlo, A.; Johnson, S.P. Boswellia occulta (Burseraceae), a new species of frankincense tree from Somalia (Somaliland). Phytotaxa 2019, 394, 219–224. [Google Scholar] [CrossRef]
- Schulz, S.; Toft, S. Branched long chain alkyl methyl ethers: A new class of lipids from spider silk. Tetrahedron 1993, 49, 6805–6820. [Google Scholar] [CrossRef]
- Schulz, S. Composition of the silk lipids of the spider Nephila clavipes. Lipids 2001, 36, 637–647. [Google Scholar] [CrossRef]
- Geffroy-Rodier, C.; Grasset, L.; Sternberg, R.; Buch, A.; Amblès, A. Thermochemolysis in search for organics in extraterrestrial environments. J. Anal. Appl. Pyrol. 2009, 85, 454–459. [Google Scholar] [CrossRef]
- Nuylert, A.; Kuwahara, Y.; Hongpattarakere, T.; Asano, Y. Identification of saturated and unsaturated 1-methoxyalkanes from the Thai millipede Orthomorpha communis as potential “Raincoat Compounds”. Sci. Rep. 2018, 8, 11730. [Google Scholar] [CrossRef]
- Dictionary of Natural Products on DVD; v. 6.1; CRC Press: Boca Raton, FL, USA, 2018.
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- Satyal, P. Development of GC-MS Database of Essential Oil Components by the Analysis of Natural Essential Oils and Synthetic Compounds and Discovery of Biologically Active Novel Chemotypes in Essential Oils. Ph.D. Thesis, University of Alabama in Huntsville, Huntsville, AL, USA, 2015. [Google Scholar]





| Compound | Boswellia carteria | Boswellia occulta | |||
|---|---|---|---|---|---|
| Chemotype Ia | Chemotype Ib | Chemotype Ic | Chemotype II | ||
| α-Pinene | 27.6 (14.2–39.8) | 45.3 (43.2–50.2) | 26.8 (24.1–32.3) | 8.1 (2.3–14.8) | 0.5 (0.1–2.4) |
| Limonene | 7.9 (0.8–14.9) | 11.5 (5.9–18.6) | 32.5 (28.0–44.8) | 2.0 (1.0–4.8) | 0.1 (trace–0.1) |
| α-Thujene | 5.0 (trace–14.0) | 4.8 (0.8–11.7) | 2.1 (0.1–3.6) | 40.6 (32.9–50.6) | 0.3 (0.1–0.6) |
| Sabinene | 8.1 (1.4–25.7) | 3.7 (2.3–7.0) | 3.8 (0.4–4.9) | 7.4 (5.6–10.9) | 3.5 (0.1–8.3) |
| Myrcene | 10.9 (0.4–25.7) | 3.5 (2.3–12.1) | 2.9 (0.0–4.2) | 0.8 (0.0–2.9) | 0.1 (0.0–0.1) |
| p-Cymene | 5.3 (0.7–11.8) | 2.7 (1.7–5.0) | 3.2 (1.7–4.1) | 13.0 (4.3–19.7) | 0.3 (0.1–0.8) |
| 1-Methoxydecane | 0.0 | 0.0 | 0.0 | 0.0 | 35.1 (26.6–47.9) |
| Serratol | 0.1 (0.0–0.4) | 1.0 (0.0–5.9) | 0.2 (0.0–0.6) | 0.0 | 15.1 (2.7–31.8) |
| Unidentified guaiol b | 0.0 | 0.0 | 0.0 | 0.0 | 9.7 (1.2–15.1) |
| 1-Methoxyoctane | 0.0 | 0.0 | 0.0 | 0.0 | 6.4 (3.6–9.2) |
| Compound | Non-Certified Commercial Samples | Certified “Organic” Samples | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| α-Pinene | 26.6 | 31.5 | 32.6 | 24.4 | 40.4 | 39.5 | 39.9 | 34.8 | 42.3 | 47.5 | 37.3 | 35.1 | 35.7 |
| Limonene | 15.7 | 25.7 | 5.7 | 5.1 | 6.3 | 9.7 | 19.2 | 9.9 | 4.5 | 4.5 | 5.8 | 8.1 | 17.5 |
| α-Thujene | 11.2 | 7.3 | 1.6 | 1.2 | 11.9 | 10.6 | 6.1 | 7.2 | 1.4 | 2.2 | 1.3 | 1.8 | 6.2 |
| Sabinene | 5.8 | 4.1 | 5.4 | 6.2 | 1.3 | 4.2 | 6.0 | 6.2 | 4.8 | 5.1 | 8.1 | 5.9 | 4.8 |
| Myrcene | 4.3 | 4.3 | 3.7 | 5.9 | 2.4 | 2.5 | 5.3 | 3.4 | 3.0 | 3.1 | 6.5 | 8.1 | 5.0 |
| p-Cymene | 5.3 | 5.0 | 2.9 | 1.9 | 7.4 | 2.7 | 4.6 | 3.5 | 2.1 | 2.0 | 2.3 | 4.0 | 4.2 |
| 1-Methoxydecane | 0.1 | 0.0 | 17.6 | 0.0 | 0.0 | 0.9 | 0.4 | 2.2 | 12.1 | 9.0 | 7.8 | 9.2 | 0.0 |
| Serratol | 2.2 | 0.1 | 0.5 | 5.9 | 0.2 | 0.9 | 0.2 | 1.1 | 0.3 | 0.3 | 0.4 | 0.6 | 0.8 |
| Unident. guaiol | 0.0 | 0.0 | 0.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.2 | 0.3 | 0.2 | 0.0 |
| 1-Methoxyoctane | 0.0 | 0.0 | 5.5 | 0.0 | 0.0 | 0.3 | trace | 0.7 | 3.8 | 2.6 | 2.1 | 2.7 | 0.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, S.; DeCarlo, A.; Satyal, P.; Dosoky, N.S.; Sorensen, A.; Setzer, W.N. Organic Certification is Not Enough: The Case of the Methoxydecane Frankincense. Plants 2019, 8, 88. https://doi.org/10.3390/plants8040088
Johnson S, DeCarlo A, Satyal P, Dosoky NS, Sorensen A, Setzer WN. Organic Certification is Not Enough: The Case of the Methoxydecane Frankincense. Plants. 2019; 8(4):88. https://doi.org/10.3390/plants8040088
Chicago/Turabian StyleJohnson, Stephen, Anjanette DeCarlo, Prabodh Satyal, Noura S. Dosoky, Aaron Sorensen, and William N. Setzer. 2019. "Organic Certification is Not Enough: The Case of the Methoxydecane Frankincense" Plants 8, no. 4: 88. https://doi.org/10.3390/plants8040088