Seed Germination in Cistus ladanifer: Heat Shock, Physical Dormancy, Soil Temperatures and Significance to Natural Regeneration
Abstract
:1. Introduction
2. Results
2.1. Experiment 1: Effects of Temperature and Light Regime on Final Seed Germination of Cistus ladanifer
2.2. Experiment 2: Effects of Heat Shock on Seed Germination of Cistus ladanifer
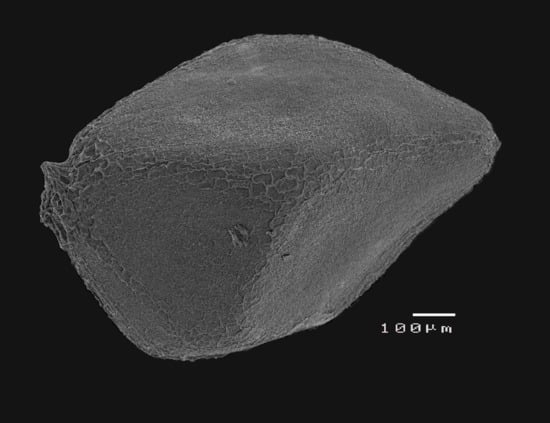
2.3. Experiment 3: Effects of Heat Shock on Seed Coat Morphology of Cistus ladanifer
2.4. Experiment 4: Weight Increase of Nongerminated Imbibing Seeds of Cistus ladanifer
2.5. Experiment 5: Dye Uptake by Imbibing Seeds of Cistus ladanifer
2.6. Experiment 6: Effects of Heat Shock on Volume Increase of Imbibing Seeds of Cistus ladanifer
2.7. Depth of Emergence of Cistus ladanifer Seedlings and Soil Temperatures
2.7.1. Depth of Emergence of Cistus ladanifer Seedlings
2.7.2. Soil Temperatures during Fires
3. Discussion
3.1. Responses of Seed Germination to Temperature and Light Regime
3.2. Responses of Seed Germination to Heat Shock
3.3. Responses of Water Uptake by Seeds to Heat Shock
3.4. Ecological Significance of Seeds Response to Heat Shock
4. Materials and Methods
4.1. Seed Collection
4.2. Experiments 1 and 2: Germination Experiments
4.2.1. Experiment 1: Effects of Temperature and Light Regime on Final Seed Germination
4.2.2. Experiment 2: Effects of Heat Shock on Seed Germination
4.3. Experiment 3: Effects of Heat Shock on Seed Coat Morphology
4.4. Experiments 4–6: Water Uptake by Seeds
4.4.1. Experiment 4: Weight Increase of Nongerminated Imbibing Seeds
4.4.2. Experiment 5: Dye Uptake by Imbibing Seeds
4.4.3. Experiment 6: Effects of Heat Shock on Volume Increase of Imbibing Seeds
4.5. Depth of Emergence and Soil Temperatures
4.6. Data Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Keeley, J.E.; Fotheringham, C.J. Role of fire in regeneration from seed. In Seeds. The Ecology of Regeneration in Plant Communities, 2nd ed.; Fenner, M., Ed.; CABI Publishing: Wallingford, UK, 2000; pp. 311–330. ISBN 0-85199-432-6. [Google Scholar]
- Quintana, J.R.; Cruz, A.; Fernández-González, F.; Moreno, J.M. Time of germination and establishment success after fire of three obligate seeders in a Mediterranean shrubland of central Spain. J. Biogeogr. 2004, 31, 241–249. [Google Scholar] [CrossRef]
- Thanos, C.A.; Georghiou, K. Ecophysiology of fire-stimulated seed germination in Cistus incanus ssp. creticus (L.) Heywood and C. salvifolius L. Plant Cell Environ. 1988, 11, 841–849. [Google Scholar] [CrossRef]
- Thanos, C.A.; Georghiou, K.; Kadis, C.; Pantazi, C. Cistaceae: A plant family with hard seeds. Isr. J. Bot. 1992, 41, 251–263. [Google Scholar] [CrossRef]
- Sanders, T.W. Sander’s Encyclopaedia of Gardening, 22nd ed.; W.H. and L. Collingridge: London, UK, 1956. [Google Scholar]
- Brickell, C. The Royal Horticultural Society Gardeners’ Encyclopedia of Plants & Flowers; Dorling Kindersley: London, UK, 1994; ISBN 0-7513-014-77. [Google Scholar]
- Braun-Blanquet, J.; Silva, A.R.P.; Rozeira, A. Résultat de trois excursions géobotaniques à travers le Portugal septentrionel et moyen. III Landes à Cistes et Ericacées (Cisto-Lavanduletea et Calluno-Ulicetea). Agron. Lusit. 1961, 23, 229–312. [Google Scholar]
- Goday, S.R. Vegetación y Flórula de la Cuenca Extremeña del Guadiana; Diputacion Provincial de Badajoz: Madrid, España, 1964. [Google Scholar]
- Tárrega, R.; Luis-Calabuig, E.; Valbuena, L. Eleven years of recovery dynamic after experimental burning in two Cistus communities. Acta Oecol. 2001, 22, 277–283. [Google Scholar] [CrossRef]
- Du Plessis, S.P.; Rink, A.; Goodall, V.; Kaplan, H.; Jubase, N.; Van Wyk, E. Assessment and management of the invasive shrub, Cistus ladanifer, in South Africa. S. Afr. J. Bot. 2017, 117, 85–94. [Google Scholar] [CrossRef]
- Martin, L.B.; Juhren, M. Cistus and its response to fire. Lasca Leaves 1954, 4, 65–67. [Google Scholar]
- Montgomery, K.R.; Strid, T.W. Regeneration of introduced species of Cistus (Cistaceae) after fire in Southern California. Madroño 1976, 23, 417–427. [Google Scholar]
- Clemente, A.S.; Rego, F.C.; Correia, O.A. Demographic patterns and productivity of post-fire regeneration of portuguese mediterranean maquis. Int. J. Wildland Fire 1996, 6, 5–12. [Google Scholar] [CrossRef]
- Castroviejo, S.; Aedo, C.; Cirujano, S.; Laínz, M.; Montserrat, P.; Morales, R.; Garmendia, F.M.; Navarro, C.; Paiva, J.; Soriano, C. Flora Iberica: Plantas Vasculares de la Península Ibérica e Islas Baleares. Volume III. Plumbaginaceae (Partim)—Capparaceae; Real Jardín Botánico and CSIC: Madrid, Spain, 1993; ISBN 84-00-07375-4. [Google Scholar]
- Corral, R.; Perez-Garcia, F.; Pita, J.M. Seed morphology and histology in four species of Cistus L. (Cistaceae). Phytomorphology 1989, 39, 75–80. [Google Scholar]
- Juhren, G. The use of Cistus in erosion control. Lasca Leaves 1956, 6, 26–29. [Google Scholar]
- Ching, F.T. Slow burning plant research project. A progress report. Lasca Leaves 1959, 9, 75–80. [Google Scholar]
- Knapp, R. Rock roses—Cistus. Components of the shrub vegetation and their usefulness in soil conservation. Lasca Leaves 1962, 12, 77–79. [Google Scholar]
- The PLANTS Database. Available online: http://plants.usda.gov (accessed on 1 December 2018).
- Laure, G.; Oieni, S.; Zinke, P. A burning test on Cistus chaparral in Sicily. Lasca Leaves 1961, 11, 67–72. [Google Scholar]
- Bolaños, M.M.; Lopez, E.G. Jarales y Jaras (Cistografia Hispanica); Ediciones Ares: Madrid, Spain, 1949. [Google Scholar]
- Corral, R.; Pita, J.M.; Pérez-García, F. Some aspects of seed germination in four species of Cistus L. Seed Sci. Technol. 1990, 18, 321–325. [Google Scholar]
- Valbuena, L.; Tárrega, R.; Luis, E. Influence of heat on seed germination of Cistus laurifolius and Cistus ladanifer. Int. J. Wildland Fire 1992, 2, 15–20. [Google Scholar] [CrossRef]
- Pérez-Garcia, F. Germination of Cistus ladanifer seeds in relation to parent material. Plant. Ecol. 1997, 133, 57–62. [Google Scholar] [CrossRef]
- Ferrandis, P.; Herranz, J.M.; Martínez-Sánchez, J.J. Effect of fire on hard-coated Cistaceae seed banks and its influence on techniques for quantifying seed banks. Plant Ecol. 1999, 144, 103–114. [Google Scholar] [CrossRef]
- Chamorro-Moreno, S.; Rosúa-Campos, J.L. The relationships between lengthening capacity of seedlings and the post-fire germinative behaviour of six Cistus species. Rev. Ecol. 2004, 59, 409–424. [Google Scholar]
- Margaris, N.S. Adaptive strategies in plants dominating Mediterranean-type ecosystems. In Mediterranean-type Shrublands; Castri, F., Goodal, D.W., Specht, R.L., Eds.; Elsevier: Amsterdam, The Netherlands, 1981; pp. 309–315. ISBN 0-444-41858-X. [Google Scholar]
- Vuillemin, J.; Bulard, C. Ecophysiologie de la germination de Cistus albidus L. et Cistus monspeliensis L. Nat. Monspel. Sér. Bot. 1981, 46, 1–11. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds. Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 1998; ISBN 0-12-080260-0. [Google Scholar]
- Moreira, B.; Tormo, J.; Estrelles, E.; Pausas, J.G. Disentangling the role of heat and smoke as germination cues in Mediterranean Basin flora. Ann. Bot. 2010, 105, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Krollmann, P.; Eich, C.; Gülz, P.-G. Epicuticular waxes of seed coats from species of the genus Cistus L. (Cistaceae). Z. Nat. 1984, 39c, 521–524. [Google Scholar] [CrossRef]
- Delgado, J.A.; Serrano, J.M.; López, F.; Acosta, F.J. Seed size and seed germination in the Mediterranean fire-prone shrub Cistus ladanifer. Plant Ecol. 2008, 197, 269–276. [Google Scholar] [CrossRef]
- DeBano, L.F.; Dunn, P.H.; Conrad, C.E. Fire’s effect on physical and chemical properties of chaparral soils. In Proceedings of the Symposium on the Environmental Consequences of Fire and Fuel Management in Mediterranean Ecosystems, Palo Alto, CA, USA, 1–5 August 1977; USDA Forest Service: Washington, DC, USA, 1977; pp. 65–74. [Google Scholar]
- Trabaud, L. Etude du comportement du feu dans la Garrigue de Chêne kermes à partir des temperatures et des vitesses de propagation. Ann. Sci. 1979, 36, 13–38. [Google Scholar] [CrossRef]
- Vélez, R. Los incendios forestales en España. Ecología 1990, 1, 213–221. [Google Scholar]
- Odion, D.C.; Davis, F.W. Fire, soil heating, and the formation of vegetation patterns in chaparral. Ecol. Monogr. 2000, 70, 149–169. [Google Scholar] [CrossRef]
- Baeza, M.J.; Raventós, J.; Escarré, A. Ulex parviflorus germination after experimental burning: Effects of temperature and soil depth. In Fire and Biological Processes; Trabaud, L., Prodon, R., Eds.; Backhuis Publishers: Leiden, The Netherlands, 2002; pp. 83–91. ISBN 90-5782-116-8. [Google Scholar]
- Santana, V.M.; Baeza, M.J.; Vallejo, V.R. Fuel structural traits modulating soil temperatures in different species patches of Mediterranean Basin shrublands. Int. J. Wildland Fire 2011, 20, 668–677. [Google Scholar] [CrossRef]
- Lutes, D.; Keane, R. FOFEM 6.4. First Order Fire Effects Model; Fire Modeling Institute: Missoula, MT, USA, 2017. [Google Scholar]
- Sikkink, P.G.; Lutes, D.C.; Keane, R.E. Field Guide for Identifying Fuel Loading Models; United States Department of Agriculture, Forest Service: Rocky Mountain, BC, USA, 2009.
- Krollmann, P.; Gülz, P.-G. Composition of seed lipids from species of the genus Cistus L. (Cistaceae). Z. Planzenphysiol. 1983, 110, 469–474. [Google Scholar] [CrossRef]
- Parsons, R.F. Incidence and ecology of very fast germination. Seed Sci. Res. 2012, 22, 161–167. [Google Scholar] [CrossRef]
- Parsons, R.F.; Vandelook, F.; Janssens, S.B. Very fast germination: Additional records and relationship to embryo size and phylogeny. Seed Sci. Res. 2014, 24, 159–163. [Google Scholar] [CrossRef]
- Limpert, E.; Stahel, W.A.; Abbt, M. Log-normal distributions across the sciences: Keys and clues. BioScience 2001, 51, 341–352. [Google Scholar] [CrossRef]
- Auld, T.D.; Ooi, M.K.J. Heat increases germination of water-permeable seeds of obligate-seeding Darwinia species (Myrtaceae). Plant Ecol. 2009, 200, 117–127. [Google Scholar] [CrossRef]
- Pereiras, J.; Puentes, M.A.; Casal, M. Efecto de las altas temperaturas sobre la germinacion de las semillas del tojo (Ulex europaeus L.). Studia Oecol. 1985, 6, 125–133. [Google Scholar]
- Auld, T.D.; O’Connell, M.A. Predicting patterns of post-fire germination in 35 eastern Australian Fabaceae. Aust. J. Ecol. 1991, 16, 53–70. [Google Scholar] [CrossRef]
- Herrero, C.; San Martin, R.; Bravo, F. Effect of heat and ash treatments on germination of Pinus pinaster and Cistus laurifolius. J. Arid Environ. 2007, 70, 540–548. [Google Scholar] [CrossRef]
- Bolin, J.F. Heat shock germination responses of three eastern North American temperate species. Castanea 2009, 74, 160–167. [Google Scholar] [CrossRef]
- Reyes, O.; Trabaud, L. Germination behaviour of 14 Mediterranean species in relation to fire factors: Smoke and heat. Plant Ecol. 2009, 202, 113–121. [Google Scholar] [CrossRef]
- Bradshaw, S.D.; Dixon, K.W.; Hopper, S.D.; Lambers, H.; Turner, S.R. Little evidence for fire-adapted plant traits in Mediterranean climate regions. Trends Plant Sci. 2011, 16, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Keeley, J.E.; Pausas, J.G.; Rundel, P.W.; Bond, W.J.; Bradstock, R.A. Fire as an evolutionary pressure shaping plant traits. Trends Plant Sci. 2011, 16, 406–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradshaw, S.D.; Dixon, K.W.; Hopper, S.D.; Lambers, H.; Turner, S.R. Response to Keely et al.: Fire as an evolutionary pressure shaping plant traits. Trends Plant Sci. 2011, 16, 405. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, G.K. Are wildfires an adapted ecological cue breaking physical dormancy in the Mediterranean basin? Seed Sci. Res. 2015, 25, 120–126. [Google Scholar] [CrossRef]
- Stone, E.C.; Juhren, G. The effect of fire on the germination of the seed of Rhus ovata Wats. Am. J. Bot. 1951, 38, 368–372. [Google Scholar] [CrossRef]
- Egley, G.H.; Paul, R.N.; Lax, A.R. Seed coat imposed dormancy: Histochemistry of the region controlling onset of water entry into Sida spinosa seeds. Physiol. Plant. 1986, 67, 320–327. [Google Scholar] [CrossRef]
- Briggs, C.L.; Morris, E.C.; Ashford, A.E. Investigations into seed dormancy in Grevillea linearifolia, G. buxifolia and G. sericea: Anatomy and histochemistry of the seed coat. Ann. Bot. 2005, 96, 965–980. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.W.; Cocks, P.S.; Kailis, S.G.; Kuo, J. The role of fractures and lipids in the seed coat in the loss of hardseededness of six Mediterranean legume species. J. Agric. Sci. 2005, 143, 43–55. [Google Scholar] [CrossRef]
- Briggs, C.L.; Morris, E.C. Seed-coat dormancy in Grevillea linearifolia: Little change in permeability to an apoplastic tracer after treatment with smoke and heat. Ann. Bot. 2008, 101, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Egerton-Warburton, L.E. A smoke-induced alteration of the sub-testa cuticle in seeds of the post-fire recruiter, Emmenanthe penduliflora Benth. (Hydrophyllaceae). J. Exp. Bot. 1998, 49, 1317–1327. [Google Scholar] [CrossRef]
- Keeley, J.E.; Fotheringham, C.J. Mechanisms of smoke-induced seed germination in a post-fire chaparral annual. J. Ecol. 1998, 86, 27–36. [Google Scholar] [CrossRef]
- Trabaud, L.; Oustric, J. Heat requirements for seed germination of three Cistus species in the garrigue of southern France. Flora 1989, 183, 321–325. [Google Scholar] [CrossRef]
- Roy, J.; Sonié, L. Germination and population dynamics of Cistus species in relation to fire. J. Appl. Ecol. 1992, 29, 647–655. [Google Scholar] [CrossRef]
- Pérez-Fernández, M.A.; Rodríguez-Echeverría, S. Effect of smoke, charred wood, and nitrogen compounds on seed germination of ten species from woodland in central-western Spain. J. Chem. Ecol. 2003, 29, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.P.; Bhati, P.R.; Sen, D.N. Differential specificity in water imbibition of Indian arid zone seeds. Biol. Plant. 1980, 22, 327–331. [Google Scholar] [CrossRef]
- Larson, M.M. The effect soaking pea seeds with or without seedcoats has on seedling growth. Plant Physiol. 1968, 43, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.C.; Oliveira, D.M.T.; Oliveira, F.A.O. A new seed coat water-impermeability mechanism in Chaetostoma armatum (Melastomataceae): Evolutionary and biogeographical implications of physiophysical dormancy. Seed Sci. Res. 2015, 25, 194–202. [Google Scholar] [CrossRef]
- Talavera, S.; Gibbs, P.E.; Herrera, J. Reproductive biology of Cistus ladanifer (Cistaceae). Plant Syst. Evol. 1993, 186, 123–134. [Google Scholar] [CrossRef]
- Alonso, I.; Luis, E.; Tárrega, R. First phases of regeneration of Cistus laurifolius and Cistus ladanifer after burning and cutting in experimental plots. Int. J. Wildland Fire 1992, 2, 7–14. [Google Scholar] [CrossRef]
- Alías, J.C.; Sosa, T.; Escudero, J.C.; Chaves, N. Autotoxicity against germination and seedling emergence in Cistus ladanifer L. Plant Soil 2006, 282, 327–332. [Google Scholar] [CrossRef]
- Traba, J.; Azcárate, F.M.; Peco, B. From what depth do seeds emerge? A soil seed bank experiment with Mediterranean grassland species. Seed Sci. Res. 2004, 14, 297–303. [Google Scholar] [CrossRef]
- Luna, B.; Moreno, J.M. Light and nitrate effects on seed germination of Mediterranean plant species of several functional groups. Plant Ecol. 2009, 203, 123–135. [Google Scholar] [CrossRef]
- Bodí, M.B.; Cerdà, A.; Mataix-Solera, J.; Doerr, S.H. Efectos de los incendios forestales en la vegetación y el suelo en la cuenca mediterránea: Revisión bibliográfica. Bol. Asoc. Geógr. Esp. 2012, 58, 33–55. [Google Scholar] [CrossRef]
- Bradstock, R.A.; Auld, T.D. Soil temperatures during experimental bushfires in relation to fire intensity: Consequences for legume germination and fire management in south-eastern Australia. J. Appl. Ecol. 1995, 32, 76–84. [Google Scholar] [CrossRef]
- Thanos, C.A. Fire effects on forest vegetation, the case of Mediterranean pine forests in Greece. In Wildfire Management; Eftichidis, G., Balabanis, P., Ghazi, A., Eds.; Algosystems & European Comission DGXII: Athens, Greece, 1999; pp. 323–334. [Google Scholar]
- Agencia Estatal de Meteorología, Ministerio de Medio Ambiente y Medio Rural y Marino; Instituto de Meteorologia de Portugal. Atlas Climático Ibérico, Temperatura del Aire y Precipitación (1971–2000)/Atlas Climático Ibérico, Temperatura do Ar e Precipitação (1971–2000)/Iberian Climate Atlas, Air Temperature and Precipitation (1971–2000); Agencia Estatal de Meteorología, Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, Spain; Instituto de Meteorologia de Portugal: Lisboa, Portugal, 2011; ISBN 978-84-7837-079-5. [Google Scholar]
- Rietveld, W.J. Phytotoxic Grass Residues Reduce Germination and Initial Root Growth of Ponderosa Pine; United States Department of Agriculture Forest Service: Fort Collins, CO, USA, 1975.
- Dias, L.S. Describing phytotoxic effects on cumulative germination. J. Chem. Ecol. 2001, 27, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Spólnik, P.; Stopa, B.; Piekarska, B.; Jagusiak, A.; Konieczny, L.; Rybarska, J.; Król, M.; Roterman, I.; Urbanowicz, B.; Zieba-Palus, J. The use of rigid, fibrillar Congo red nanostructures for scaffolding protein assemblies and inducing the formation of amyloid-like arrangement of molecules. Chem. Biol. Drug Des. 2007, 70, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Al-Thabaiti, S.A.; Aazam, E.S.; Khan, Z.; Bashir, O. Aggregation of Congo red with surfactants and Ag-nanoparticles in an aqueous solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 156, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Bond, W.J.; Honig, M.; Maze, K.E. Seed size and seedling emergence: An allometric relationship and some ecological implications. Oecologia 1999, 120, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Luna, B.; Chamorro, D. Germination sensitivity to water stress of eight Cistaceae species from the Western Mediterranean. Seed Sci. Res. 2016, 26, 101–110. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An analysis of transformations. J. R. Stat. Soc. B 1964, 26, 211–252. [Google Scholar] [CrossRef]
- Chew, V. Comparing means: A compendium. HortScience 1976, 11, 348–357. [Google Scholar]
- Ury, H.K. A comparison of four procedures for multiple comparisons among means (pairwise contrasts) for arbitrary sample sizes. Technometrics 1976, 18, 89–97. [Google Scholar] [CrossRef]
- Draper, N.R.; Smith, H. Applied Regression Analysis, 3rd ed.; John Wiley: New York, NY, USA, 1998; ISBN 0-471-17082-8. [Google Scholar]
- Weibull, W. A statistical distribution function of wide applicability. J. Appl. Mech. 1951, 18, 293–297. [Google Scholar]
- Marquardt, D.W. An algorithm for least-squares estimation of nonlinear parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Dubey, S.D. Normal and Weibull distributions. Nav. Res. Logist. Q. 1967, 14, 69–79. [Google Scholar] [CrossRef]
- Bonner, F.T.; Dell, T.R. The Weibull function: A new method of comparing seed vigor. J. Seed Technol. 1976, 1, 96–103. [Google Scholar]
- Dias, A.S.; Pereira, I.P.; Dias, L.S. Investigating and modeling the combined effects of pH and osmotic pressure on seed germination for use in phytoactivity and allelopathic research. Plant Biosyst. 2017, 151, 657–664. [Google Scholar] [CrossRef]
- Hazony, Y. Algorithms for parallel processing: Curve and surface definition with Q-splines. Comput. Gr. 1979, 4, 165–176. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva Dias, L.; Pires Pereira, I.; Soveral Dias, A. Seed Germination in Cistus ladanifer: Heat Shock, Physical Dormancy, Soil Temperatures and Significance to Natural Regeneration. Plants 2019, 8, 63. https://doi.org/10.3390/plants8030063
Silva Dias L, Pires Pereira I, Soveral Dias A. Seed Germination in Cistus ladanifer: Heat Shock, Physical Dormancy, Soil Temperatures and Significance to Natural Regeneration. Plants. 2019; 8(3):63. https://doi.org/10.3390/plants8030063
Chicago/Turabian StyleSilva Dias, Luís, Isabel Pires Pereira, and Alexandra Soveral Dias. 2019. "Seed Germination in Cistus ladanifer: Heat Shock, Physical Dormancy, Soil Temperatures and Significance to Natural Regeneration" Plants 8, no. 3: 63. https://doi.org/10.3390/plants8030063