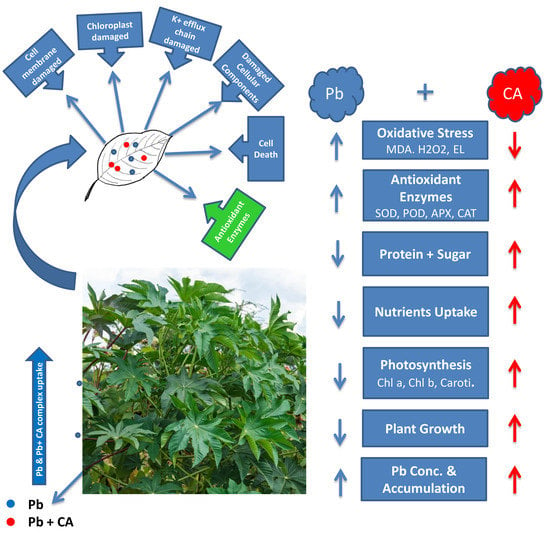
Citric Acid Enhances Plant Growth, Photosynthesis, and Phytoextraction of Lead by Alleviating the Oxidative Stress in Castor Beans
Abstract
:1. Introduction
2. Results
2.1. Plant Growth and Biomass
2.2. Photosynthetic Pigments and Gas Exchange Parameters
2.3. Antioxidant Enzymes Activities
2.4. Effect of CA Application on EL, H2O2, and MDA
2.5. Lead Concentration and Uptake in Castor Beans
3. Discussion
3.1. Plant Growth and Biomass
3.2. Photosynthetic Pigments
3.3. Antioxidant Enzymes
3.4. H2O2, EL, and MDA Contents
3.5. The Accumulation of Pb in Roots and Leaves
4. Materials and Methods
4.1. Soil Sampling and Analysis
4.2. Pot Experiment
4.3. Plants Harvesting
4.4. Chlorophyll Contents and Gas Exchange Parameters
4.5. Estimation of MDA, EL, H2O2 and Antioxidants Enzymes
4.6. Estimation of Pb Contents
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tabelin, C.B.; Igarashi, T.; Villacorte-Tabelin, M.; Park, I.; Opiso, E.M.; Ito, M.; Hiroyoshi, N. Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: A review of their sources, modes of enrichment, mechanisms of release, and mitigation strategies. Sci. Total Environ. 2018, 645, 1522–1553. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Tong, P.; Xue, K.S.; Williams, P.L.; Wang, J.S.; Tang, L. High-throughput assessment of toxic effects of metal mixtures of cadmium (Cd), lead (Pb), and manganese (Mn) in nematode Caenorhabditis elegans. Chemosphere 2019. [Google Scholar] [CrossRef] [PubMed]
- Bali, S.; Jamwal, V.L.; Kaur, P.; Kohli, S.K.; Ohri, P.; Gandhi, S.G.; Bhardwaj, R.; Al-Huqail, A.A.; Siddiqui, M.H.; Ahmad, P. Role of P-type ATPase metal transporters and plant immunity induced by jasmonic acid against Lead (Pb) toxicity in tomato. Ecotoxicol. Environ. Saf. 2019, 174, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Lamhamdi, M.; El Galiou, O.; Bakrim, A.; Nóvoa-Muñoz, J.C.; Arias-Estevez, M.; Aarab, A.; Lafont, R. Effect of lead stress on mineral content and growth of wheat (Triticum aestivum) and spinach (Spinacia oleracea) seedlings. Saudi J. Boil. Sci. 2013, 20, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Kasim, W.A.; Abokassem, E.M.; Ragab, G.A.; Sewelam, N.A. Alleviation of lead stress toxicity in Vigna unguiculata by salicylic acid. Egypt. J. Exp. Biol. 2014, 10, 37–49. [Google Scholar]
- Ali, S.; Bharwana, S.A.; Rizwan, M.; Farid, M.; Kanwal, S.; Ali, Q.; Ibrahim, M.; Gill, R.A.; Khan, M.D. Fulvic acid mediates chromium (Cr) tolerance in wheat (Triticum aestivum L.) through lowering of Cr uptake and improved antioxidant defense system. Environ. Sci. Pollut. Res. 2015, 22, 10601–10609. [Google Scholar] [CrossRef]
- Ali, S.; Chaudhary, A.; Rizwan, M.; Anwar, H.T.; Adrees, M.; Farid, M.; Irshad, M.K.; Hayat, T.; Anjum, S.A. Alleviation of chromium toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed chromium uptake and oxidative stress in wheat (Triticum aestivum L.). Environ. Sci. Pollut. Res. 2015, 22, 10669–10678. [Google Scholar] [CrossRef]
- Ali, S.; Gill, R.A.; Ulhassan, Z.; Najeeb, U.; Kanwar, M.K.; Abid, M.; Mwamba, T.M.; Huang, Q.; Zhou, W. Insights on the responses of Brassica napus cultivars against the cobalt-stress as revealed by carbon assimilation, anatomical changes and secondary metabolites. Environ. Exp. Bot. 2018, 156, 183–196. [Google Scholar] [CrossRef]
- Kanwal, U.; Ali, S.; Shakoor, M.B.; Farid, M.; Hussain, S.; Yasmeen, T.; Adrees, M.; Bharwana, S.A.; Abbas, F. EDTA ameliorates phytoextraction of lead and plant growth by reducing morphological and biochemical injuries in Brassica napus L. under lead stress. Environ. Sci. Pollut. Res. 2014, 21, 9899–9910. [Google Scholar] [CrossRef]
- Ehsan, S.; Ali, S.; Noureen, S.; Mahmood, K.; Farid, M.; Ishaque, W.; Shakoor, M.B.; Rizwan, M. Citric acid assisted phytoremediation of cadmium by Brassica napus L. Ecotoxicol. Environ. Saf. 2014, 106, 164–172. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Rizwan, M.; Farid, M.; Shakoor, M.B.; Gill, R.A.; Najeeb, U.; Iqbal, N.; Ahmad, R. Citric acid assisted phytoremediation of copper by Brassica napus L. Ecotoxicol. Environ. Saf. 2015, 120, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Mahmud, J.A.; Alharby, H.F.; Fujita, M. Exogenous glutathione attenuates lead-induced oxidative stress in wheat by improving antioxidant defense and physiological mechanisms. J. Plant Interact. 2018, 13, 203–212. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Rizwan, M.; Ali, Q.; Abbas, F.; Bukhari, S.A.H.; Saeed, R.; Wu, L. Citric acid assisted phytoextraction of chromium by sunflower; morpho-physiological and biochemical alterations in plants. Ecotoxicol. Environ. Saf. 2017, 145, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, I.S. Comparison of the ability of organic acids and EDTA to enhance the phytoextraction of metals from a multi-metal contaminated soil. Bulletin Environ. Contamin. Toxicol. 2010, 84, 255–259. [Google Scholar] [CrossRef]
- Afshan, S.; Ali, S.; Bharwana, S.A.; Rizwan, M.; Farid, M.; Abbas, F.; Ibrahim, M.; Mehmood, M.A.; Abbasi, G.H. Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica napus L. Environ. Sci. Pollut. Res. 2015, 22, 11679–11689. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Ali, S.; Hameed, A.; Farid, M.; Hussain, S.; Yasmeen, T.; Najeeb, U.; Bharwana, S.A.; Abbasi, G.H. Citric acid improves lead (Pb) phytoextraction in Brassica napus L. by mitigating Pb-induced morphological and biochemical damages. Ecotoxicol. Environ. Saf. 2014, 109, 38–47. [Google Scholar] [CrossRef]
- Olivares, A.R.; Carrillo-González, R.; González-Chávez, M.D.C.A.; Hernández, R.M.S. Potential of castor bean (Ricinus communis L.) for phytoremediation of mine tailings and oil production. J. Environ. Manag. 2013, 114, 316–323. [Google Scholar] [CrossRef]
- Romeiro, S.; Lagôa, A.M.; Furlani, P.R.; Abreu, C.A.D.; Abreu, M.F.D.; Erismann, N.M. Lead uptake and tolerance of Ricinus communis L. Brazilian J. Plant Physiol. 2006, 18, 483–489. [Google Scholar] [CrossRef]
- Liu, J.N.; Zhou, Q.X.; Sun, T.; Ma, L.Q.; Wang, S. Identification and chemical enhancement of two ornamental plants for phytoremediation. Bulletin Environ. Contamin. Toxicol. 2008, 80, 260–265. [Google Scholar] [CrossRef]
- Singh, R.; Tripathi, R.D.; Dwivedi, S.; Kumar, A.; Trivedi, P.K.; Chakrabarty, D. Lead bioaccumulation potential of an aquatic macrophyte Najas indica are related to antioxidant system. Biores. Technol. 2010, 101, 3025–3032. [Google Scholar] [CrossRef]
- Piotrowska, A.; Bajguz, A.; Godlewska-Żyłkiewicz, B.; Czerpak, R.; Kamińska, M. Jasmonic acid as modulator of lead toxicity in aquatic plant Wolffia arrhiza (Lemnaceae). Environ. Exp. Bot. 2009, 66, 507–513. [Google Scholar] [CrossRef]
- Okant, M.; Kaya, C. The role of endogenous nitric oxide in melatonin-improved tolerance to lead toxicity in maize plants. Environ. Sci. Pollut. Res. 2019, 26, 11864–11874. [Google Scholar] [CrossRef] [PubMed]
- Gopal, R.; Rizvi, A.H. Excess lead alters growth, metabolism and translocation of certain nutrients in radish. Chemosphere 2008, 70, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Islam, E.; Yang, X.; Li, T.; Liu, D.; Jin, X.; Meng, F. Effect of Pb toxicity on root morphology, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J. Hazard. Mat. 2007, 147, 806–816. [Google Scholar] [CrossRef]
- Arias, J.A.; Peralta-Videa, J.R.; Ellzey, J.T.; Ren, M.; Viveros, M.N.; Gardea-Torresdey, J.L. Effects of Glomus deserticola inoculation on Prosopis: Enhancing chromium and lead uptake and translocation as confirmed by X-ray mapping, ICP-OES and TEM techniques. Environ. Exp. Bot. 2010, 68, 139–148. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, D. Pb-induced cellular defense system in the root meristematic cells of Allium sativum L. BMC Plant. Biol. 2010, 10, 40. [Google Scholar] [CrossRef]
- Malar, S.; Manikandan, R.; Favas, P.J.; Sahi, S.V.; Venkatachalam, P. Effect of lead on phytotoxicity, growth, biochemical alterations and its role on genomic template stability in Sesbania grandiflora: A potential plant for phytoremediation. Ecotoxicol. Environ. Saf. 2014, 108, 249–257. [Google Scholar] [CrossRef]
- Fahr, M.; Laplaze, L.; El Mzibri, M.; Doumas, P.; Bendaou, N.; Hocher, V.; Bogusz, D.; Smouni, A. Assessment of lead tolerance and accumulation in metallicolous and non-metallicolous populations of Hirschfeldia incana. Environ. Exp. Bot. 2015, 109, 186–192. [Google Scholar] [CrossRef]
- Ali, B.; Gill, R.A.; Yang, S.; Gill, M.B.; Farooq, M.A.; Liu, D.; Daud, M.K.; Ali, S.; Zhou, W. Regulation of cadmium-induced proteomic and metabolic changes by 5-aminolevulinic acid in leaves of Brassica napus L. PLoS ONE 2015, 10, e0123328. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, L.; Gu, J.; Zhao, J.; Fu, J. Citric acid and EDTA on the growth, photosynthetic properties and heavy metal accumulation of Iris halophila Pall. cultivated in Pb mine tailings. Inter. Biodeter. Biodegrad. 2018, 128, 15–21. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, M.N.V.; Sytar, O. Lead toxicity, defense strategies and associated indicative biomarkers in Talinum triangulare grown hydroponically. Chemosphere 2012, 89, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Al Mahmud, J.; Hasanuzzaman, M.; Nahar, K.; Bhuyan, M.B.; Fujita, M. Insights into citric acid-induced cadmium tolerance and phytoremediation in Brassica juncea L.: coordinated functions of metal chelation, antioxidant defense and glyoxalase systems. Ecotoxicol. Environ. Saf. 2018, 147, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.; Santos, C.; Azevedo, R.; Moutinho-Pereira, J.; Correia, C.; Dias, M.C. Chromium (VI) induces toxicity at different photosynthetic levels in pea. Plant. Physiol. Biochem. 2012, 53, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Park, B.C.; Ahn, B.K.; Lee, J.H. Thallium uptake and translocation in barley and sunflower grown in hydroponic conditions. Inter. J. Environ. Res. 2016, 10, 575–582. [Google Scholar]
- Huang, S.; Han, Y.; Yu, S.; Gu, J.; Zhang, L. The effect of lead and copper on the growth and physiological response of water flower Iris pseudacorus L. Fresenius Environ. Bulletin. 2011, 20, 2246–2250. [Google Scholar]
- Huang, X.H.; Zhu, F.; Yan, W.D.; Chen, X.Y.; Wang, G.J.; Wang, R.J. Effects of Pb and Zn toxicity on chlorophyll fluorescence and biomass production of Koelreuteria paniculata and Zelkova schneideriana young plants. Photosynthetica 2019, 57, 688–697. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.Y.; Tong, H.Y.; Huang, S.Z.; Yuan, H.Y. Effects of citric acid and oxalic acid on the growth and physiology of Iris lactea var. chinensis under Pb stress. Chin. J. Ecol. 2010, 29, 1340–1346. [Google Scholar]
- Ruley, A.T.; Sharma, N.C.; Sahi, S.V.; Singh, S.R.; Sajwan, K.S. Effects of lead and chelators on growth, photosynthetic activity and Pb uptake in Sesbania drummondii grown in soil. Environ. Pollut. 2006, 144, 11–18. [Google Scholar] [CrossRef]
- Dias, M.C.; Mariz-Ponte, N.; Santos, C. Lead induces oxidative stress in Pisum sativum plants and changes the levels of phytohormones with antioxidant role. Plant Physiol. Biochem. 2019, 137, 121–129. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Zia-ur-Rehman, M.; Irshad, M.K.; Bharwana, S.A. The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Pollut. Res. 2015, 22, 8148–8162. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Saeed, R.; Rizwan, M.; Bukhari, S.A.H.; Abbasi, G.H.; Hussain, A.; Ali, B.; Zamir, M.S.I.; Ahmad, I. Combined application of citric acid and 5-aminolevulinic acid improved biomass, photosynthesis and gas exchange attributes of sunflower (Helianthus annuus L.) grown on chromium contaminated soil. Inter. J. Phytorem. 2019, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V. Reactive oxygen species and oxidative stress in plants. Plant Stress Physiol. 2012, 24–58. [Google Scholar]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Adrees, M.; Ibrahim, M.; Zia-ur-Rehman, M.; Farid, M.; Abbas, F. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J. Hazard. Mat. 2017, 322, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Abbas, F.; Adrees, M.; Zia-ur-Rehman, M.; Farid, M.; Gill, R.A.; Ali, B. Role of organic and inorganic amendments in alleviating heavy metal stress in oil seed crops. Oil Seed Crops Yield Adap. Under Environ. Stress. 2017, 12, 224–235. [Google Scholar]
- Noman, A.; Ali, S.; Naheed, F.; Ali, Q.; Farid, M.; Rizwan, M.; Irshad, M.K. Foliar application of ascorbate enhances the physiological and biochemical attributes of maize (Zea mays L.) cultivars under drought stress. Arch. Agron. Soil Sci. 2015, 61, 1659–1672. [Google Scholar] [CrossRef]
- Arshad, M.; Ali, S.; Noman, A.; Ali, Q.; Rizwan, M.; Farid, M.; Irshad, M.K. Phosphorus amendment decreased cadmium (Cd) uptake and ameliorates chlorophyll contents, gas exchange attributes, antioxidants, and mineral nutrients in wheat (Triticum aestivum L.) under Cd stress. Arch. Agron. Soil Sci. 2016, 62, 533–546. [Google Scholar] [CrossRef]
- Hu, Z.H.; Zhuo, F.; Jing, S.H.; Li, X.; Yan, T.X.; Lei, L.L.; Lu, R.R.; Zhang, X.F.; Jing, Y.X. Combined application of arbuscular mycorrhizal fungi and steel slag improves plant growth and reduces Cd, Pb accumulation in Zea mays. Int. J. Phytorem. 2019, 21, 1–9. [Google Scholar] [CrossRef]
- Islam, F.; Yasmeen, T.; Arif, M.S.; Riaz, M.; Shahzad, S.M.; Imran, Q.; Ali, I. Combined ability of chromium (Cr) tolerant plant growth promoting bacteria (PGPB) and salicylic acid (SA) in attenuation of chromium stress in maize plants. Plant. Physiol. Biochem. 2016, 108, 456–467. [Google Scholar] [CrossRef]
- Rathika, R.; Khalifa, A.Y.; Srinivasan, P.; Praburaman, L.; Kamala-Kannan, S.; Selvankumar, T.; Kim, W.; Govarthanan, M. Effect of citric acid and vermi-wash on growth and metal accumulation of Sorghum bicolor cultivated in lead and nickel contaminated soil. Chemosphere 2019, 243, 125327. [Google Scholar] [CrossRef]
- Aderholt, M.; Vogelien, D.L.; Koether, M.; Greipsson, S. Phytoextraction of contaminated urban soils by Panicum virgatum L. enhanced with application of a plant growth regulator (BAP) and citric acid. Chemosphere 2017, 175, 85–96. [Google Scholar] [CrossRef]
- Kiran, B.R.; Prasad, M.N.V. Biochar and rice husk assisted phytoremediation potentials of Ricinus communis L. for Lead-spiked soils. Ecotoxicol. Env. Saf. 2019, 183, 109574. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, A.; Ali, S.; Rizwan, M.; Ishaque, W.; Rasool, N.; Rehman, M.Z.; Bashir, A.; Abid, M.; Wu, L. Management of tannery wastewater for improving growth attributes and reducing chromium uptake in spinach through citric acid application. Environ. Sci. Pollut. Res. 2018, 25, 10848–10856. [Google Scholar] [CrossRef] [PubMed]
- Rostami, S.; Azhdarpoor, A. The application of plant growth regulators to improve phytoremediation of contaminated soils: A review. Chemosphere 2019, 220, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Yadav, P.; Sharma, A.; Thukral, A.K.; Kumar, V.; Kohli, S.K.; Bhardwaj, R. Castasterone and citric acid treatment restores photosynthetic attributes in Brassica juncea L. under Cd (II) toxicity. Ecotoxicol. Environ. Saf. 2017, 145, 466–475. [Google Scholar] [CrossRef]
- Karami, N.; Clemente, R.; Moreno-Jiménez, E.; Lepp, N.W.; Beesley, L. Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. J. Hazard. Mat. 2011, 191, 41–48. [Google Scholar] [CrossRef]
- Soltanpour, P.N. Use of ammonium bicarbonate DTPA soil test to evaluate elemental availability and toxicity. Communic. Soil Sci. Plant. Anal. 1985, 16, 323–338. [Google Scholar] [CrossRef]
- US Salinity Laboratory Staff. Diagnosis and Improvement of Saline and Alkali Soils. Agriculture Handbook 60; United States Salinity Laboratory, USDA: Washington, DC, USA, 1954; p. 160.
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties; ASA, Inc.; SSSA, Inc.: Madison, WI, USA, 1982. [Google Scholar]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analyses of soils 1. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chem. Analysis; Constable and Company, Ltd.: London, UK, 1962; p. 497. [Google Scholar]
- Moodie, C.D.; Smith, H.W.; McCreery, R.A. Laboratory Manual for Soil Fertility, (Mimeographed); Washington State College: Seattle, WA, USA, 1959. [Google Scholar]
- Lichtenthaler, H.K. [34] Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Zhang, J.; Kirkham, M.B. Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant. Cell Physiol. 1994, 35, 785–791. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Zia-ur-Rehman, M.; Qayyum, M.F.; Abbas, F.; Hannan, F.; Rinklebe, J.; Ok, Y.S. Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol. Environ. Saf. 2017, 140, 37–47. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant. Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Jana, S.; Choudhuri, M.A. Glycolate metabolism of three submersed aquatic angiosperms: Effect of heavy metals. Aquatic Bot. 1981, 11, 67–77. [Google Scholar] [CrossRef]
- Zhang, X.Z. The Measurement and Mechanism of Lipid Peroxidation and SOD, POD and CAT Activities in Biological System; Research Methodology of Crop Physiology; Agriculture Press: Beijing, China, 1992; pp. 208–211. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Rehman, M.Z.U.; Rizwan, M.; Ghafoor, A.; Naeem, A.; Ali, S.; Sabir, M.; Qayyum, M.F. Effect of inorganic amendments for in situ stabilization of cadmium in contaminated soils and its phyto-availability to wheat and rice under rotation. Environ. Sci. Pollut. Res. 2015, 22, 16897–16906. [Google Scholar] [CrossRef] [PubMed]






| Texture | Sandy Loam |
|---|---|
| Silt | 15.0% |
| Sand | 67.9% |
| Clay | 17.1% |
| EC | 1.96 dS m−1 |
| pH | 7.61 |
| Sodium adsorption ratio (SAR) | 1.89 (mmol L−1)1/2 |
| Available P | 2.11 mg kg−1 |
| Organic matter | 0.59% |
| HCO3 | 2.51 mmol L−1 |
| SO4−2 | 11.44 mmol L−1 |
| Cl- | 5.45 mmol L−1 |
| Ca2+ + Mg2+ | 13.98 mmol L−1 |
| K+ | 0.04 mmol L−1 |
| Na2 | 5.23 mmol L−1 |
| Available Zn2 | 0.77 mg kg−1 |
| Available Cu2+ | 0.31 mg kg−1 |
| Available Cr +6 | 0.16 mg kg−1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallhi, Z.I.; Rizwan, M.; Mansha, A.; Ali, Q.; Asim, S.; Ali, S.; Hussain, A.; Alrokayan, S.H.; Khan, H.A.; Alam, P.; et al. Citric Acid Enhances Plant Growth, Photosynthesis, and Phytoextraction of Lead by Alleviating the Oxidative Stress in Castor Beans. Plants 2019, 8, 525. https://doi.org/10.3390/plants8110525
Mallhi ZI, Rizwan M, Mansha A, Ali Q, Asim S, Ali S, Hussain A, Alrokayan SH, Khan HA, Alam P, et al. Citric Acid Enhances Plant Growth, Photosynthesis, and Phytoextraction of Lead by Alleviating the Oxidative Stress in Castor Beans. Plants. 2019; 8(11):525. https://doi.org/10.3390/plants8110525
Chicago/Turabian StyleMallhi, Zahid Imran, Muhammad Rizwan, Asim Mansha, Qasim Ali, Sadia Asim, Shafaqat Ali, Afzal Hussain, Salman H. Alrokayan, Haseeb A. Khan, Pravej Alam, and et al. 2019. "Citric Acid Enhances Plant Growth, Photosynthesis, and Phytoextraction of Lead by Alleviating the Oxidative Stress in Castor Beans" Plants 8, no. 11: 525. https://doi.org/10.3390/plants8110525