Serratia marcescens LYGN1 Reforms the Rhizosphere Microbial Community and Promotes Cucumber and Pepper Growth in Plug Seedling Cultivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Bacterial Strain
2.2. Experimental Design
2.3. Sampling and Determination of Seedlings Growth
2.4. Root System Architecture Analysis
2.5. Microbial Community of Cucumber and Pepper Rhizosphere
2.6. Statistical Analysis
3. Results
3.1. The Cucumber and Pepper Seedling Traits and Root Morphology
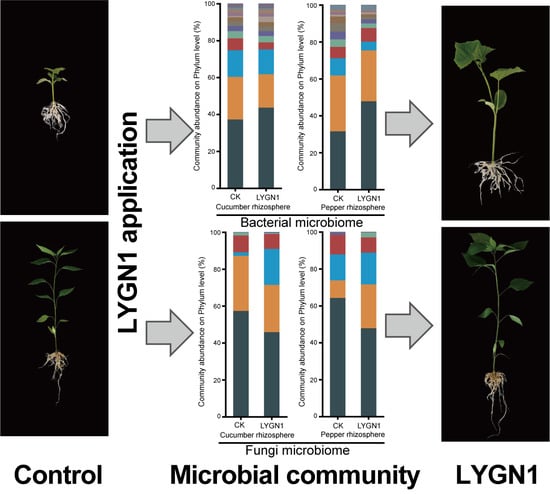
3.2. Microbial Community Diversity of Cucumber and Pepper Rhizosphere
3.3. Correlation Analysis of Phylum-Level Microbial Groups and Seedlings Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, X.; Si, L.; Jin, X.; Li, P.; Yun, Z.; Gao, K. Classification of Plug Seedling Quality by Improved Convolutional Neural Network with an Attention Mechanism. Front. Plant Sci. 2022, 13, 967706. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Mo, M.; Gao, Y.; Ma, H.; Xiang, D.; Ma, G.; Mao, H. Effects of New Compounds into Substrates on Seedling Qualities for Efficient Transplanting. Agronomy 2022, 12, 983. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and Biotic Stress Combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.W.; Wu, Z.H.; Feng, Y.X.; Shi, X.F.; Li, P.L.; Shang, Q.M. Research Progress on Vegetables Seedling Culture Technique during ‘The Thirteenth Five-Year Plan’ in China. China Vege 2021, 1, 18–26. [Google Scholar]
- Singh, M.; Singh, J.P.; Pandey, S.K.; Mahay, D.; Shrivastva, V. Factors Affecting the Performance of Greenhouse Cucumber Cultivation-A Review. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2304–2323. [Google Scholar] [CrossRef]
- Qin, Y.; Shang, Q.; Zhang, Y.; Li, P.; Chai, Y. Bacillus Amyloliquefaciens L-S60 Reforms the Rhizosphere Bacterial Community and Improves Growth Conditions in Cucumber Plug Seedling. Front. Microbiol. 2017, 8, 2620. [Google Scholar] [CrossRef]
- Kloepper, J.W. Plant Growth-Promoting Rhizobacteria and Plant Growth Under Gnotobiotic Conditions. Phytopathology 1981, 71, 642. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant Growth-Promoting Rhizobacteria (PGPR): Emergence in Agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Shultana, R.; Zuan, A.T.K.; Naher, U.A.; Islam, A.K.M.M.; Rana, M.M.; Rashid, M.H.; Irin, I.J.; Islam, S.S.; Rim, A.A.; Hasan, A.K. The PGPR Mechanisms of Salt Stress Adaptation and Plant Growth Promotion. Agronomy 2022, 12, 2266. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Ma, C.; Wu, F.; Jin, X.; Dini-Andreote, F.; Wei, Z. Biochar Amendment Reduces Cadmium Uptake by Stimulating Cadmium-Resistant PGPR in Tomato Rhizosphere. Chemosphere 2022, 307, 136138. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yuan, X.; Xing, Y.; Dao, J.; Zhao, D.; Li, Y.; Li, W.; Wang, Z. A Meta-Analysis on Morphological, Physiological and Biochemical Responses of Plants with PGPR Inoculation under Drought Stress. Plant Cell Environ. 2023, 46, 199–214. [Google Scholar] [CrossRef]
- Zahir, Z.A.; Arshad, M.; Frankenberger, W.T. Plant Growth Promoting Rhizobacteria: Applications and Perspectives In Agriculture. Adv. Agron. 2003, 81, 97–168. [Google Scholar] [CrossRef]
- Grimont, P.A.D.; Grimont, F. The Genus Serratia. Annu. Rev. Microbiol. 1978, 32, 221–248. [Google Scholar] [CrossRef]
- Zhang, X.; Song, M.; Li, J.; Liu, X.; Gao, L.; Tian, Y. Changes in Soil Nematode and Microbial Community in Cucumber Root-Zone Soil Shaped by Intercropping with Amaranth. Horticulturae 2023, 9, 924. [Google Scholar] [CrossRef]
- Lavania, M.; Chauhan, P.S.; Chauhan, S.V.S.; Singh, H.B.; Nautiyal, C.S. Induction of Plant Defense Enzymes and Phenolics by Treatment With Plant Growth–Promoting Rhizobacteria Serratia Marcescens NBRI1213. Curr. Microbiol. 2006, 52, 363–368. [Google Scholar] [CrossRef]
- Niu, H.; Sun, Y.; Zhang, Z.; Zhao, D.; Wang, N.; Wang, L.; Guo, H. The Endophytic Bacterial Entomopathogen Serratia marcescens Promotes Plant Growth and Improves Resistance against Nilaparvata lugens in Rice. Microbiol. Res. 2022, 256, 126956. [Google Scholar] [CrossRef]
- Matteoli, F.P.; Passarelli-Araujo, H.; Reis, R.J.A.; da Rocha, L.O.; de Souza, E.M.; Aravind, L.; Olivares, F.L.; Venancio, T.M. Genome Sequencing and Assessment of Plant Growth-Promoting Properties of a Serratia marcescens Strain Isolated from Vermicompost. BMC Genom. 2018, 19, 750. [Google Scholar] [CrossRef]
- Chakraborty, U.; Chakraborty, B.N.; Chakraborty, A.P. Influence of Serratia marcescens TRS-1 on Growth Promotion and Induction of Resistance in Camellia sinensis against Fomes lamaoensis. J. Plant Interact. 2010, 5, 261–272. [Google Scholar] [CrossRef]
- George, P.; Gupta, A.; Gopal, M.; Thomas, L.; Thomas, G.V. Multifarious Beneficial Traits and Plant Growth Promoting Potential of Serratia marcescens KiSII and Enterobacter Sp. RNF 267 Isolated from the Rhizosphere of Coconut Palms (Cocos Nucifera L.). World J. Microbiol. Biotechnol. 2013, 29, 109–117. [Google Scholar] [CrossRef]
- Singh, R.P.; Manchanda, G.; Yang, Y.; Singh, D.; Srivastava, A.K.; Dubey, R.C.; Zhang, C. Deciphering the Factors for Nodulation and Symbiosis of Mesorhizobium Associated with Cicer Arietinum in Northwest India. Sustainability 2019, 11, 7216. [Google Scholar] [CrossRef]
- Li, H.; La, S.; Zhang, X.; Gao, L.; Tian, Y. Salt-Induced Recruitment of Specific Root-Associated Bacterial Consortium Capable of Enhancing Plant Adaptability to Salt Stress. ISME J. 2021, 15, 2865–2882. [Google Scholar] [CrossRef]
- Dong, D.M.; Wang, J.F. Relationship between greenhouse cucumber planting density and leaf area. Chin. Veg. 1988, 3, 14–17. [Google Scholar]
- Dickson, A.; Leaf, A.L.; Hosner, J.F. Seedling Quality—Soil Fertility Relationships of White Spruce, and Red and White Pine in Nurseries. For. Chron. 1960, 36, 237–241. [Google Scholar] [CrossRef]
- Niu, B.; Paulson, J.N.; Zheng, X.; Kolter, R. Simplified and Representative Bacterial Community of Maize Roots. Proc. Natl. Acad. Sci. USA 2017, 114, E2450–E2459. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS Primers with Enhanced Specificity for Basidiomycetes—Application to the Identification of Mycorrhizae and Rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE Database for Molecular Identification of Fungi: Handling Dark Taxa and Parallel Taxonomic Classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; DeSantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A Flexible Tool for Aligning Sequences to a Template Alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Shi, H.; Lu, L.; Ye, J.; Shi, L. Effects of Two Bacillus velezensis Microbial Inoculants on the Growth and Rhizosphere Soil Environment of Prunus Davidiana. Int. J. Mol. Sci. 2022, 23, 13639. [Google Scholar] [CrossRef]
- Chang, C.-L.; Chang, K.-P. The Growth Response of Leaf Lettuce at Different Stages to Multiple Wavelength-Band Light-Emitting Diode Lighting. Sci. Hortic. 2014, 179, 78–84. [Google Scholar] [CrossRef]
- Chen, J.-M.; Feng, W.-M.; Yan, H.; Liu, P.; Zhou, G.-S.; Guo, S.; Yu, G.; Duan, J.-A. Explore the Interaction between Root Metabolism and Rhizosphere Microbiota during the Growth of Angelica sinensis. Front. Plant Sci. 2022, 13, 1005711. [Google Scholar] [CrossRef]
- Kotoky, R.; Nath, S.; Kumar Maheshwari, D.; Pandey, P. Cadmium Resistant Plant Growth Promoting Rhizobacteria Serratia marcescens S2I7 Associated with the Growth Promotion of Rice Plant. Environ. Sustain. 2019, 2, 135–144. [Google Scholar] [CrossRef]
- Pérez-Rodriguez, M.M.; Piccoli, P.; Anzuay, M.S.; Baraldi, R.; Neri, L.; Taurian, T.; Lobato Ureche, M.A.; Segura, D.M.; Cohen, A.C. Native Bacteria Isolated from Roots and Rhizosphere of Solanum lycopersicum L. Increase Tomato Seedling Growth under a Reduced Fertilization Regime. Sci. Rep. 2020, 10, 15642. [Google Scholar] [CrossRef]
- Verbon, E.H.; Liberman, L.M. Beneficial Microbes Affect Endogenous Mechanisms Controlling Root Development. Trends Plant Sci. 2016, 21, 218–229. [Google Scholar] [CrossRef]
- López-Bucio, J.; Cruz-Ramírez, A.; Herrera-Estrella, L. The Role of Nutrient Availability in Regulating Root Architecture. Curr. Opin. Plant Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef]
- Sun, M.; Liu, X.; Shi, K.; Peng, F.; Xiao, Y. Effects of Root Zone Aeration on Soil Microbes Species in a Peach Tree Rhizosphere and Root Growth. Microorganisms 2022, 10, 1879. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The Role of Mycorrhizae and Plant Growth Promoting Rhizobacteria (PGPR) in Improving Crop Productivity under Stressful Environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The Rhizosphere Microbiome and Plant Health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.-K.; Wang, Q.-Y.; Li, J.-S.; Yan, H.-W.; Chen, Q.-J.; Sun, J.; Liu, C.-J.; Han, Y.-Y.; Zou, Y.-J.; Zhang, G.-Q. Biochar Immobilized Plant Growth-Promoting Rhizobacteria Enhanced the Physicochemical Properties, Agronomic Characters and Microbial Communities during Lettuce Seedling. Front. Microbiol. 2023, 14, 1218205. [Google Scholar] [CrossRef] [PubMed]
- Bruto, M.; Prigent-Combaret, C.; Muller, D.; Moënne-Loccoz, Y. Analysis of Genes Contributing to Plant-Beneficial Functions in Plant Growth-Promoting Rhizobacteria and Related Proteobacteria. Sci. Rep. 2014, 4, 6261. [Google Scholar] [CrossRef]
- Bokhari, A.; Essack, M.; Lafi, F.F.; Andres-Barrao, C.; Jalal, R.; Alamoudi, S.; Razali, R.; Alzubaidy, H.; Shah, K.H.; Siddique, S.; et al. Bioprospecting Desert Plant Bacillus Endophytic Strains for Their Potential to Enhance Plant Stress Tolerance. Sci. Rep. 2019, 9, 18154. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, J.; Bai, Z.; Wu, S.; Li, X.; Wang, N.; Du, X.; Fan, H.; Zhuang, G.; Bohu, T.; et al. Unraveling Mechanisms and Impact of Microbial Recruitment on Oilseed Rape (Brassica napus L.) and the Rhizosphere Mediated by Plant Growth-Promoting Rhizobacteria. Microorganisms 2021, 9, 161. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Peng, J.; Hao, X.; Feng, G.; Shen, Y.; Wang, G.; Chen, Z. Serratia marcescens LYGN1 Reforms the Rhizosphere Microbial Community and Promotes Cucumber and Pepper Growth in Plug Seedling Cultivation. Plants 2024, 13, 592. https://doi.org/10.3390/plants13050592
Zhang X, Peng J, Hao X, Feng G, Shen Y, Wang G, Chen Z. Serratia marcescens LYGN1 Reforms the Rhizosphere Microbial Community and Promotes Cucumber and Pepper Growth in Plug Seedling Cultivation. Plants. 2024; 13(5):592. https://doi.org/10.3390/plants13050592
Chicago/Turabian StyleZhang, Xu, Jinxin Peng, Xiaodong Hao, Guifang Feng, Yanhui Shen, Guanghui Wang, and Zhiqun Chen. 2024. "Serratia marcescens LYGN1 Reforms the Rhizosphere Microbial Community and Promotes Cucumber and Pepper Growth in Plug Seedling Cultivation" Plants 13, no. 5: 592. https://doi.org/10.3390/plants13050592