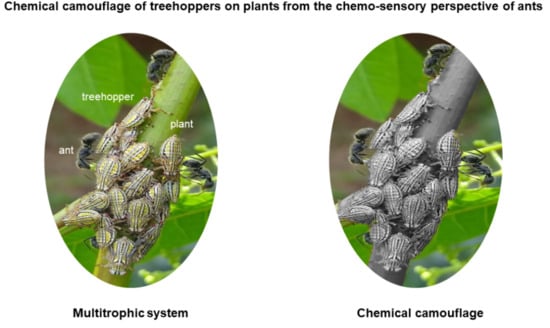
Chemical Camouflage Induced by Diet in a Pest Treehopper on Host Plants
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Site and Organisms
4.2. Chemical Analyses
4.3. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Massad, T.J.; Fincher, R.M.; Smilanich, A.M.; Dyer, L. A quantitative evaluation of major plant defense hypotheses, nature versus nurture, and chemistry versus ants. Arthropod-Plant Interact. 2011, 5, 125–139. [Google Scholar] [CrossRef]
- Yactayo-Chang, J.P.; Tang, H.V.; Mendoza, J.; Christensen, S.A.; Block, A.K. Plant defense chemicals against insect pests. Agronomy 2020, 10, 1156. [Google Scholar] [CrossRef]
- Sendoya, S.F.; Freitas, A.V.L.; Oliveira, P.S. Egg-laying butterflies distinguish predaceous ants by sight. Am. Nat. 2009, 174, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Silveira, H.C.P.; Oliveira, P.S.; Trigo, J.R. Attracting predators without falling prey: Chemical camouflage protects honeydew-producing treehoppers from ant predation. Am. Nat. 2010, 175, 261–268. [Google Scholar] [CrossRef]
- Lange, D.; Calixto, E.S.; Rosa, B.B.; Sales, T.A.; Del-Claro, K. Natural history and ecology of foraging of the Camponotus crassus Mayr, 1862 (Hymenoptera: Formicidae). J. Nat. Hist. 2019, 53, 1737–1749. [Google Scholar] [CrossRef]
- Floren, A.; Biun, A.; Linsenmair, E.K. Arboreal ants as key predators in tropical lowland rainforest trees. Oecologia 2002, 131, 137–144. [Google Scholar] [CrossRef]
- Barbero, F. Cuticular lipids as a cross-talk among ants, plants and butterflies. Int. J. Mol. Sci. 2016, 17, 1966. [Google Scholar] [CrossRef] [PubMed]
- Depa, Ł.; Kaszyca-Taszakowska, N.; Taszakowski, A.; Kanturski, M. Ant-induced evolutionary patterns in aphids. Biol. Rev. 2020, 95, 1574–1589. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Bagnères, A.-G. Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology; Cambridge University Press: Cambridge, UK, 2010; 506p. [Google Scholar]
- Chung, H.; Carroll, S.B. Wax, sex and the origin of species: Dual roles of insect cuticular hydrocarbons in adaptation and mating. BioEssays 2015, 37, 822–830. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Ginzel, M.D. Chemical ecology, biochemistry, and molecular biology of insect hydrocarbons. Ann. Rev. Entomol. 2021, 66, 45–60. [Google Scholar] [CrossRef]
- Wang, Z.; Receveur, J.P.; Pu, J.; Cong, H.; Richards, C.; Liang, M.; Chung, H. Desiccation resistance differences in Drosophila species can be largely explained by variations in cuticular hydrocarbons. Elife 2022, 11, e80859. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Pu, J.; Richards, C.; Giannetti, E.; Cong, H.; Lin, Z.; Chung, H. Evolution of a fatty acyl–CoA elongase underlies desert adaptation in Drosophila. Sci. Adv. 2023, 9, eadg0328. [Google Scholar] [CrossRef] [PubMed]
- Geiselhardt, S.; Otte, T.; Hilker, M. Looking for a similar partner: Host plants shape mating preferences of herbivorous insects by altering their contact pheromones. Ecol. Lett. 2012, 15, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Otte, T.; Hilker, M.; Geiselhardt, S. Phenotypic plasticity of cuticular hydrocarbon profiles in insects. J. Chem. Ecol. 2018, 44, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, P.P.; Menzel, F. Cuticular hydrocarbons in ants (Hymenoptera: Formicidae) and other insects: How and why they differ among individuals, colonies, and species. Myrmecol. News 2020, 30, 1–26. [Google Scholar]
- Li, D.-T.; Pei, X.-J.; Ye, Y.-X.; Wang, X.-Q.; Wang, Z.-C.; Chen, N.; Liu, T.-X.; Fan, Y.-L.; Zhang, C.-X. Cuticular hydrocarbon plasticity in three rice planthopper species. Int. J. Mol. Sci. 2021, 22, 7733. [Google Scholar] [CrossRef] [PubMed]
- Hölldobler, B.; Wilson, E.O. The ants; The Belknap Press of Harvard University Press: Cambridge, USA, 1990; 732p. [Google Scholar]
- Akino, T. Chemical strategies to deal with ants: A review of mimicry, camouflage, propaganda, and phytomimesis by ants (Hymenoptera: Formicidae) and other arthropods. Myrmecol. News 2008, 11, 173–181. [Google Scholar]
- Brückner, A. Using weapons instead of perfume: Chemical association strategies of the myrmecophilous bug Scolopostethus pacificus (Rhyparochromidae). Chemoecology 2022, 32, 147–157. [Google Scholar] [CrossRef]
- Oliveira, O.S.; Freitas, A.V.L.; Del-Claro, K. Ant foraging on plant foliage: Contrasting effects on the behavioral ecology of insect herbivores. In The Cerrados of Brazil: Ecology and Natural History of a Neotropical Savanna; Oliveira, P.S., Marquis, R.J., Eds.; Columbia University Press: New York, NY, USA, 2002; pp. 287–305. [Google Scholar]
- Pierce, N.E.; Braby, M.F.; Heath, A.; Lohman, D.J.; Mathew, J.; Rand, D.B.; Travassos, M.A. The ecology and evolution of ant association in the Lycaenidae (Lepidoptera). Annu. Rev. Entomol. 2002, 47, 733–771. [Google Scholar] [CrossRef]
- Hughes, D.P.; Pierce, N.E.; Boomsma, J.J. Social insect symbionts: Evolution in homeostatic fortresses. Trends Ecol. Evol. 2008, 23, 672–677. [Google Scholar] [CrossRef]
- Gibernau, M.; Dejean, A. Ant protection of a Heteropteran trophobiont against a parasitoid wasp. Oecologia 2001, 126, 53–57. [Google Scholar] [CrossRef]
- Choe, D.-H.; Rust, M.K. Homopteran chemical signatures reduce aggression of tending ants. Chemoecology 2006, 16, 175–178. [Google Scholar] [CrossRef]
- Hayashi, M.; Nakamuta, K.; Nomura, M. Ants learn aphid species as mutualistic partners: Is the learning behavior species-specific? J. Chem. Ecol. 2015, 41, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Pierce, M.P. The ecological and evolutionary importance of nectar-secreting galls. Ecosphere 2019, 10, e02670. [Google Scholar] [CrossRef]
- Pringle, E.G. Ant-Hemiptera associations. In Encyclopedia of Social Insects; Starr, C., Ed.; Springer Nature: Cham, Switzerland, 2020; pp. 1–5. [Google Scholar]
- Camacho, L.F.; Avilés, L. Resource exchange and partner recognition mediate mutualistic interactions between prey and their would-be predators. Biol. Letters 2021, 17, 20210316. [Google Scholar] [CrossRef]
- Nelson, A.S.; Mooney, K.A. The evolution and ecology of interactions between ants and honeydew-producing hemipteran insects. Annu. Rev. Ecol. Evol. S. 2022, 53, 379–402. [Google Scholar] [CrossRef]
- Sakata, H. Density-dependent predation of the ant Lasius niger (Hymenoptera: Formicidae) on two attended aphids Lachnus tropicalis and Myzocallis kuricola (Homoptera: Aphididae). Res. Popul. Ecol. 1995, 37, 159–164. [Google Scholar] [CrossRef]
- Fischer, M.K.; Hoffmann, K.H.; Völkl, W. Competition for mutualists in an ant–homopteran interaction mediated by hierarchies of ant attendance. Oikos 2001, 92, 531–541. [Google Scholar] [CrossRef]
- Wang, B.; Lu, M.; Cook, J.M.; Yang, D.-R.; Dunn, D.W.; Wang, R.-W. Chemical camouflage: A key process in shaping an ant-treehopper and fig-fig wasp mutualistic network. Sci. Rep. 2018, 8, 1833. [Google Scholar] [CrossRef]
- von Beeren, C.; Pohl, S.; Witte, V. On the use of adaptive resemblance terms in chemical ecology. Psyche 2012, 2012, 635761. [Google Scholar] [CrossRef]
- Lima, L.D.; Kaminski, L.A. Camouflage. In Encyclopedia of Animal Cognition and Behavior; Vonk, J., Shackelford, T., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 1–9. [Google Scholar]
- Cavalleri, A.; Kaminski, L.A.; Mendonça Jr, M.D.S. Ectoparasitism in Aulacothrips (Thysanoptera: Heterothripidae) revisited: Host diversity on honeydew-producing Hemiptera and description of a new species. Zool. Anz. 2010, 249, 209–221. [Google Scholar] [CrossRef]
- Cavalleri, A.; Mendonca Jr, M.D.S. Ectoparasitic thrips affect the behaviour of their aetalionid treehopper hosts. Austral Entomol. 2020, 59, 794–801. [Google Scholar] [CrossRef]
- Züst, T.; Agrawal, A.A. Plant chemical defense indirectly mediates aphid performance via interactions with tending ants. Ecology 2017, 98, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.S.; Acosta, N.C.; Mooney, K.A. Plant chemical mediation of ant behavior. Curr. Opin. Insect Sci. 2019, 32, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Akino, T.; Nakamura, K.-i.; Wakamura, S. Diet-induced chemical phytomimesis by twig-like caterpillars of Biston robustum Butler (Lepidoptera: Geometridae). Chemoecology 2004, 14, 165–174. [Google Scholar] [CrossRef]
- Akino, T. Chemical and behavioral study on the phytomimetic giant geometer Biston robustum Butler (Lepidoptera: Geometridae). Appl. Entomol. Zool. 2005, 40, 497–505. [Google Scholar] [CrossRef]
- Lima, L.D.; Trigo, J.R.; Kaminski, L.A. Chemical convergence between a guild of facultative myrmecophilous caterpillars and host plants. Ecol. Entomol. 2021, 46, 66–75. [Google Scholar] [CrossRef]
- Espelie, K.E.; Bernays, E.A.; Brown, J.J. Plant and insect cuticular lipids serve as behavioral cues for insects. Arch. Insect Biochem. 1991, 17, 223–233. [Google Scholar] [CrossRef]
- Whitehead, S.R.; Reid, E.; Sapp, J.; Poveda, K.; Royer, A.M.; Posto, A.L.; Kessler, A. A specialist herbivore uses chemical camouflage to overcome the defenses of an ant-plant mutualism. PLoS ONE 2014, 9, e102604. [Google Scholar] [CrossRef]
- Coronado-Rivera, J.; Solís-Del Valle, M.; Amador-Vargas, S. True bugs living on ant-defended acacias: Evasion strategies and ant species preferences, in Costa Rica and Panama. Rev. Biol. Trop. 2020, 68, 415–425. [Google Scholar] [CrossRef]
- Portugal, A.H.A.; Trigo, J.R. Similarity of cuticular lipids between a caterpillar and its host plant: A way to make prey undetectable for predatory ants? J. Chem. Ecol. 2005, 31, 2551–2561. [Google Scholar]
- Weir, T.L.; Newbold, S.; Vivanco, J.M.; van Haren, M.; Fritchman, C.; Dossey, A.T.; Bartram, S.; Boland, W.; Cosio, E.G.; Kofer, W. Plant-inhabiting ant utilizes chemical cues for host discrimination. Biotropica 2012, 44, 246–253. [Google Scholar] [CrossRef]
- Lang, C.; Menzel, F. Lasius niger ants discriminate aphids based on their cuticular hydrocarbons. Anim. Behav. 2011, 82, 1245–1254. [Google Scholar] [CrossRef]
- Lima, L.D.; Ceballos-González, A.V.; Prato, A.; Kaminski, L.A.; Nascimento, F.S. Plant-treehopper convergence may trick butterflies into trophic oviposition mistakes. Biotropica 2023, 55, 292–298. [Google Scholar] [CrossRef]
- Massuda, K.F.; Trigo, J.R. Hiding in plain sight: Cuticular compound profile matching conceals a larval tortoise beetle in its host chemical cloud. J. Chem. Ecol. 2014, 40, 341–354. [Google Scholar] [CrossRef]
- Espelie, K.E.; Brown, J.J. Cuticular hydrocarbons of species which interact on four trophic levels: Apple, Malus pumila Mill.; codling moth, Cydia pomonella L.; a hymenopteran parasitoid, Ascogaster quadridentata Wesmael; and a hyperparasite, Perilampus fulvicornis Ashmead. Comp. Biochem. Phys. B. 1990, 95, 131–136. [Google Scholar] [CrossRef]
- Piskorski, R.; Trematerra, P.; Dorn, S. Cuticular hydrocarbon profiles of codling moth larvae, Cydia pomonella (Lepidoptera: Tortricidae), reflect those of their host plant species. Biol. J. Linn. Soc. 2010, 101, 376–384. [Google Scholar] [CrossRef]
- Del-Claro, K.; Rico-Gray, V.; Torezan-Silingardi, H.M.; Alves-Silva, E.; Fagundes, R.; Lange, D.; Dáttilo, W.; Vilela, A.A.; Aguirre, A.; Rodriguez-Morales, D. Loss and gains in ant–plant interactions mediated by extrafloral nectar: Fidelity, cheats, and lies. Insect. Soc. 2016, 63, 207–221. [Google Scholar] [CrossRef]
- Aleixo, K.P.; Faria, L.B.; Groppo, M.; Castro, M.M.N.; Silva, C.I. Spatiotemporal distribution of floral resources in a Brazilian city: Implications for the maintenance of pollinators, especially bees. Urban For. Urban Gree. 2014, 13, 689–696. [Google Scholar] [CrossRef]
- Pais, M.P.; Varanda, E.M. Arthropod recolonization in the restoration of a semideciduous forest in southeastern Brazil. Neotrop. Entomol. 2010, 39, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Maschwitz, U.; Fiala, B.; Saw, L.G.; Norma-Rashid, Y.; Idris, A.H. Ficus obscura var. borneensis (Moraceae), a new non-specific ant-plant from Malesia. Malay. Nature J. 1994, 47, 409–416. [Google Scholar]
- Melo, Y.; Machado, S.R.; Alves, M. Anatomy of extrafloral nectaries in Fabaceae from dry-seasonal forest in Brazil. Bot. J. Linn. Soc. 2010, 163, 87–98. [Google Scholar] [CrossRef]
- Rodrigues, D.; Kaminski, L.A.; Freitas, A.V.L.; Oliveira, P.S. Trade-offs underlying polyphagy in a facultative ant-tended florivorous butterfly: The role of host plant quality and enemy-free space. Oecologia 2010, 163, 719–728. [Google Scholar] [CrossRef]
- Carlson, D.A.; Bernier, U.R.; Sutton, B.D. Elution patterns from capillary GC for methyl-branched alkanes. J. Chem. Ecol. 1998, 24, 1845–1865. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecological Methodology; Addison Wesley Longman: Menlo Park, CA, USA, 1999; 624p. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]



| Species | |||||||
|---|---|---|---|---|---|---|---|
| Compounds | Retention Time | Ficus clusiifolia | Aetalion reticulatum on Ficus clusiifolia | Luehea grandiflora | Aetalion reticulatum on Luehea grandiflora | Senegalia polyphylla | Aetalion reticulatum on Senegalia polyphylla |
| n-C18 | 14.261 | 0.48 ± 0.23 | - | 0.10 ± 0.05 | 0.01 ± 0.01 | - | - |
| n-C19 | 15.376 | 0.27 ± 0.13 | - | 0.10 ± 0.05 | 0.02 ± 0.01 | - | - |
| Unknown 1 | 16.375 | - | - | 0.21 ± 0.09 | 0.02 ± 0.01 | - | - |
| n-C20 | 16.509 | 0.55 ± 0.24 | 0.06 ± 0.02 | 0.21 ± 0.10 | 0.05 ± 0.02 | - | - |
| n-C21 | 17.855 | - | - | 0.18 ± 0.11 | 0.03 ± 0.01 | - | - |
| 3MeC21 | 18.715 | - | - | 0.16 ± 0.06 | 0.02 ± 0.01 | - | - |
| n-C22 | 18.785 | 0.49 ± 0.21 | - | 0.22 ± 0.11 | 0.05 ± 0.03 | - | - |
| z-C23 | 20.008 | - | - | 0.43 ± 0.49 | - | - | - |
| n-C23 | 20.106 | - | - | 1.51 ± 1.04 | 0.18 ± 0.07 | 0.59 ± 0.24 | - |
| n-C24 | 21.028 | 0.43 ± 0.34 | - | 0.25 ± 0.13 | 0.06 ± 0.03 | 0.09 ± 0.03 | - |
| C25:1 | 22.114 | - | - | 0.54 ± 1.23 | - | - | - |
| n-C25 | 22.165 | 1.09 ± 0.74 | 0.07 ± 0.03 | 1.30 ± 1.01 | 0.24 ± 0.22 | 1.77 ± 0.39 | 0.18 ± 0.10 |
| n-C26 | 23.329 | 0.53 ± 0.31 | - | 0.77 ± 0.65 | 0.12 ± 0.06 | 0.15 ± 0.03 | - |
| C27:1 | 24.490 | - | - | 1.41 ± 2.80 | - | - | - |
| n-C27 | 24.516 | 5.38 ± 2.81 | 0.48 ± 0.28 | 12.45 ± 4.63 | 2.62 ± 1.37 | 5.46 ± 0.43 | 0.68 ± 0.28 |
| 13,11MeC27 | 24.923 | - | - | 0.10 ± 0.05 | - | 0.16 ± 0.08 | - |
| 3MeC27 | 25.418 | - | - | 0.15 ± 0.08 | - | 0.11 ± 0.05 | - |
| n-C28 | 25.705 | 2.20 ± 0.93 | 1.20 ± 0.24 | 3.18 ± 1.74 | 1.26 ± 0.27 | 3.56 ± 0.65 | 1.11 ± 0.24 |
| Unknown 2 | 26.481 | - | 0.78 ± 0.56 | - | 1.52 ± 0.78 | - | 0.99 ± 0.32 |
| z-C29 | 26.215 | - | - | 0.76 ± 1.20 | - | 0.14 ± 0.03 | - |
| n-C29 | 26.938 | 41.50 ± 15.32 | 23.00 ± 5.10 | 43.89 ± 9.46 | 39.27 ± 5.43 | 36.10 ± 7.71 | 23.97 ± 2.16 |
| 15-;13-;11-;9MeC29 | 27.308 | - | 0.21 ± 0.09 | - | 0.38 ± 0.17 | - | 0.66 ± 0.47 |
| 7,11diMeC29 | 27.762 | - | - | - | - | 0.25 ± 0.19 | - |
| 3MeC29 | 27.821 | - | - | - | 0.22 ± 0.44 | 0.08 ± 0.03 | - |
| n-C30 | 28.096 | 5.12 ± 1.86 | 2.73 ± 0.45 | 2.83 ± 1.44 | 3.14 ± 1.90 | 2.39 ± 0.25 | 2.57 ± 0.38 |
| Unknown 3 | 28.860 | - | 0.86 ± 0.07 | 0.12 ± 0.06 | 0.52 ± 0.16 | - | 0.50 ± 0.12 |
| C31:1 a | 29.039 | 0.70 ± 1.28 | - | 0.64 ± 0.99 | - | - | - |
| C31:1 b | 29.422 | - | - | 0.32 ± 0.16 | - | - | - |
| n-C31 | 29.547 | 35.54 ± 10.67 | 10.12 ± 3.56 | 15.51 ± 6.82 | 12.00 ± 3.81 | 1.45 ± 0.36 | 11.86 ± 2.44 |
| Unknown 4 | 29.607 | - | - | 2.94 ± 2.02 | - | - | - |
| 15-;13-;9MeC31 | 29.644 | - | 2.76 ± 0.87 | - | 2.08 ± 1.20 | - | 2.46 ± 0.94 |
| 7MeC31 | 29.780 | - | 0.31 ± 0.27 | - | 0.16 ± 0.15 | - | - |
| 11,15diMeC31 | 29.960 | - | 0.89 ± 0.26 | - | 0.79 ± 0.26 | - | 1.54 ± 1.05 |
| 5,17diMeC31 | 30.219 | - | 1.68 ± 0.69 | - | 1.62 ± 0.55 | - | 1.65 ± 0.94 |
| n-C32 | 30.464 | 1.68 ± 1.17 | 0.33 ± 0.08 | 0.98 ± 1.25 | 0.24 ± 0.12 | - | 0.27 ± 0.11 |
| 16,15,14MeC32 | 30.795 | - | 0.79 ± 0.17 | - | 0.38 ± 0.19 | - | 0.52 ± 0.18 |
| 3,11diMeC32 | 31.109 | - | 0.71 ± 0.46 | - | - | - | 0.62 ± 0.45 |
| 3,10diMeC32 | 31.540 | - | - | - | - | - | 0.38 ± 0.49 |
| z-C33 | 31.339 | 1.00 ± 1.01 | - | - | - | 9.03 ± 0.66 | - |
| n-C33 | 31.602 | 3.07 ± 2.18 | 1.20 ± 0.40 | 1.28 ± 0.91 | 0.94 ± 0.64 | - | 1.22 ± 0.62 |
| Unknown 5 | 31.863 | - | - | 6.76 ± 4.35 | - | 38.67 ± 8.54 | - |
| 17-;15-;13MeC33 | 31.935 | - | 5.98 ± 0.68 | - | 5.38 ± 2.63 | - | 5.87 ± 1.52 |
| 15,19diMeC33 | 32.240 | - | 7.42 ± 2.64 | - | 7.77 ± 5.11 | - | 9.00 ± 3.07 |
| 5,17diMeC33 | 32.488 | - | 0.63 ± 0.21 | - | 0.54 ± 0.17 | - | 1.12 ± 0.66 |
| 15,14MeC34 | 33.043 | - | 1.00 ± 0.15 | - | 0.38 ± 0.16 | - | 0.82 ± 0.19 |
| 14,XdiMeC34 | 33.285 | - | - | - | 0.87 ± 0.42 | - | - |
| 4,XdiMeC34 | 33.313 | - | 1.58 ± 0.23 | - | - | - | 1.73 ± 0.30 |
| 3,10diMeC34 | 33.766 | - | 0.48 ± 0.23 | - | - | - | 0.75 ± 0.28 |
| Unknown 6 | 33.835 | - | 0.40 ± 0.14 | - | - | - | - |
| n-C35 | 33.988 | - | 0.13 ± 0.04 | 0.19 ± 0.33 | - | - | - |
| 17-;15MeC35 | 34.136 | - | 6.19 ± 0.85 | - | 4.00 ± 0.60 | - | 5.78 ± 0.44 |
| 15,19diMeC35 | 34.404 | - | 10.10 ± 1.82 | - | 8.12 ± 2.91 | - | 11.23 ± 1.91 |
| Unknown 7 | 34.561 | - | - | 0.14 ± 0.07 | - | - | - |
| 5,17diMeC35 | 34.663 | - | 1.42 ± 0.50 | - | 0.66 ± 0.34 | - | 2.27 ± 1.23 |
| n-C36 | 35.217 | - | - | 0.27 ± 0.29 | - | - | - |
| 13MeC36 | 35.247 | - | 0.74 ± 0.18 | - | - | - | 0.54 ± 0.20 |
| 4,17diMeC36 | 35.515 | - | 1.18 ± 0.30 | - | - | - | 0.64 ± 0.17 |
| 4,10diMeC36 | 36.049 | - | 0.32 ± 0.11 | - | - | - | - |
| n-C37 | 36.368 | - | - | 0.12 ± 0.16 | - | - | - |
| 17-,15-,13MeC37 | 36.487 | - | 4.47 ± 0.73 | - | 2.01 ± 0.45 | - | 3.79 ± 0.45 |
| 17,21diMeC37 | 36.785 | - | 7.20 ± 0.56 | - | 2.33 ± 0.69 | - | 4.71 ± 1.39 |
| Unknown 8 | 37.086 | - | 0.71 ± 0.45 | - | - | - | 0.58 ± 0.29 |
| 17-,15MeC39 | 39.538 | - | 0.25 ± 0.08 | - | - | - | - |
| Unknown 9 | 39.923 | - | 1.67 ± 0.56 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, L.D.; Ceballos-González, A.V.; Prato, A.; Cavalleri, A.; Trigo, J.R.; Nascimento, F.S.d. Chemical Camouflage Induced by Diet in a Pest Treehopper on Host Plants. Plants 2024, 13, 216. https://doi.org/10.3390/plants13020216
Lima LD, Ceballos-González AV, Prato A, Cavalleri A, Trigo JR, Nascimento FSd. Chemical Camouflage Induced by Diet in a Pest Treehopper on Host Plants. Plants. 2024; 13(2):216. https://doi.org/10.3390/plants13020216
Chicago/Turabian StyleLima, Luan Dias, Amalia Victoria Ceballos-González, Amanda Prato, Adriano Cavalleri, José Roberto Trigo, and Fábio Santos do Nascimento. 2024. "Chemical Camouflage Induced by Diet in a Pest Treehopper on Host Plants" Plants 13, no. 2: 216. https://doi.org/10.3390/plants13020216