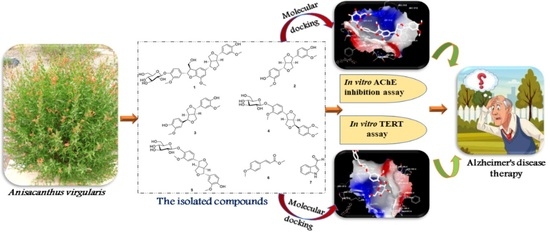
Furofuranoid-Type Lignans and Related Phenolics from Anisacanthus virgularis (Salisb.) Nees with Promising Anticholinesterase and Anti-Ageing Properties: A Study Supported by Molecular Modelling
Abstract
:1. Introduction
2. Results
2.1. Structural Elucidation
2.2. Bioactivity of the Isolated Lignans 1–5
2.2.1. Anti-AChE Activity
2.2.2. Anti-Ageing Activity
2.3. Molecular Modelling Studies
2.3.1. Molecular Docking
2.3.2. Structure–Activity Relationship Using Rapid Overlay Chemical Similarity (ROCS) and Shape Alignment
3. Discussion
- The sugar moiety at the C-4 position of the furofuranoid lignan skeleton is crucial in the compound’s potent activity provided that no HB can be formed with the oxygen atom of the hydroxyl group of C-4 of the furofuranoid skeleton;
- The HB formation of key amino acids with the hydroxyl groups at C-4 or C-4′ is better than their formation with the methoxy groups at C-3 or C-3′;
- The direct attachment of the sugar moiety at the C-4 position of the furofuranoid lignan system without a spacer (dihydrofuran moiety) is preferable;
- The ligands’ interaction with the key amino acids ARG: 24A, TYR: 77A, VAL: 340A, and PHE: 346A are crucial for the anticholinesterase activity.
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Spectroscopic Data of Isolated Compounds
- Anisacanthin (1): yellow residue; +25 (c 0.08, CH3OH); 1H and 13C NMR data (Table 1); HRESIMS m/z 721.2442 [M + Na]+ (calcd for C36H42NaO14, 721.2472).
- Spectroscopic data of the known compound 2–7: see Supplementary Materials.
4.5. Cholinesterase-Inhibitory Assay
4.6. TERT Activity Assay
4.7. Statistical Analysis
4.8. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bach Knudsen, K.E.; Nørskov, N.; Bolvig, A.K.; Hedemann, M.S.; Lærke, H.N. Lignans. In Dietary Polyphenols: Their Metabolism and Health Effects; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 365–406. [Google Scholar] [CrossRef]
- Smeds, A.I.; Eklund, P.C.; Sjöholm, R.E.; Willför, S.M.; Nishibe, S.; Deyama, T.; Holmbom, B.R. Quantification of a broad spectrum of lignans in cereals, oilseeds, and nuts. J. Agricult. Food Chem. 2007, 55, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Milder, I.E.J.; Arts, I.C.W.; Putte, B.; van de Putte, B.; Hollman, P.C.H. Lignan contents of Dutch plant foods: A database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol. Br. J. Nutr. 2005, 93, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Peñalvo, J.L.; Adlercreutz, H.; Uehara, M.; Ristimaki, A.; Watanabe, S. Lignan content of selected foods from Japan. J. Agricult. Food Chem. 2008, 56, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Kuhnle, G.G.C.; Dell’Aquila, C.; Aspinall, S.M.; Runswick, S.A.; Mulligan, A.A.; Bingham, S.A. Phytoestrogen content of foods of animal origin: Dairy products, eggs, meat, fish, and seafood, J. Agricult. Food Chem. 2008, 56, 10099–10104. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Maia, M.; Rodrigues, G.C.S.; de Sousa, N.F.; Scotti, M.T.; Scotti, L.; Mendonça-Junior, F.J.B. Identification of new targets and the virtual screening of lignans against Alzheimer’s disease. Oxid. Med. Cell. Longev. 2020, 2020, 3098673. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Wen, Y.; Liu, Z.; Zhai, J.; Li, S.; Yin, J. Advances in the roles and mechanisms of lignans against Alzheimer’s disease. Front. Pharmacol. 2022, 13, 960112. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Han, W.; Han, H.; Liu, Y.; Guan, W.; Kuang, H. Lignans from Schisandra chinensis rattan stems suppresses primary Aβ1-42-induced microglia activation via NF-κB/MAPK signaling pathway, Nat. Prod. Res. 2019, 33, 2726–2729. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Ni, C.; Song, G. Honokiol attenuates oligomeric amyloid β1-42-induced Alzheimer’s disease in mice through attenuating mitochondrial apoptosis and inhibiting the nuclear factor kappa-B signaling pathway. Cell. Physiol. Biochem. 2017, 43, 69–81. [Google Scholar] [CrossRef]
- Swarup, V.; Ghosh, J.; Mishra, M.K.; Basu, A. Novel strategy for treatment of Japanese encephalitis using arctigenin, a plant lignan. J. Antimicrob. Chemother. 2008, 61, 679–688. [Google Scholar] [CrossRef]
- Jung, Y.J.; Park, J.H.; Cho, J.G.; Seo, K.H.; Lee, D.S.; Kim, Y.C.; Kang, H.C.; Song, M.C.; Baek, N.I. Lignan and flavonoids from the stems of Zea mays and their anti-inflammatory and neuroprotective activities. Arch. Pharmacal Res. 2015, 38, 178–185. [Google Scholar] [CrossRef]
- Haam, J.; Yakel, J.L. Cholinergic modulation of the hippocampal region and memory function. J. Neurochem. 2017, 142, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Rios, M.Y.; Ocampo-Acuña, Y.D.; Ramírez-Cisneros, M.Á.; Salazar-Rios, M.E. Furofuranone lignans from Leucophyllum ambiguum. J. Nat. Prod. 2020, 83, 424–1431. [Google Scholar] [CrossRef] [PubMed]
- El-Hassan, A.; El-Sayed, M.; Hamed, A.I.; Rhee, I.K.; Ahmed, A.A.; Zeller, K.P.; Verpoorte, R. Bioactive constituents of Leptadenia arborea. Fitoterapia 2003, 74, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Kim, S.; Lee, J.; Kim, J.I.; Oh, E.; Kim, S.W.; Lee, E.; Cho, K.S.; Kim, C.S.; Lee, M.H. Lignan-Rich Sesame (Sesamum indicum L.) Cultivar Exhibits In Vitro Anti-Cholinesterase Activity, Anti-Neurotoxicity in Amyloid-β Induced SH-SY5Y Cells, and Produces an In Vivo Nootropic Effect in Scopolamine-Induced Memory Impaired Mice. Antioxidants 2023, 12, 1110. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.H.; Zhao, P.; Wang, M.; Liang, Q. Naturally occurring furofuran lignans: Structural diversity and biological activities. Nat. Prod. Res. 2019, 33, 1357–1373. [Google Scholar] [CrossRef] [PubMed]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet. 2023. Available online: http://www.plantsoftheworldonline.org (accessed on 22 December 2023).
- El-Domiaty, M.; Abdel Aal, M.; El-Shafae, A.; Abou-Hashem, M. Macro-and micromorphological study of the root, stem, leaves and inflorescence of Anisacanthus virgularis nees (Acanthaceae). ZJPS 1999, 8, 22–39. [Google Scholar] [CrossRef]
- Michael, H.N.; Salib, J.Y.; Shafik, N.H. New flavone glycosides from Anisacanthus virgularis. Egypt. J. Chem. 2007, 50, 523–529. [Google Scholar]
- Refaey, M.S.; Abdelhamid, R.A.; Orabi, M.A.A.; Ali, A.A.; Abdelhameed, R.F.A.; Mousa, E.A.; Hamano, S.; Yamada, K. A new iridoid glucoside from Anisacanthus virgularis and its antiamoebic activity. Heterocycles 2019, 98, 1229–1235. [Google Scholar] [CrossRef]
- El-Domiaty, M.M.; El-Shafae, A.M.; Abdel-Aal, M.M.; Abou-Hashem, M.M. The first report on the occurrence of furofuranoid lignan glucosides in Acanthaceae. Pharm. Biol. 2002, 40, 96–102. [Google Scholar] [CrossRef]
- Refaey, M.S.; Abdelhamid, R.A.; Elimam, H.; Elshaier, Y.A.; Ali, A.A.; Orabi, M.A. Bioactive constituents from Thunbergia erecta as potential anticholinesterase and anti-ageing agents: Experimental and in silico studies. Bioorg. Chem. 2021, 108, 104643. [Google Scholar] [CrossRef]
- Abouelela, M.E.; Orabi, M.A.; Abdelhamid, R.A.; Abdelkader, M.S.; Darwish, F.M.; Hotsumi, M.; Konno, H. Anti-Alzheimer’s flavanolignans from Ceiba pentandra aerial parts. Fitoterapia 2020, 143, 104541. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Jiang, Q.; McDermott, J.; Han, J.D.J. Aging and Alzheimer’s disease: Comparison and associations from molecular to system level. Aging Cell 2018, 17, e12802. [Google Scholar] [CrossRef] [PubMed]
- Weidling, I.W.; Swerdlow, R.H. Mitochondria in Alzheimer’s disease and their potential role in Alzheimer’s proteostasis. Exp. Neurol. 2020, 330, 113321. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Li, Z.L.; Chen, H.; Liu, X.Q.; Zhou, W.; Hua, H.M. Four new cytotoxic tetrahydrofuranoid lignans from Sinopodophyllum emodi. Planta Med. 2012, 78, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Qin, N.; Xue, J.; Li, S.; Huang, X.; Sun, J.; Xu, F.; Li, Z.; Li, D.; Hua, H. Dehydrodiconiferyl alcohol from Silybum marianum (L.) Gaertn accelerates wound healing via inactivating NF-κB pathways in macrophages. J. Pharm. Pharmacol. 2020, 72, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.R.; Chao, Y.C.; Teng, C.M.; Wu, Y.C. Chemical Constituents from Cassytha filiformis II. J. Nat. Prod. 1998, 61, 863–866. [Google Scholar] [CrossRef]
- Gréger, H.; Hofer, O. New unsymmetrically substituted tetrahydrofurofuran lignans from Artemisia absinthium: Assignment of the relative stereochemistry by lanthanide induced chemical shifts. Tetrahedron 1980, 36, 3551–3558. [Google Scholar] [CrossRef]
- Shao, S.Y.; Yang, Y.N.; Feng, Z.M.; Jiang, J.S.; Zhang, P.C. An efficient method for determining the relative configuration of furofuran lignans by 1H NMR spectroscopy. J. Nat. Prod. 2018, 81, 1023–1028. [Google Scholar] [CrossRef]
- Tshitenge, D.T.; Feineis, D.; Awale, S.; Bringmann, G. Gardenifolins A–H, scalemic neolignans from Gardenia ternifolia: Chiral resolution, configurational assignment, and cytotoxic activities against the HeLa cancer cell line. J. Nat. Prod. 2017, 80, 1604–1614. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Hisada, S.; Nishibe, S. Lignans from bark of Fraxinus mandshurica var. japonica and F. japonica. Chem. Phar. Bull. 1984, 32, 4482–4489. [Google Scholar] [CrossRef]
- Nishibe, S.; Tsukamoto, H.; Hisada, S. Effects of O-Methylation and O-Glucosylation on Carbon-13 Nuclear Magnetic Resonance Chemical Shifts of Matairesinol, (+)-Pinoresinol and (+)-Epipinoresinol. Chem. Pharm. Bull. 1984, 32, 4653–4657. [Google Scholar] [CrossRef]
- Rahman, M.M.A.; Dewick, P.M.; Jackson, D.E.; Lucas, J.A. Lignans of Forsythia intermedia. Phytochemistry 1990, 29, 1971–1980. [Google Scholar] [CrossRef]
- Dae, K.K.; Jong, P.L.; Jin, W.K.; Hee, W.P.; Jae, S.E. Antitumor and antiinflammatory constituents from Celtis sinensis. Arch. Pharm. Res. 2005, 28, 39–43. [Google Scholar] [CrossRef]
- Gao, L.; Xu, X.; Nan, H.; Yang, J.; Sun, G.; Wu, H.; Zhong, M. Isolation of cinnamic acid derivatives from the root of Rheum tanguticum Maxim. ex Balf. And its significance. J. Med. Plant Res. 2012, 6, 929–931. [Google Scholar] [CrossRef]
- El-Sawy, E.; Abo-Salem, H.; Mandour, A. 1H-Indole-3-carboxaldehyde: Synthesis and reactions. Egypt. J. Chem. 2017, 60, 723–751. [Google Scholar] [CrossRef]
- Umezawa, T. Diversity in lignan biosynthesis. Phytochem. Rev. 2003, 2, 371–390. [Google Scholar] [CrossRef]
- Suzuki, S.; Umezawa, T. Biosynthesis of lignans and norlignans. J. Wood Sci. 2007, 53, 273–284. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres Jr, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Holohan, B.; Wright, W.E.; Shay, J.W. Telomeropathies: An emerging spectrum disorder. J. Cell Biol. 2012, 205, 289–299. [Google Scholar] [CrossRef]
- Bernardes de Jesus, B.; Vera, E.; Schneeberger, K.; Tejera, A.M.; Ayuso, E.; Bosch, F.; Blasco, M.A. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol. Med. 2012, 4, 691–704. [Google Scholar] [CrossRef] [PubMed]
- González-Suárez, E.; Geserick, C.; Flores, J.M.; Blasco, M.A. Antagonistic effects of telomerase on cancer and aging in K5-mTert transgenic mice. Oncogene 2005, 24, 2256–2270. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Loba, A.; Flores, I.; Fernández-Marcos, P.J.; Cayuela, M.L.; Maraver, A.; Tejera, A.; Borrás, C.; Matheu, A.; Klatt, P.; Flores, J.M.; et al. Telomerase Reverse Transcriptase Delays Aging in Cancer-Resistant Mice. Cell 2008, 135, 609–622. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Timchenko, N.; Suwa, T.; Hornsby, P.J.; Campisi, J.; Medrano, E.E. The human melanocyte: A model system to study the complexity of cellular aging and transformation in non-fibroblastic cells. Exp. Gerontol. 2001, 36, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Bielak-Zmijewska, A.; Grabowska, W.; Ciolko, A.; Bojko, A.; Mosieniak, G.; Bijoch, Ł.; Sikora, E. The Role of Curcumin in the Modulation of Ageing. Int. J. Mol. Sci. 2019, 20, 1239. [Google Scholar] [CrossRef] [PubMed]
- De Boer, D.; Nguyen, N.; Mao, J.; Moore, J.; Sorin, E.J. A comprehensive review of cholinesterase modeling and simulation. Biomolecules 2021, 11, 580. [Google Scholar] [CrossRef]
- Nachon, F.; Carletti, E.; Ronco, C.; Trovaslet, M.; Nicolet, Y.; Jean, L.; Renard, P.Y. Crystal structures of human cholinesterases in complex with huprine W and tacrine: Elements of specificity for anti-Alzheimer’s drugs targeting acetyl- and butyryl-cholinesterase. Biochem. J. 2013, 453, 393–399. [Google Scholar] [CrossRef]
- Kearnes, S.; Pande, V. ROCS-derived features for virtual screening. J. Comput. Aided Mol. Des. 2016, 30, 609–617. [Google Scholar] [CrossRef]
- Halls, S.C.; Davin, L.B.; Kramer, D.M.; Lewis, N.G. Kinetic study of coniferyl alcohol radical binding to the (+)-pinoresinol forming dirigent protein. Biochemistry 2004, 43, 2587–2595. [Google Scholar] [CrossRef]
- Albertson, A.K.; Lumb, J.P. A bio-inspired total synthesis of tetrahydrofuran lignans. Angew. Chem. Int. Ed. 2015, 54, 2204–2208. [Google Scholar] [CrossRef]
- Yu, J.; Kwon, H.; Cho, E.; Jeon, J.; Kang, R.H.; Youn, K.; Jun, M.; Lee, Y.C.; Ryu, J.H.; Kim, D.H. The effects of pinoresinol on cholinergic dysfunction-induced memory impairments and synaptic plasticity in mice. Food Chem. Toxicol. 2019, 125, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Köse, L.P.; Gulcin, I. Inhibition effects of some lignans on carbonic anhydrase, acetylcholinesterase and butyrylcholinesterase enzymes. Rec. Nat. Prod. 2017, 11, 558–561. [Google Scholar] [CrossRef]
- Su, S.; Wink, M. Natural lignans from Arctium lappa as antiaging agents in Caenorhabditis elegans. Phytochemistry 2015, 117, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Munsch, T.; Lanoue, A.; Garros, L.; Tungmunnithum, D.; Messaili, S.; Destandau, E.; Billet, K.; St-Pierre, B.; Clastre, M.; et al. UPLC-HRMS analysis revealed the differential accumulation of antioxidant and anti-aging lignans and neolignans in in vitro cultures of Linum usitatissimum L. Front. Plant Sci. 2020, 11, 508658. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, S.; Perumal, P.; Palayan, M. In-vitro Screening for acetylcholinesterase enzyme inhibition potential and antioxidant activity of extracts of Ipomoea aquatica Forsk: Therapeutic lead for Alzheimer’s disease. J. Appl. Pharm. Sci. 2015, 5, 012–016. [Google Scholar] [CrossRef]
- Saber, F.R.; Mohsen, E.; El-Hawary, S.; Eltanany, B.M.; Elimam, H.; Sobeh, M.; Elmotayam, A.K. Chemometric-enhanced metabolic profiling of five Pinus species using HPLC-MS/MS spectrometry: Correlation to in vitro anti-aging, anti-Alzheimer and antidiabetic activities. J. Chromatogr. B. 2021, 1177, 122759. [Google Scholar] [CrossRef]
- v. FRED. OpenEye Scientific Software. 2006. Available online: http://www.eyesopen.com (accessed on 1 May 2023).
- v. OMEGA. OpenEye Scientific Software, Santa Fe, NM (USA). Available online: http://www.eyesopen.com (accessed on 1 May 2023).
- v. VIDA. OpenEye Scientific Software, Santa Fe, NM (USA). Available online: http://www.eyesopen.com (accessed on 1 May 2023).
- Gutierrez-Lugo, M.T.; Woldemichael, G.M.; Singh, M.P.; Suarez, P.A.; Maiese, W.M.; Montenegro, G.; Timmermann, B.N. Isolation of three new naturally occurring compounds from the culture of Micromonospora sp. P1068. Nat. Prod. Res. 2006, 19, 645–652. [Google Scholar] [CrossRef]

 ) and HMBC (H
) and HMBC (H C) correlations of compound 1.
C) correlations of compound 1.





| Position | δH in ppm, Multiplicity (J in Hz) | δC in ppm |
|---|---|---|
| 1 | 136.1 | |
| 2 | 6.95, d (J = 1.5) | 110.9 |
| 3 | 149.1 | |
| 4 | 147.3 | |
| 5 | 6.77, d (J = 8.0) | 116 |
| 6 | 6.92, dd (J = 8.0, 2.0) | 119.3 |
| 7 | 4.74 a, d (J = 4.5) | 87.6 |
| 8 | 3.17 b, m | 55.6 |
| 9 | 4.23 c, ddd (J = 11.5, 7.0, 2.0) 3.85 * (ov)d | 72.7 |
| 1′ | - | 133.7 |
| 2′ | 6.92, brs. | 112.1 |
| 3′ | 145.5 | |
| 4′ | 149 | |
| 5′ | 129.9 | |
| 6′ | 6.90, brs. | 116 |
| 7′ | 4.72 a, d (J = 4.5) | 87.4 |
| 8′ | 3.15 b, m | 55.3 |
| 9′ | 4.25 c, ddd (J = 11.5, 7.0, 2.0)3.84 * (ov)d | 72.5 |
| 1″ | - | 138.1 |
| 2″ | 7.03, d (J = 1.6) | 111.1 |
| 3″ | - | 150.9 |
| 4″ | - | 147.6 |
| 5″ | 7.14, d (J = 8.5) | 118 |
| 6″ | 6.82, dd (J = 8.5, 1.5) | 120 |
| 7″ | 5.58, d (J = 5.5) | 88.6 |
| 8″ | 3.48, m | 55.6 |
| 9″ | 3.78, dd (J = 11.0, 7.0) 3.85 * (ov)d | 64.9 |
| 1‴ | 4.89, d (J = 7.5) | 102.7 |
| 2‴ | 3.47, dd (J = 10, 7.5) | 74.9 |
| 3‴ | 3.39, t (J = 10) | 78.2 |
| 4‴ | 3.39, m | 71.3 |
| 5‴ | 3.45, m | 77.9 |
| 6‴ | 3.68, ddd (J = 12.0, 5.5,1.5) 3.87 * (ov) | 62.4 |
| 3′-OCH3 | 3.85, s | 56.4 |
| 3″-OCH3 | 3.83, s | 56.7 |
| 3-OCH3 | 3.88, s | 56.8 |
| Compounds | Concentrations (µM) | IC50 (nM) a | |||
|---|---|---|---|---|---|
| % Inhibition | |||||
| 0.01 | 0.1 | 1 | 10 | ||
| 1 | 24 ± 1 | 61 ± 1 | 75 ± 2 | 83 ± 2 | 85 ± 4 |
| 2 | 26 ± 1 | 41 ± 1 | 60 ± 3 | 75 ± 2 | 292 ± 9 |
| 3 | 23 ± 1 | 44 ± 1 | 60 ± 2 | 82 ± 3 | 242 ± 9 |
| 4 | 26 ± 3 | 41 ± 2 | 64 ± 2 | 73 ± 2 | 279 ± 11 |
| 5 | 28 ± 3 | 59 ± 4 | 79 ± 1 | 88 ± 3 | 64 ± 3 |
| Donepezil | 34 ± 3 | 69 ± 3 | 79 ± 2 | 89 ± 2 | 31 ± 3 |
| Compound | TERT Concentration Mean ± SD in ng/mL | Relative Increase |
|---|---|---|
| 1 | 2.8 ± 0.04 | 1.64 |
| 2 | 2.2 ± 0.03 | 1.28 |
| 3 | 2.0 ± 0.06 | 1.18 |
| 4 | 2.9 ± 0.06 | 1.72 |
| 5 | 2.8 ± 0.09 | 1.66 |
| Curcumin | 2.8 ± 0.03 | 1.62 |
| HFB4 (control) | 1.78 ± 0.07 | 1 |
| Compound Name | Tanimoto Combo | Shape Tanimoto | Colour Tanimoto |
|---|---|---|---|
| Donepezil | 2.00 | 1.00 | 1.00 |
| 2 | 0.93 | 0.71 | 0.23 |
| 5 | 0.87 | 0.74 | 0.14 |
| 3 | 0.82 | 0.21 | 0.62 |
| Isomer of 1 | 0.80 | 0.66 | 0.14 |
| 4 | 0.79 | 0.60 | 0.18 |
| 1 | 0.61 | 0.49 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orabi, M.A.A.; Abdelhamid, R.A.; Elimam, H.; Elshaier, Y.A.M.M.; Ali, A.A.; Aldabaan, N.; Alhasaniah, A.H.; Refaey, M.S. Furofuranoid-Type Lignans and Related Phenolics from Anisacanthus virgularis (Salisb.) Nees with Promising Anticholinesterase and Anti-Ageing Properties: A Study Supported by Molecular Modelling. Plants 2024, 13, 150. https://doi.org/10.3390/plants13020150
Orabi MAA, Abdelhamid RA, Elimam H, Elshaier YAMM, Ali AA, Aldabaan N, Alhasaniah AH, Refaey MS. Furofuranoid-Type Lignans and Related Phenolics from Anisacanthus virgularis (Salisb.) Nees with Promising Anticholinesterase and Anti-Ageing Properties: A Study Supported by Molecular Modelling. Plants. 2024; 13(2):150. https://doi.org/10.3390/plants13020150
Chicago/Turabian StyleOrabi, Mohamed A. A., Reda A. Abdelhamid, Hanan Elimam, Yaseen A. M. M. Elshaier, Ahmed A. Ali, Nayef Aldabaan, Abdulaziz Hassan Alhasaniah, and Mohamed S. Refaey. 2024. "Furofuranoid-Type Lignans and Related Phenolics from Anisacanthus virgularis (Salisb.) Nees with Promising Anticholinesterase and Anti-Ageing Properties: A Study Supported by Molecular Modelling" Plants 13, no. 2: 150. https://doi.org/10.3390/plants13020150