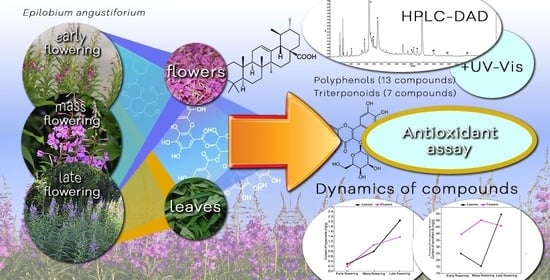
Phenological Variations in the Content of Polyphenols and Triterpenoids in Epilobium angustifolium Herb Originating from Ukraine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Variation in the Total Content of Phenolic Compounds
2.2. HPLC Metabolite Profiling
2.3. The Quantification of Core Metabolites
2.3.1. Phenolic Acids
2.3.2. Flavonoids
2.3.3. Tannins
2.3.4. Triterpenoids
2.4. Validation of the HPLC Method
2.5. Chemical Antioxidant Profile
2.6. Effects of the Flowering Phase on the Content of Metabolites
2.6.1. Quantitative Dynamics of Polyphenols
2.6.2. Quantitative Dynamics of Triterpenoids
3. Discussion
4. Materials and Methods
4.1. Plant Collection and Authentication
4.2. Chemicals and Solvents
4.3. Preparation of Extracts
4.4. Standard Solutions
4.5. Determination of Total Phenol Content
4.6. HPLC Chromatographic Analysis
4.7. Validation of the Analytical Method
4.8. Antioxidant Profile
4.8.1. ABTS Radical Scavenging Assay
4.8.2. FRAP Radical Scavenging Effects Assay
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Metabolite | Factor | Sum of Squares | df | Mean Square | F | p |
|---|---|---|---|---|---|---|
| Gallic acid | Plant part | 0.0544 | 1 | 0.0544 | 72.60 | <0.001 |
| Phase | 0.0129 | 2 | 0.0064 | 8.58 | 0.005 | |
| Interaction | 0.1303 | 2 | 0.0065 | 86.87 | <0.001 | |
| Within | 0.0090 | 12 | 0.0001 | |||
| Total | 0.0266 | 17 | ||||
| Neochlorogenic acid | Plant part | 0.0660 | 1 | 0.0660 | 56.04 | <0.001 |
| Phase | 1.3073 | 2 | 0.6536 | 555.00 | <0.001 | |
| Interaction | 2.7751 | 2 | 1.3876 | 1178.00 | <0.001 | |
| Within | 0.0141 | 12 | 0.0012 | |||
| Total | 4.1626 | 17 | ||||
| Oenothein B | Plant part | 831.95 | 1 | 831.95 | 11,330.00 | <0.001 |
| Phase | 1258.79 | 2 | 629.39 | 8571.00 | <0.001 | |
| Interaction | 1426.10 | 2 | 713.05 | 9710.00 | <0.001 | |
| Within | 0.88 | 12 | 0.07 | |||
| Total | 3517.72 | 17 | ||||
| Oenothein A | Plant part | 9.6478 | 1 | 9.6478 | 3177.00 | <0.001 |
| Phase | 50.7050 | 2 | 25.3525 | 8348.00 | <0.001 | |
| Interaction | 61.8870 | 2 | 30.9435 | 10,190.00 | <0.001 | |
| Within | 0.0364 | 12 | 0.0030 | |||
| Total | 122.276 | 17 | ||||
| Chlorogenic acid | Plant part | 0.5940 | 1 | 0.5940 | 136.20 | <0.001 |
| Phase | 2.4310 | 2 | 1.2155 | 278.70 | <0.001 | |
| Interaction | 2.2987 | 2 | 1.1493 | 263.50 | <0.001 | |
| Within | 0.0523 | 12 | 0.0044 | |||
| Total | 5.3761 | 17 | ||||
| Ellagic acid | Plant part | 0.1166 | 1 | 0.1166 | 44.44 | <0.001 |
| Phase | 0.0388 | 2 | 0.0194 | 7.40 | 0.008 | |
| Interaction | 0.0393 | 2 | 0.0197 | 7.50 | 0.007 | |
| Within | 0.0315 | 12 | 0.0026 | |||
| Total | 0.2263 | 17 | ||||
| Rutin | Plant part | 0.0390 | 1 | 0.0390 | 21.81 | <0.001 |
| Phase | 10.0197 | 2 | 5.0098 | 2800.00 | <0.001 | |
| Interaction | 0.8145 | 2 | 0.4072 | 227.60 | <0.001 | |
| Within | 0.0215 | 12 | 0.0018 | |||
| Total | 10.8947 | 17 | ||||
| Hyperoside | Plant part | 0.1262 | 1 | 0.1262 | 99.06 | <0.001 |
| Phase | 6.4952 | 2 | 3.2476 | 2550.00 | <0.001 | |
| Interaction | 0.6669 | 2 | 0.3335 | 261.80 | <0.001 | |
| Within | 0.0153 | 12 | 0.0013 | |||
| Total | 7.3035 | 17 | ||||
| Isoquercitrin | Plant part | 3.7174 | 1 | 3.7174 | 483.10 | <0.001 |
| Phase | 46.9873 | 2 | 23.4937 | 3053.00 | <0.001 | |
| Interaction | 39.1820 | 2 | 19.5910 | 2546.00 | <0.001 | |
| Within | 0.0923 | 12 | 0.0077 | |||
| Total | 89.9790 | 17 | ||||
| Quercitrin | Plant part | 10.3983 | 1 | 10.3983 | 5658.00 | <0.001 |
| Phase | 11.5140 | 2 | 5.7570 | 3132.00 | <0.001 | |
| Interaction | 5.2254 | 2 | 2.6127 | 1422.00 | <0.001 | |
| Within | 0.0220 | 12 | 0.0018 | |||
| Total | 27.1597 | 17 | ||||
| Quercetin | Plant part | 0.0000 | 1 | 0.0000 | 0.9134 | |
| Phase | 0.0057 | 2 | 0.0028 | <0.001 | ||
| Interaction | 0.0026 | 2 | 0.0013 | <0.001 | ||
| Within | 0.0008 | 12 | 0.0007 | |||
| Total | 0.0091 | 17 | ||||
| Afzelin | Plant part | 49.6008 | 1 | 49.6008 | 54,770.00 | <0.001 |
| Phase | 25.2428 | 2 | 12.6214 | 13,940.00 | <0.001 | |
| Interaction | 25.7776 | 2 | 12.8888 | 14,230.00 | <0.001 | |
| Within | 0.0109 | 12 | 0.0009 | |||
| Total | 100.6320 | 17 | ||||
| Guaijaverin | Plant part | 0.2594 | 1 | 0.2594 | 170.20 | <0.001 |
| Phase | 5.1129 | 2 | 2.5564 | 1677.00 | <0.001 | |
| Interaction | 0.9601 | 2 | 0.4800 | 314.90 | <0.001 | |
| Within | 0.0183 | 12 | 0.0015 | |||
| Total | 6.3507 | 17 | ||||
| All phenolic compounds | Plant part | 1503.35 | 1 | 1503.35 | 25,270.00 | <0.001 |
| Phase | 4119.62 | 2 | 2059.81 | 34,630.00 | <0.001 | |
| Interaction | 2452.70 | 2 | 1226.35 | 20,610.00 | <0.001 | |
| Within | 0.7139 | 12 | 0.0595 | |||
| Total | 8076.38 | 17 |
| Metabolite | Factor | Sum of Squares | df | Mean Square | F | p |
|---|---|---|---|---|---|---|
| Maslinic acid | Plant part | 0.0004 | 1 | 0.0004 | 0.8889 | 0.3644 |
| Phase | 0.2103 | 2 | 0.1051 | 262.80 | <0.001 | |
| Interaction | 0.0854 | 2 | 0.0427 | 106.80 | <0.001 | |
| Within | 0.0048 | 12 | 0.0004 | |||
| Total | 0.3008 | 17 | ||||
| Corosolic acid | Plant part | 0.6013 | 1 | 0.6013 | 339.30 | <0.001 |
| Phase | 6.4816 | 2 | 3.2408 | 1829.00 | <0.001 | |
| Interaction | 1.0422 | 2 | 0.5211 | 294.00 | <0.001 | |
| Within | 0.0213 | 12 | 0.0018 | |||
| Total | 8.1464 | 17 | ||||
| Oleanolic acid | Plant part | 0.0280 | 1 | 0.0280 | 42.36 | <0.001 |
| Phase | 0.5697 | 2 | 0.2849 | 430.90 | <0.001 | |
| Interaction | 0.1934 | 2 | 0.0967 | 146.30 | <0.001 | |
| Within | 0.0079 | 12 | 0.0007 | |||
| Total | 0.7990 | 17 | ||||
| Ursolic acid | Plant part | 12.8863 | 1 | 12.8863 | 27,950.00 | <0.001 |
| Phase | 0.6752 | 2 | 0.3376 | 732.20 | <0.001 | |
| Interaction | 1.5075 | 2 | 0.7538 | 1635.00 | <0.001 | |
| Within | 0.0055 | 12 | 0.0005 | |||
| Total | 15.0746 | 17 | ||||
| Betulin | Plant part | 0.0533 | 1 | 0.0533 | 96.04 | <0.001 |
| Phase | 0.0588 | 2 | 0.0294 | 52.92 | <0.001 | |
| Interaction | 0.0782 | 2 | 0.0391 | 70.36 | <0.001 | |
| Within | 0.0067 | 12 | 0.0005 | |||
| Total | 0.1970 | 17 | ||||
| Erythrodiol | Plant part | 0.0047 | 1 | 0.0047 | 2.59 | 0.1337 |
| Phase | 0.0053 | 2 | 0.0027 | 1.48 | 0.2664 | |
| Interaction | 0.0053 | 2 | 0.0027 | 1.48 | 0.2664 | |
| Within | 0.0217 | 12 | 0.0018 | |||
| Total | 0.0370 | 17 | ||||
| Uvaol | Plant part | 0.0002 | 1 | 0.0002 | 9.24 | 0.0102 |
| Phase | 0.0004 | 2 | 0.0002 | 9.24 | 0.0037 | |
| Interaction | 0.0004 | 2 | 0.0002 | 9.24 | 0.0037 | |
| Within | 0.0002 | 12 | 0.0000 | |||
| Total | 0.0011 | 17 | ||||
| All triterpenoids | Plant part | 19.0550 | 1 | 19.0550 | 3076.00 | <0.001 |
| Phase | 13.6317 | 2 | 6.8158 | 1100.00 | <0.001 | |
| Interaction | 0.0261 | 2 | 0.0131 | 2.11 | 0.1639 | |
| Within | 0.0743 | 12 | 0.0062 | |||
| Total | 32.7872 | 17 |
References
- Stace, C. New Flora of the British Isles, 4th ed.; C&M Floristics: Iver, UK, 2019; p. 1266. [Google Scholar]
- Wagner, W.L.; Hoch, P.C.; Raven, P.H. Revised classification of the Onagraceae. Syst. Bot. Monogr. 2007, 83, 1–240. [Google Scholar]
- Sõukand, R.; Pieroni, A.; Biró, M.; Dénes, A.; Dogan, Y.; Hajdari, A.; Kalle, R.; Reade, B.; Mustafa, B.; Nedelcheva, A.; et al. An ethnobotanical perspective on traditional fermented plant foods and beverages in Eastern Europe. J. Ethnopharmacol. 2015, 170, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, S.; Süntar, I.; Yakinci, O.F.; Sytar, O.; Ceribasi, S.; Dursunoglu, B.; Ozbek, H.; Guvenalp, Z. In vivo bioactivity assessment on Epilobium species: A particular focus on Epilobium angustifolium and its components on enzymes connected with the healing process. J. Ethnopharmacol. 2020, 262, 113207–113220. [Google Scholar] [CrossRef]
- Granica, S.; Piwowarski, J.P.; Czerwińska, M.E.; Kiss, A.K. Phytochemistry, pharmacology and traditional uses of different Epilobium species (Onagraceae): A review. J. Ethnopharmacol. 2014, 156, 316–346. [Google Scholar] [CrossRef] [PubMed]
- Uminska, K.; Gudžinskas, Z.; Ivanauskas, L.; Georgiyants, V.; Kozurak, A.; Skibytska, M.; Mykhailenko, O. Amino acid profiling in wild Chamaenerion angustifolium populations applying chemometric analysis. J. Appl. Pharm. Sci. 2023, 13, 171–180. [Google Scholar] [CrossRef]
- Adamczak, A.; Dreger, M.; Seidler-Łożykowska, K.; Wielgus, K. Fireweed (Epilobium angustifolium L.): Botany, phytochemistry and traditional uses. A review. Herba Pol. 2019, 65, 51–63. [Google Scholar] [CrossRef]
- Slobodianiuk, L.; Budniak, L.; Feshchenko, H.; Sverstiuk, A.; Palaniza, Y. Quantitative analysis of fatty acids and monosaccharides composition in Chamerion angustifolium L. by GC/MS method. Pharmacia 2022, 69, 167–174. [Google Scholar] [CrossRef]
- Granica, S.; Bazylko, A.; Kiss, A.K. Determination of macrocyclic ellagitannin oenothein B in plant materials by HPLC-DAD-MS: Method development and validation. Phytochem. Anal. 2012, 23, 582–587. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Petrikaite, V.; Marksa, M.; Ivanauskas, L.; Jakstas, V.; Raudone, L. Fractionation and characterization of triterpenoids from Vaccinium vitis-idaea L. cuticular waxes and their potential as anticancer agents. Antioxidants 2023, 12, 465. [Google Scholar] [CrossRef]
- Piwowarski, J.P.; Bobrowska-Korczak, B.; Stanisławska, I.; Bielecki, W.; Wrzesien, R.; Granica, S.; Krupa, K.; Kiss, A.K. Evaluation of the effect of Epilobium angustifolium aqueous extract on LNCaP cell proliferation in in vitro and in vivo models. Planta Med. 2017, 83, 1159–1168. [Google Scholar] [CrossRef]
- Vitalone, A.; Allkanjari, O. Epilobium spp.: Pharmacology and phytochemistry. Phytother. Res. 2018, 32, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zong, W.; Tao, X.; Liu, S.; Feng, Z.; Lin, Y.; Liao, Z.; Chen, M. Evaluation of the therapeutic effect against benign prostatic hyperplasia and the active constituents from Epilobium angustifolium L. J. Ethnopharmacol. 2019, 232, 1–10. [Google Scholar] [CrossRef] [PubMed]
- EMA/HMPC/712510/2014; Assessment Report on Epilobium angustifolium L. and/or Epilobium parviflorum Schreb., Herba. European Medicines Agency: London, UK, 2016; p. 20.
- Deng, L.Q.; Zhou, S.Y.; Mao, J.X.; Liu, S.; Lan, X.Z.; Liao, Z.H.; Chen, M. HPLC-ESI-MS/MS analysis of phenolics and in vitro antioxidant activity of Epilobium angustifolium L. Nat. Prod. Res. 2018, 32, 1432–1435. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Ramstead, A.G.; Kirpotina, L.N.; Voyich, J.M.; Jutila, M.A.; Quinn, M.T. Therapeutic potential of polyphenols from Epilobium angustifolium (Fireweed). Phytother. Res. 2016, 30, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; Santarcangelo, C.; Masselli, R.; Buonomo, G.; Nicotra, G.; Insolia, V.; D’Avino, M.; Caruso, G.; Buonomo, A.R.; Sacchi, R.; et al. Epilobium angustifolium L. extract with high content in oenothein B on benign prostatic hyperplasia: A monocentric, randomized, double-blind, placebo-controlled clinical trial. Biomed. Pharmacother. 2021, 138, 111414. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zidorn, C. Seasonal variations of natural products in European herbs. Phytochem. Rev. 2022, 21, 1549–1575. [Google Scholar] [CrossRef]
- Mykhailenko, O.; Ivanauskas, L.; Bezruk, I.; Petrikaitė, V.; Georgiyants, V. Application of Quality by Designapproach to the pharmaceutical development of anticancer crude extracts of Crocus sativus perianth. Sci. Pharm. 2022, 90, 19. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R. Phenolic compounds from new natural sources-plant genotype and ontogenetic variation. Molecules 2023, 28, 1731. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Ștefănescu, D.; Stoenescu, A.M. Variability of phenological behaviours of wild fruit tree species based on discriminant analysis. Plants 2021, 11, 45. [Google Scholar] [CrossRef]
- Schröder, W.; Schmidt, G.; Schönrock, S. Modelling and mapping of plant phenological stages as bio-meteorological indicators for climate change. Environ. Sci. Eur. 2014, 26, 5. [Google Scholar] [CrossRef]
- Mykhailenko, O.; Saidov, N.B.; Ivanauskas, L.; Georgiyants, V. Model implementation of the legal regulation on medicinal plant cultivation for pharmaceutical purposes. Case study of Crocus sativus cultivation in Ukraine. Botanica 2022, 28, 27–38. [Google Scholar] [CrossRef]
- Iversen, M.; Fauchald, P.; Langeland, K.; Ims, R.A.; Yoccoz, N.G.; Bråthen, K.A. Phenology and cover of plant growth forms predict herbivore habitat selection in a high latitude ecosystem. PLoS ONE 2014, 9, e100780. [Google Scholar] [CrossRef] [PubMed]
- de Albuquerque Ugoline, B.C.; de Souza, J.; Ferrari, F.C.; Ferraz-Filha, Z.S.; Coelho, G.B.; Saúde-Guimarães, D.A. The influence of seasonality on the content of goyazensolide and on anti-inflammatory and anti-hyperuricemic effects of the ethanolic extract of Lychnophora passerina (Brazilian arnica). J. Ethnopharmacol. 2017, 198, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Kazlauskaite, J.A.; Ivanauskas, L.; Marksa, M.; Bernatoniene, J. The effect of traditional and cyclodextrin-assisted extraction methods on Trifolium pratense L. (Red Clover) extracts antioxidant potential. Antioxidants 2022, 11, 435. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M.; et al. Polyphenols in metabolic diseases. Molecules 2022, 27, 6280. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.; Fuentes, E.; Ávila, F.; Alarcón, M.; Palomo, I. Roles of phenolic compounds in the reduction of risk factors of cardiovascular diseases. Molecules 2019, 24, 366. [Google Scholar] [CrossRef] [PubMed]
- Jürgenson, S.; Matto, V.; Raal, A. Vegetational variation of phenolic compounds in Epilobium angustifolium. Nat. Prod. Res. 2012, 26, 1951–1953. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, Z. Accumulation and dynamic trends of triterpenoid saponin in vegetative organs of Achyranthus bidentata. J. Integr. Plant Biol. 2009, 51, 122–129. [Google Scholar] [CrossRef]
- Agnieszka, G.; Mariola, D.; Anna, P.; Piotr, K.; Natalia, W.; Aneta, S.; Marcin, O.; Bogna, O.; Zdzisław, Ł.; Aurelia, P.; et al. Qualitative and quantitative analyses of bioactive compounds from ex vitro Chamaenerion angustifolium (L.) (Epilobium augustifolium) herb in different harvest times. Ind. Crops Prod. 2018, 123, 208–220. [Google Scholar] [CrossRef]
- Dreger, M.; Seidler-Łożykowska, K.; Szalata, M.; Adamczak, A.; Wielgus, K. Phytochemical variability during vegetation of Chamerion angustifolium (L.) Holub genotypes derived from in vitro cultures. Plant Cell Tissue Organ Cult. 2021, 147, 619–633. [Google Scholar] [CrossRef]
- Kowalik, K.; Polak-Berecka, M.; Prendecka-Wróbel, M.; Pigoń-Zając, D.; Niedźwiedź, I.; Szwajgier, D.; Baranowska-Wójcik, E.; Waśko, A. Biological activity of an Epilobium angustifolium L. (Fireweed) infusion after in vitro digestion. Molecules 2022, 27, 1006. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Zagórska-Dziok, M.; Ossowicz-Rupniewska, P.; Makuch, E.; Duchnik, W.; Kucharski, Ł.; Adamiak-Giera, U.; Prowans, P.; Czapla, N.; Bargiel, P.; et al. Epilobium angustifolium L. extracts as valuable ingredients in cosmetic and dermatological products. Molecules 2021, 26, 3456. [Google Scholar] [CrossRef] [PubMed]
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Křen, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Sobreiro, M.A.; Della Torre, A.; de Araújo, M.E.; Canella, P.R.; de Carvalho, J.E.; Carvalho, P.D.; Ruiz, A.L. Enzymatic hydrolysis of rutin: Evaluation of kinetic parameters and anti-proliferative, mutagenic and anti-mutagenic effects. Life 2023, 13, 549. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Chen, A.; Zhao, L.; Cao, F.; Ding, G.; Xiao, W. One-pot synthesis of hyperoside by a three-enzyme cascade using a UDP-galactose regeneration system. J. Agric. Food Chem. 2017, 65, 6042–6048. [Google Scholar] [CrossRef] [PubMed]
- Al Zahrani, N.A.; El-Shishtawy, R.M.; Asiri, A.M. Recent developments of gallic acid derivatives and their hybrids in medicinal chemistry: A review. Eur. J. Med. Chem. 2020, 204, 112609. [Google Scholar] [CrossRef] [PubMed]
- Ishikura, N.; Hayashida, S.; Tazaki, K. Biosynthesis of gallic and ellagic acids with14C-labeled compounds in Acer and Rhus leaves. Bot. Mag. Shokubutsu-Gaku-Zasshi 1984, 97, 355–367. [Google Scholar] [CrossRef]
- de la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Chapter 12—Phenolic Compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Woodhead Publishing: Sawston, UK, 2019; Charpter 12; pp. 253–271. [Google Scholar]
- Salminen, J.P.; Roslin, T.; Karonen, M.; Sinkkonen, J.; Pihlaja, K.; Pulkkinen, P. Seasonal variation in the content of hydrolyzable tannins, flavonoid glycosides, and proanthocyanidins in oak leaves. J. Chem. Ecol. 2004, 30, 1693–1711. [Google Scholar] [CrossRef]
- Ono, N.N.; Qin, X.; Wilson, A.E.; Li, G.; Tian, L. Two UGT84 family Glycosyltransferasescatalyze a critical reaction of hydrolyzable tannin biosynthesis in pomegranate (Punica granatum). PLoS ONE 2016, 11, e0156319. [Google Scholar] [CrossRef]
- Yang, X.; Tomás-Barberán, F.A. Tea is a significantdietary source of ellagitannins and ellagic acid. J. Agric. Food Chem. 2019, 67, 5394–5404. [Google Scholar] [CrossRef]
- Gudoityte, E.; Arandarcikaite, O.; Mazeikiene, I.; Bendokas, V.; Liobikas, J. Ursolic and oleanolic acids: Plant metabolites with neuroprotective potential. Int. J. Mol. Sci. 2021, 22, 4599. [Google Scholar] [CrossRef]
- Geană, E.I.; Ciucure, C.T.; Ionete, R.E.; Ciocârlan, A.; Aricu, A.; Ficai, A.; Andronescu, E. Profiling of phenolic compounds and triterpene acids of twelve apple (Malus domestica Borkh.) cultivars. Foods 2021, 10, 267. [Google Scholar] [CrossRef]
- Yeo, H.J.; Park, C.H.; Park, Y.E.; Hyeon, H.; Kim, J.K.; Lee, S.Y.; Park, S.U. Metabolic profiling and antioxidant activity during flower development in Agastache rugosa. Physiol. Mol. Biol. Plants 2021, 27, 445–455. [Google Scholar] [CrossRef]
- Chrétien, L.T.S.; Khalil, A.; Gershenzon, J.; Lucas-Barbosa, D.; Dicke, M.; Giron, D. Plant metabolism and defence strategies in the flowering stage: Time-dependent responses of leaves and flowers under attack. Plant Cell Environ. 2022, 45, 2841–2855. [Google Scholar] [CrossRef]
- Takibayeva, A.T.; Zhumabayeva, G.K.; Bakibaev, A.A.; Demets, O.V.; Lyapunova, M.V.; Mamaeva, E.A.; Yerkassov, R.S.; Kassenov, R.Z.; Ibrayev, M.K. Methods of analysis and identification of betulin and its derivatives. Molecules 2023, 28, 5946. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- The World Flora Online. The International Plant Names Index and World Checklist of Vascular Plants. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:92411-2 (accessed on 15 November 2023).
- Shuber, P. Features of climate dynamics in the Alpine belt of the Ukrainian Carpathians in the second half of the 20th and early 21st centuries. Visnyk Lviv. Univ. Ser. Geogr. 2014, 48, 187–193. (In Ukrainian) [Google Scholar] [CrossRef]
- Volochai, V.; Denesenko, V.; Chabovska, O.; Klimovich, N.; Kozurak, A.; Skibitska, M.; Gurenko, N.; Chetvernia, S.; Skliar, V.; Mykhailenko, O. The study of growth conditions of Epilobium angustifolium L. (Onagraceae Juss.) in natural populations on the territory of Ukraine. Agroecol. J. 2020, 1, 166–172. [Google Scholar]
- Fedoronchuk, M.M.; Klimovych, N.B. Uchast vydiv rodu Epilobium (Onagraceae) u biotopakh Ukrainy [Participation of species of the genus Epilobium (Onagraceae) in the biotopes of Ukraine]. Chornomors’k. Bot. Z. 2020, 16, 55–61. (In Ukrainian) [Google Scholar]
- Bernatoniene, J.; Cizauskaite, U.; Ivanauskas, L.; Jakstas, V.; Kalveniene, Z.; Kopustinskiene, D.M. Novel approaches to optimize extraction processes of ursolic, oleanolic and rosmarinic acids from Rosmarinus officinalis leaves. Ind. Crops Prod. 2016, 84, 72–79. [Google Scholar] [CrossRef]
- Stabrauskiene, J.; Marksa, M.; Ivanauskas, L.; Viskelis, P.; Viskelis, J.; Bernatoniene, J. Citrus paradisi L. fruit waste: The impact of eco-friendly extraction techniques on the phytochemical and antioxidant potential. Nutrients 2023, 15, 1276. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]





| Indicator | Early Flowering | Mass Flowering | Late Flowering | ||||
|---|---|---|---|---|---|---|---|
| Flowers | Leaves | Flowers | Leaves | Rhizomes | Flowers | Leaves | |
| Total amount of polyphenols, mg GA/g dw | 1.13 ± 0.02 | 1.29 ± 0.02 | 1.19 ±0.02 | 1.54 ± 0.03 | 0.56 ± 0.01 | 1.14 ± 0.02 | 1.17 ± 0.02 |
| ABTS radical scavenging effects, μg TE/g dw | 8744.44 ± 153.20 | 9755.56 ± 171.15 | 8750.44 ± 153.85 | 8655.56 ± 152.18 | 2577.78 ± 45.32 | 8742.41 ± 153.71 | 9865.10 ± 177.34 |
| Ferric reducing antioxidant power, mg FE(II)/g dw | 2287.50 ± 40.22 | 11,677.51 ± 205.32 | 3352.50 ± 58.95 | 9097.50 ± 159.96 | 2552.50 ± 44.87 | 3052.52 ± 53.67 | 3532.51 ± 62.11 |
| RT | Metabolite | Early Flowering | Mass Flowering | Late Flowering | ||||
|---|---|---|---|---|---|---|---|---|
| Raw Material | Flowers | Leaves | Flowers | Leaves | Rhizomes | Flowers | Leaves | |
| Polyphenols | ||||||||
| 6.06 | Gallic acid | 0.60 ± 0.01 | 0.51 ± 0.01 | 0.77 ± 0.01 | 0.41 ± 0.01 | 0.14 ± 0.01 | 0.57 ± 0.02 | 0.64 ± 0.01 |
| 9.53 | Neochlorogenic acid | 0.73 ± 0.01 | 1.35 ± 0.02 | 1.54 ± 0.03 | 0.54 ± 0.01 | 0.01 ± 0.00 | 1.23 ± 0.03 | 1.99 ± 0.04 |
| 10.68 | Oenothein B | 39.70 ± 0.70 | 24.90 ± 0.44 | 50.26 ± 0.88 | 15.45 ± 0.27 | 1.95 ± 0.03 | 45.95 ± 0.81 | 54.62 ± 0.96 |
| 11.68 | Chlorogenic acid | 0.91 ± 0.02 | 1.61 ± 0.03 | 1.77 ± 0.03 | 1.10 ± 0.02 | 0.01 ± 0.00 | 1.55 ± 0.03 | 2.60 ± 0.05 |
| 13.04 | Oenothein A | 3.96 ± 0.07 | 4.40 ± 0.08 | 8.44 ± 0.15 | 1.85 ± 0.03 | 0.13 ± 0.01 | 7.26 ± 0.13 | 9.00 ± 0.16 |
| 22.72 | Ellagic acid | 0.41 ± 0.01 | 0.27 ± 0.01 | 0.51 ± 0.02 | 0.23 ± 0.01 | 0.17 ± 0.02 | 0.52 ± 0.02 | 0.41 ± 0.01 |
| 22.73 | Rutin | 0.18 ± 0.00 | 0.01 ± 0.00 | 1.19 ± 0.03 | 0.90 ± 0.02 | - | 1.56 ± 0.03 | 2.26 ± 0.04 |
| 23.65 | Hyperoside | 0.16 ± 0.00 | 0.29 ± 0.01 | 1.05 ± 0.02 | 0.80 ± 0.01 | - | 1.38 ± 0.02 | 2.00 ± 0.04 |
| 24.90 | Isoquercitrin | 4.77 ± 0.08 | 8.08 ± 0.14 | 7.98 ± 1.14 | 4.69 ± 0.08 | 0.05 ± 0.00 | 8.41 ± 0.15 | 11.18 ± 0.20 |
| 31.11 | Quercitrin | 2.75 ± 0.05 | 0.53 ± 0.01 | 0.72 ± 0.03 | - | - | 2.92 ± 0.05 | 0.56 ± 0.01 |
| 31.13 | Quercetin | 0.03 ± 0.00 | 0.04 ± 0.00 | 0.05 ± 0.01 | 0.02 ± 0.01 | - | 0.06 ± 0.00 | 0.08 ± 0.01 |
| 37.74 | Afzelin | 3.24 ± 0.06 | 0.41 ± 0.01 | 0.66 ± 0.02 | - | - | 6.47 ± 0.12 | - |
| 27.82 | Guaijaverin | - | 0.50 ± 0.02 | 1.63 ± 0.03 | 1.24 ± 0.02 | - | 0.99 ± 0.02 | 1.58 ± 0.03 |
| Total phenolic acid content | 0.66 ± 0.01 | 0.94 ± 0.03 | 1.15 ± 0.02 | 0.57 ± 0.01 | 0.08 ± 0.00 | 0.97 ± 0.03 | 1.41 ± 0.03 | |
| Total tannin content | 21.83 ± 0.40 | 14.65 ± 0.26 | 29.35 ± 0.51 | 8.65 ± 0.15 | 1.04 ± 0.02 | 26.61 ± 0.47 | 31.81 ± 0.56 | |
| Total flavonoid content | 1.86 ± 0.03 | 1.41 ± 0.02 | 1.90 ± 0.03 | 1.53 ± 0.03 | 0.05 ± 0.01 | 3.11 ± 0.06 | 2.94 ± 0.05 | |
| Total polyphenol content | 57.44 ± 1.01 | 42.90 ± 0.75 | 75.85 ± 1.33 | 27.24 ± 0.48 | 2.45 ± 0.04 | 78.87 ± 1.39 | 86.92 ± 1.53 | |
| Triterpenoids | ||||||||
| 6.278 | Maslinic acid | 0.50 ± 0.01 | 0.26 ± 0.01 | 0.13± 0.00 | 0.17 ± 0.01 | 0.10 ± 0.00 | 0.06 ± 0.00 | 0.21 ± 0.01 |
| 6.790 | Corosolic acid | 1.05 ± 0.02 | 0.81 ± 0.01 | 1.32 ± 0.02 | 2.23 ± 0.04 | 0.22 ± 0.01 | 0.12 ± 0.01 | 0.53 ± 0.01 |
| 12.06 | Oleanolic acid | 0.61 ± 0.01 | 0.54 ± 0.01 | 0.13 ± 0.00 | 0.50 ± 0.01 | 0.51 ± 0.01 | 0.19 ± 0.01 | 0.10 ± 0.00 |
| 12.69 | Ursolic acid | 1.08 ± 0.02 | 3.51 ± 0.06 | 2.20 ± 0.04 | 3.20 ± 0.06 | 0.11 ± 0.00 | 1.46 ± 0.03 | 3.12 ± 0.05 |
| 13.75 | Betulin | 0.51 ± 0.01 | 0.52 ± 0.02 | 0.53 ± 0.01 | 0.22 ± 0.01 | 0.08 ± 0.00 | 0.42 ± 0.01 | 0.38 ± 0.01 |
| 19.75 | Erythrodiol | - | 0.02 ± 0.00 | - | 0.02 ± 0.00 | - | - | - |
| 21.05 | Uvaol | - | - | - | 0.02 ± 0.00 | - | - | - |
| Total content | 3.75 ± 0.07 | 5.66 ± 0.10 | 4.31 ± 0.08 | 6.36 ± 0.11 | 1.02 ± 0.02 | 2.25 ± 0.04 | 4.34 ± 0.08 | |
| Metabolite | RT, min | Coefficient of Determination R2 a | Equation | Linearity Range (µg/mL) | LOD (µg/mL) b | LOQ (µg/mL) c | Repeatability RT/Area (%) | Precision RT/Area (%) |
|---|---|---|---|---|---|---|---|---|
| Gallic acid | 6.10 | 0.99996 | y = 3.39 × 104x + 4.28 × 103 | 0.679–174.000 | 0.154 | 0.662 | 0.2/0.7 | 0.3/0.8 |
| Neochlorogenic acid | 9.53 | 0.99982 | y = 5.50 × 104x − 2.29 × 103 | 0.195–25.000 | 0.102 | 0.307 | 0.3/0.7 | 0.4/0.8 |
| Oenothein B | 10.66 | 0.99764 | y = 1.81 × 104x − 9.31 × 103 | 0.781–100.000 | 0.210 | 0.424 | 0.1/0.8 | 0.3/1.2 |
| Chlorogenic acid | 11.95 | 0.99980 | y = 3.10 × 104x − 7.62 × 103 | 0.406–208.200 | 0.049 | 0.147 | 0.2/0.6 | 0.4/0.8 |
| Ellagic acid | 22.48 | 0.99999 | y = 1.00 × 105x − 6.18 × 103 | 0.185–23.700 | 0.067 | 0.126 | 0.1/0.3 | 0.2/0.5 |
| Rutin | 22.80 | 0.99993 | y = 1.85 × 104x − 5.68 × 103 | 2.456–78.600 | 0.200 | 0.731 | 0.3/0.7 | 0.4/0.8 |
| Hyperoside | 23.62 | 0.99985 | y = 2.10 × 104x − 5.17 × 103 | 1.514–193.800 | 0.126 | 0.378 | 0.2/0.6 | 0.3/0.8 |
| Isoquercitrin | 24.50 | 0.99978 | y = 2.18 × 104x − 7.31 × 103 | 1.462–187.200 | 0.278 | 0.772 | 0.2/0.7 | 0.4/0.9 |
| Guaijaverin | 28.31 | 0.99838 | y = 1.21 × 104x + 5.33 × 103 | 1.562–100.000 | 0.260 | 0.781 | 0.1/1.0 | 0.3/1.2 |
| Quercitrin | 30.90 | 0.99991 | y = 1.98 × 104x − 9.22 × 103 | 0.802–205.500 | 0.134 | 0.401 | 0.3/0.9 | 0.4/1.0 |
| Afzelin | 37.95 | 0.99981 | y = 9.52 × 103x + 7.46 × 103 | 0.781–100.000 | 0.155 | 0.390 | 0.8/0.9 | 0.9/1.0 |
| Quercetin | 43.65 | 0.99994 | y = 3.61 × 104x − 1.42 × 103 | 1.025–131.200 | 0.162 | 0.995 | 0.1/0.5 | 0.2/0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanauskas, L.; Uminska, K.; Gudžinskas, Z.; Heinrich, M.; Georgiyants, V.; Kozurak, A.; Mykhailenko, O. Phenological Variations in the Content of Polyphenols and Triterpenoids in Epilobium angustifolium Herb Originating from Ukraine. Plants 2024, 13, 120. https://doi.org/10.3390/plants13010120
Ivanauskas L, Uminska K, Gudžinskas Z, Heinrich M, Georgiyants V, Kozurak A, Mykhailenko O. Phenological Variations in the Content of Polyphenols and Triterpenoids in Epilobium angustifolium Herb Originating from Ukraine. Plants. 2024; 13(1):120. https://doi.org/10.3390/plants13010120
Chicago/Turabian StyleIvanauskas, Liudas, Kateryna Uminska, Zigmantas Gudžinskas, Michael Heinrich, Victoriya Georgiyants, Alla Kozurak, and Olha Mykhailenko. 2024. "Phenological Variations in the Content of Polyphenols and Triterpenoids in Epilobium angustifolium Herb Originating from Ukraine" Plants 13, no. 1: 120. https://doi.org/10.3390/plants13010120