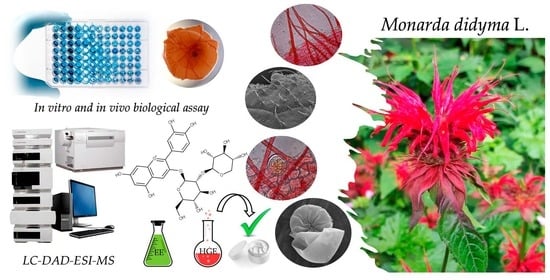
Pharmacognostic Evaluation of Monarda didyma L. Growing in Trentino (Northern Italy) for Cosmeceutical Applications
Abstract
:1. Introduction
2. Results
2.1. Distribution of Non-Glandular and Glandular Trichomes by SEM Analysis
2.1.1. Leaf
2.1.2. Bract
2.1.3. Calyx and Corolla
2.2. Phytochemical Analyses
2.3. Biological Properties
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Microscopical Analyses
4.3. Phytochemical Analyses
4.3.1. Total Phenolic Compounds
4.3.2. Total Flavonoids
4.3.3. Anthocyanins
4.3.4. Flavan-3-ols
4.3.5. Proanthocyanidins
4.3.6. LC-DAD-ESI-MS Analysis
4.4. Biological Activities
4.4.1. FRAP Assay
4.4.2. DPPH Assay
4.4.3. TEAC Assay
4.4.4. ORAC Assay
4.4.5. Albumin Denaturation Assay
4.4.6. Protease Inhibition Assay
4.4.7. CAM Assay
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bentley, R. A Manual of Botany: Including the Structure, Functions, Classification, Properties, and Uses of Plants, 1st ed.; John Churchill: London, UK, 1861; p. 611. [Google Scholar]
- Bown, D. Encyclopaedia of Herbs and Their Uses, 1st ed.; Dorling Kindersley: London, UK, 1995. [Google Scholar]
- Foster, S.; Johnson, R. Desk Reference to Nature’s Medicine, 1st ed.; National Geographic Society: Seattle, WA, USA, 2006; pp. 34–35. [Google Scholar]
- Franciosi, A. Cento Fiori Colti Nel Loro Mese, 1805–1822, Volume II. Available online: https://phaidra.cab.unipd.it/detail/o:434699 (accessed on 11 April 2022).
- Dudchenko, V.V.; Svydenko, L.V.; Markovska, O.Y.; Sydiakina, O.V. Morphobiological and Biochemical Characteristics of Monarda L. Varieties under Conditions of the Southern Steppe of Ukraine. J. Ecol. Eng. 2020, 21, 99–107. [Google Scholar] [CrossRef]
- Marchioni, I.; Najar, B.; Ruffoni, B.; Copetta, A.; Pistelli, L.; Pistelli, L. Bioactive compounds and aroma profile of some Lamiaceae edible flowers. Plants 2020, 9, 691. [Google Scholar] [CrossRef]
- Mattarelli, P.; Epifano, F.; Minardi, P.; Di Vito, M.; Modesto, M.; Barbanti, L.; Bellardi, M.G. Chemical composition and antimicrobial activity of essential oils from aerial parts of Monarda didyma and Monarda fistulosa cultivated in Italy. TEOP 2017, 20, 76–86. [Google Scholar] [CrossRef]
- Duke, J.A. Hanbook of Medicinal Herbs, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2002; p. 68. [Google Scholar]
- Roberts, M. 100 Edible & Healing Flowers: Cultivating—Cooking—Restoring Health, 2nd ed.; Struik Nature (An Imprint of Random House Struik (Pty) Ltd.): Cape Town, South Africa, 2014. [Google Scholar]
- Setzer, W.N. The Phytochemistry of Cherokee Aromatic Medicinal Plants. Medicines 2018, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- GU 224 of 26-9-2018. Available online: https://www.gazzettaufficiale.it/eli/gu/2018/09/26/224/sg/pdf (accessed on 23 May 2023).
- Wróblewska, K.; Szumny, A.; Żarowska, B.; Kromer, K.; Dębicz, R.; Fabian, S. Impact of mulching on growth essential oil composition and its biological activity in Monarda didyma L. Ind. Crops Prod. 2019, 129, 299–308. [Google Scholar] [CrossRef]
- Côté, H.; Pichette, A.; St-Gelais, A.; Legault, J. The biological activity of Monarda didyma L. essential oil and its effect as a diet supplement in mice and broiler chicken. Molecules 2021, 26, 3368. [Google Scholar] [CrossRef] [PubMed]
- Fraternale, D.; Dufat, H.; Albertini, M.C.; Bouzidi, C.; D’Adderio, R.; Coppari, S.; Di Giacomo, B.; Melandri, D.; Ramakrishna, S.; Colomba, M. Chemical composition, antioxidant and anti-inflammatory properties of Monarda didyma L. essential oil. PeerJ 2022, 10, e14433. [Google Scholar] [CrossRef]
- Maslennikov, P.V.; Chupakhina, G.N.; Skrypnik, L.N. The Content of Phenolic Compounds in Medicinal Plants of a Botanical Garden (Kaliningrad Oblast). Biol. Bull. Russ. Acad. Sci. 2014, 41, 133–138. [Google Scholar] [CrossRef]
- Stefaniak, A.; Grzeszczuk, M. Nutritional and Biological Value of Five Edible Flower Species. Not. Bot. Horti. Agrobo. 2019, 47, 128–134. [Google Scholar] [CrossRef]
- Ricci, D.; Epifano, F.; Fraternale, D. The Essential Oil of Monarda didyma L. (Lamiaceae) Exerts Phytotoxic Activity in Vitro against Various Weed Seed. Molecules 2017, 22, 222. [Google Scholar] [CrossRef]
- Ascensão, L.; Marques, N.; Pais, M.S. Glandular Trichomes on Vegetative and Reproductive Organs of Leonotis leonurus (Lamiaceæ). Ann. Bot. 1995, 75, 619–626. [Google Scholar] [CrossRef]
- Baran, P.; Özdemir, C.; Aktaş, K. Structural investigation of the glandular trichomes of Salvia argentea. Biologia 2010, 65, 33–38. [Google Scholar] [CrossRef]
- Jordheim, M.; Calcott, K.; Gould, K.S.; Davies, K.M.; Schwinn, K.E.; Andersen, Ø.M. High concentrations of aromatic acylated anthocyanins found in cauline hairs in Plectranthus ciliates. Phytochemistry 2016, 128, 27–34. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.B.; de Mendonça, M.S.; Meira, R.M.S.A. Anatomy of vegetative organs of Scutellaria agrestis, a medicinal plant cultivated by riverine populations of the Brazilian Amazon. Braz. J. Pharmacogn. 2013, 23, 386–397. [Google Scholar] [CrossRef]
- Saito, N.; Harborne, J.B. Correlations between anthocyanin type, pollinator and flower colour in the labiatae. Phytochemistry 1992, 31, 3009–3015. [Google Scholar] [CrossRef]
- Uphof, J.C.T.; Hummel, K.; Staesche, K. Plant Hairs—Die Verbreitung der Haartypen in den Natürlichen Verwandtschaftsgruppen; Gebrüder Borntraeger: Berlin-Nikolassee, Germany, 1962. [Google Scholar]
- Gostin, I.N. Glandular and Non-Glandular Trichomes from Phlomis herba-venti subsp. pungens Leaves: Light, Confocal, and Scanning Electron Microscopy and Histochemistry of the Secretory Products. Plants 2023, 12, 2423. [Google Scholar] [CrossRef] [PubMed]
- Serrato-Valenti, G.; Bisio, A.; Cornara, L.; Ciarallo, G. Structural and Histochemical Investigation of the Glandular Trichomes of Salvia aurea L. Leaves, and Chemical Analysis of the Essential Oil. Ann. Bot. 1997, 79, 329–336. [Google Scholar] [CrossRef]
- Muravnik, L.E. The Structural Peculiarities of the Leaf Glandular Trichomes: A Review. In Plant Cell and Tissue Differentiation and Secondary Metabolites; Ramawat, K.G., Ekiert, H.M., Goyal, S., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 63–97. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano Tixier, A.-S. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Leblanc, J.; et al. Re-evaluation of glycerol (E 422) as a food additive. EFSA J. 2017, 15, e04720. [Google Scholar] [CrossRef]
- Manousaki, A.; Jancheva, M.; Grigorakis, S.; Makris, D. Extraction of antioxidant phenolics from agri-food waste biomass using a newly designed glycerol-based natural low-transition temperature mixture: A comparison with conventional ecofriendly solvents. Recycling 2016, 1, 194–204. [Google Scholar] [CrossRef]
- Eyiz, V.; Tontul, I.; Turker, S. Optimization of green extraction of phytochemicals from red grape pomace by homogenizer assisted extraction. J Food Meas Charact. 2020, 14, 39–47. [Google Scholar] [CrossRef]
- Kurtulbas, E.; Pekel, A.G.; Bilgin, M.; Makris, D.P.; Sahin, S. Citric acid-based deep eutectic solvent for the anthocyanin recovery from Hibiscus sabdariffa through microwave-assisted extraction. Biomass Conv. Bioref 2022, 12, 351–360. [Google Scholar] [CrossRef]
- Shehata, E.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Extraction optimization using water/glycerol for the efficient recovery of polyphenolic antioxidants from two Artemisia species. Sep. Purif. Technol. 2015, 149, 462–469. [Google Scholar] [CrossRef]
- Kowalska, G.; Wyrostek, J.; Kowalski, R.; Pankiewicz, U. Evaluation of glycerol usage for the extraction of anthocyanins from black chokeberry and elderberry fruits. J. Appl. Res. Med. Aromat. Plants 2021, 22, 100296. [Google Scholar] [CrossRef]
- Salem, Y.; Rajha, H.N.; Franjieh, D.; Hoss, I.; Manca, M.L.; Manconi, M.; Castangia, I.; Perra, M.; Maroun, R.G.; Louka, N. Stability and Antioxidant Activity of Hydro-Glyceric Extracts Obtained from Different Grape Seed Varieties Incorporated in Cosmetic Creams. Antioxidants 2022, 11, 1348. [Google Scholar] [CrossRef]
- Hoss, I.; Rajha, H.N.; El Khoury, R.; Youssef, S.; Manca, M.L.; Manconi, M.; Louka, N.; Maroun, R.G. Valorization of Wine-Making By-Products’ Extracts in Cosmetics. Cosmetics 2021, 8, 109. [Google Scholar] [CrossRef]
- Baroi, A.M.; Popitiu, M.; Fierascu, I.; Sardarescu, I.-D.; Fierascu, R.C. Grapevine Wastes: A Rich Source of Antioxidants and Other Biologically Active Compounds. Antioxidants 2022, 11, 393. [Google Scholar] [CrossRef]
- Smeriglio, A.; D’Angelo, V.; Denaro, M.; Trombetta, D.; Germanò, M.P. The Hull of Ripe Pistachio Nuts (Pistacia vera L.) as a Source of New Promising Melanogenesis Inhibitors. Plant Foods Hum. Nutr. 2021, 76, 111–117. [Google Scholar] [CrossRef]
- Laganà, G.; Barreca, D.; Smeriglio, A.; Germanò, M.P.; D’Angelo, V.; Calderaro, A.; Bellocco, E.; Trombetta, D. Evaluation of Anthocyanin Profile, Antioxidant, Cytoprotective, and Anti-Angiogenic Properties of Callistemon citrinus Flowers. Plants 2020, 9, 1045. [Google Scholar] [CrossRef]
- Smeriglio, A.; Denaro, M.; Barreca, D.; D’Angelo, V.; Germanò, M.P.; Trombetta, D. Polyphenolic profile and biological activities of black carrot crude extract (Daucus carota L. ssp. sativus var. atrorubens Alef.). Fitoterapia 2018, 124, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kanlayavattanakul, M.; Chongnativisit, W.; Chaikul, P.; Lourith, N. Phenolic-Rich Pomegranate Peel Extract: In Vitro, Cellular, and In Vivo Activities for Skin Hyperpigmentation Treatment. Planta Med. 2020, 86, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Akhtar, N. Formulation and in Vivo Evaluation for Anti-Aging Effects of an Emulsion Containing Basil Extract Using Non- Invasive Biophysical Techniques. Daru 2011, 19, 344–350. [Google Scholar] [PubMed]
- Villalva, M.; Santoyo, S.; Salas-Pérez, L.; Siles-Sánchez, M.d.l.N.; Rodríguez García-Risco, M.; Fornari, T.; Reglero, G.; Jaime, L. Sustainable Extraction Techniques for Obtaining Antioxidant and Anti-Inflammatory Compounds from the Lamiaceae and Asteraceae Species. Foods 2021, 10, 2067. [Google Scholar] [CrossRef] [PubMed]
- Kozyra, M.; Biernasiuk, A.; Wiktor, M.; Kukula-Koch, W.; Malm, A. Comparative HPLC–DAD–ESIQTOF/MS/MS Analysis of Bioactive Phenolic Compounds Content in the Methanolic Extracts from Flowering Herbs of Monarda Species and Their Free Radical Scavenging and Antimicrobial Activities. Pharmaceutics 2023, 15, 964. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Nakane, Y.; Tamura, H.; Goto, T. Structure of monardein, a bis-malonylated anthocyanin isolated from golden balm, Monarda didyma. Tetrahedron Lett. 1985, 26, 5879–5882. [Google Scholar] [CrossRef]
- Savickiene, N.; Dagilyte, A.; Barsteigiene, Z.; Kazlauskas, S.; Vaiciūniene, J. Identification of flavonoids in the flowers and leaves of Monarda didyma L. Medicina 2002, 38, 1119–1122. [Google Scholar]
- Davies, A.J.; Mazza, G. Separation and characterization of anthocyanins of Monarda fistulosa by high performance liquid chromatograph. J. Agric. Food Chem. 1992, 40, 1341–1345. [Google Scholar] [CrossRef]
- Marshall, H.H.; Scora, R.W. A new chemical race of Monarda fistulosa (Labiatae). Can. J. Bot. 1972, 50, 1845–1849. [Google Scholar] [CrossRef]
- Shanaida, M.; Jasicka-Misiak, I.; Makowicz, E.; Stanek, N.; Shanaida, V.; Wieczorek, P.P. Development of high-performance thin layer chromatography method for identification of phenolic compounds and quantification of rosmarinic acid content in some species of the Lamiaceae family. J. Pharm. Bioallied Sci. 2020, 12, 139–145. [Google Scholar] [CrossRef]
- Shanaida, M.; Hudz, N.; Jasicka-Misiak, I.; Wieczorek, P.P. Polyphenols and pharmacological screening of a Monarda fistulosa L. dry extract based on a hydrodistilled residue by-product. Front. Pharmacol. 2021, 29, 563436. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Gattuso, G.; Bellocco, E.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Laganà, G.; Daglia, M.; Meneghini, S.; Nabavi, S.M. Flavanones: Citrus phytochemical with health-promoting properties. Biofactors. 2017, 43, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Lagana, G.; Leuzzi, U.; Smeriglio, A.; Trombetta, D.; Bellocco, E. Evaluation of the nutraceutical, antioxidant and cytoprotective properties of ripe pistachio (Pistacia vera L., variety Bronte) hulls. Food Chem. 2016, 196, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Mandalari, G.; Bisignano, C.; Filocamo, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Polyphenolic content and biological properties of Avola almond (Prunus dulcis Mill. D.A. Webb) skin and its industrial byproducts. Ind. Crops Prod. 2016, 83, 283–293. [Google Scholar] [CrossRef]
- Bellocco, E.; Barreca, D.; Laganà, G.; Calderaro, A.; El Lekhlifi, Z.; Chebaibi, S.; Smeriglio, A.; Trombetta, D. Cyanidin-3-O-galactoside in ripe pistachio (Pistacia vera L. variety Bronte) hulls: Identification and evaluation of its antioxidant and cytoprotective activities. J. Funct. Foods 2016, 27, 376–385. [Google Scholar] [CrossRef]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Gacche, R.N.; Meshram, R.J.; Shegokar, H.D.; Gond, D.S.; Kamble, S.S.; Dhabadge, V.N.; Utage, B.G.; Patil, K.K.; More, R.A. Flavonoids as a scaffold for development of novel anti-angiogenic agents: An experimental and computational enquiry. Arch. Biochem. Biophys. 2015, 577–578, 35–48. [Google Scholar] [CrossRef]
- Mirossay, L.; Varinská, L.; Mojžiš, J. Antiangiogenic Effect of Flavonoids and Chalcones: An Update. Int. J. Mol. Sci. 2017, 19, 27. [Google Scholar] [CrossRef]
- Giacomelli, C.; Miranda, F.S.; Gonçalves, N.S.; Spinelli, A. Antioxidant activity of phenolic and related compounds: A density functional theory study on the O-H bond dissociation enthalpy. Redox Rep. 2004, 9, 263–269. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. Int. J. Mol. Sci. 2018, 19, 1573–1589. [Google Scholar] [CrossRef]
- Cao, W.; Hu, C.; Wu, L.; Xu, L.; Jiang, W. Rosmarinic acid inhibits inflammation and angiogenesis of hepatocellular carcinoma by suppression of NF-κB signaling in H22 tumor-bearing mice. J. Pharmacol. Sci. 2016, 132, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.C.; Kendrick, B.; Peterson, C.A. Efficient lipid staining in plant material with sudan red 7B or fluorol [correction of fluoral] yellow 088 in polyethylene glycol-glycerol. Biotech. Histochem. 1991, 66, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.P.; Snowdon, D.W. Atlas of Microscopy of Medicinal Plants, Culinary Herbs and Spices; Belhaven Press A Division of Pinter Publishers: London, UK, 1990. [Google Scholar]
- Chieco, C.; Rotondi, A.; Morrone, L.; Rapparini, F.; Baraldi, R. An ethanol-based fixation method for anatomical and micro-morphological characterization of leaves of various tree species. Biotech. Histochem. 2013, 88, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Pathan, A.K.; Bond, J.; Gaskin, R.E. Sample preparation for scanning electron microscopy of plant surfaces. Horses for courses. Micron 2008, 39, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Ingegneri, M.; Smeriglio, A.; Rando, R.; Gervasi, T.; Tamburello, M.P.; Ginestra, G.; La Camera, E.; Pennisi, R.; Sciortino, M.T.; Mandalari, G.; et al. Composition and Biological Properties of Blanched Skin and Blanch Water Belonging to Three Sicilian Almond Cultivars. Nutrients 2023, 15, 1545. [Google Scholar] [CrossRef]
- Lenucci, M.S.; Cadinu, D.; Taurino, M.; Piro, G.; Dalessandro, G. Antioxidant composition in cherry and high-pigment tomato cultivars. J. Agric. Food Chem. 2006, 54, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Rapisarda, P.; Fanella, F.; Maccarone, E. Reliability of analytical methods for determining anthocyanins in blood orange juices. J Agric Food Chem. 2000, 48, 2249–2252. [Google Scholar] [CrossRef]
- Margheri, G.; Falcieri, E. Importanza dell’evoluzione delle sostanze polifenoliche nei vini rossi di qualita’ durante l’invecchimento, Nota II. Vini d’Italia 1972, 14, 81–84. [Google Scholar]
- Spagna, G.; Tomaino, A.; Cimino, F.; Barbagallo, R.N.; Ventura, D.; Bonina, F.; Saija, A. Chemical analysis and photoprotective effect of an extract of wine from Jacquez grapes. JSFA 2022, 82, 1867–1874. [Google Scholar] [CrossRef]
- Spagna, G.; Pifferi, P.G.; Rangoni, C.; Mattivi, F.; Nicolini, G.; Palmonari, R. The stabilization of white wines by adsorption of phenolic compounds on chitin and chitosan. Food Res Inter. 1996, 29, 241–248. [Google Scholar] [CrossRef]
- Danna, C.; Bazzicalupo, M.; Ingegneri, M.; Smeriglio, A.; Trombetta, D.; Burlando, B.; Cornara, L. Anti-Inflammatory and Wound Healing Properties of Leaf and Rhizome Extracts from the Medicinal Plant Peucedanum ostruthium (L.) W. D. J. Koch. Molecules 2022, 27, 4271. [Google Scholar] [CrossRef] [PubMed]
- Cornara, L.; Sgrò, F.; Raimondo, F.M.; Ingegneri, M.; Mastracci, L.; D’Angelo, V.; Germanò, M.P.; Trombetta, D.; Smeriglio, A. Pedoclimatic Conditions Influence the Morphological, Phytochemical and Biological Features of Mentha pulegium L. Plants 2023, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Denaro, M.; D’Angelo, V.; Germanò, M.P.; Trombetta, D. Antioxidant, anti-inflammatory and anti-angiogenic properties of Citrus lumia Juice. Front. Pharmacol. 2020, 11, 593506. [Google Scholar] [CrossRef] [PubMed]
- Certo, G.; Costa, R.; D’Angelo, V.; Russo, M.; Albergamo, A.; Dugo, G.; Germanò, M.P. Anti-angiogenic activity and phytochemical screening of fruit fractions from Vitex agnus castus. Nat. Prod. Res. 2017, 31, 2850–2856. [Google Scholar] [CrossRef]









| Phytochemical Assay | EE | HGE |
|---|---|---|
| Total phenols (mg GAE a/100 mL LE b) | 64.22 ± 3.45 | 105.75 ± 5.91 * |
| Flavonoids (mg RE c/100 mL LE) | 47.70 ± 1.27 | 71.60 ± 5.09 * |
| Anthocyanins (mg CyGE d/100 mL LE) | 0.35 ± 0.02 | 3.22 ± 0.15 * |
| Vanillin index (mg CE e/100 mL LE) | 5.83 ± 0.22 | 43.89 ± 1.88 * |
| Proanthocyanidins (mg CyE f/100 mL LE) | 0.004 ± 0.00 | 0.022 ± 0.00 * |
| Polimerization index g | 1623.73 | 1971.60 * |
| Compound | RT a min | λmax nm | [M-H]− m/z | [M-H]+ m/z | EE | HGE |
|---|---|---|---|---|---|---|
| mg/100 mL LE b | ||||||
| Quinic acid c | 1.6 | 233; 310 | 191 | - | 0.87 ± 0.03 | 1.88 ± 0.05 * |
| Sinapoylquinic acid d | 6.4 | 218, 327 | 397 | - | 0.17 ± 0.01 | 0.07 ± 0.00 * |
| (-)-Epigallocatechin 3-O-gallate c | 10.1 | 274 | 457 | - | 2.29 ± 0.06 | 14.66 ± 0.85 * |
| 1-O-Feruloyl-beta-D-glucose c | 13.8 | 330 | 355 | - | 0.22 ± 0.01 | 0.19 ± 0.01 |
| Rosmadial e | 15.3 | 234, 290, 356 | 343 | - | 1.63 ± 0.03 | 2.48 ± 0.08 * |
| Anhydro-secoisolariciresinol f | 16.6 | 288 | 343 | - | 0.18 ± 0.01 | 0.15 ± 0.01 |
| Peonidin 3-O-(6″-acetyl-glicoside) g | 17.3 | 277, 526 | 504 | - | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Pelargonidin 3,5-di-beta-D-glucoside (Monardin) c | 19.8 | 276, 329, 501 | - | 595 | 0.01 ± 0.00 | 0.06 ± 0.00 * |
| 3-O-Caffeoyl quinic acid (Chlorogenic acid) c | 20.3 | 248, 326 | 353 | - | 1.83 ± 0.03 | 2.70 ± 0.06 * |
| 4-Hydroxybenzoic acid c | 22.4 | 253 | 137 | - | 6.89 ± 0.22 | 8.02 ± 0.17 * |
| (+)-Catechin 3-O-glucoside h | 22.8 | 276, 320 | - | 453 | 1.71 ± 0.04 | 15.61 ± 0.76 * |
| Bis (demalonyl) monardaein (Monardein) i | 24.0 | 286, 313, 507 | - | 742 | 0.01 ± 0.00 | 0.03 ± 0.00 * |
| Apigenin-7-O-glucuronopyranosyl(1,2)-glucuronopyranoside j | 25.2 | 267, 336 | - | 622 | 0.08 ± 0.00 | 0.13 ± 0.00 * |
| (-)-Epigallocatechin 3-O-glucuronide k | 27.3 | 272 | - | 483 | 0.25 ± 0.01 | 4.28 ± 0.12 * |
| Kaempferol 3-O-(6″-malonyl-glucoside) l | 28.6 | 265, 345 | 533 | - | 9.65 ± 0.24 | 8.53 ± 0.18 * |
| Ligstroside c | 29.1 | 235, 275 | - | 525 | 0.06 ± 0.00 | 0.07 ± 0.00 |
| Delphinidin 3-O-sambubioside c | 30.2 | 274, 523 | 596 | - | 0.01 ± 0.00 | 0.17 ± 0.01 * |
| Diosmin c | 30.4 | 260,350 | - | 609 | 0.36 ± 0.02 | 0.45 ± 0.02 * |
| Phloretin 2′-O-xylosyl-glucoside m | 30.8 | 242, 289 | - | 569 | 0.83 ± 0.04 | 0.65 ± 0.03 * |
| Malvidin 3-O-(6″-p-coumaroyl-glucoside) n | 31.2 | 274, 310, 527 | 638 | - | 0.01 ± 0.00 | 0.02 ± 0.00 |
| Chicoric acid c | 31.7 | 250, 330 | - | 475 | 0.08 ± 0.00 | 0.07 ± 0.00 |
| Kaempferol 3-O-xylosyl-glucoside l | 32.4 | 253, 266, 323, 364 | 579 | - | 2.73 ± 0.08 | 1.71 ± 0.02 * |
| Kaempferol 3-O-rhamnosyl-rhamnosyl-glucoside l | 33.6 | 253, 265, 325, 364 | - | 741 | 0.75 ± 0.03 | 0.55 ± 0.02 * |
| Cyanidin 3,5-O-diglucoside c | 33.7 | 279, 326, 514 | 610 | - | 0.03 ± 0.00 | 0.01 ± 0.00 * |
| Quercetin 3-O-xylosyl-rutinoside o | 34.0 | 258, 360 | - | 743 | 2.55 ± 0.07 | 1.55 ± 0.10 * |
| Rosmarinic acid c | 34.4 | 292, 332 | 359 | - | 0.02 ± 0.00 | 0.03 ± 0.00 |
| Delphinidin 3-O-glucoside c | 35.5 | 274, 523 | 464 | - | 0.01 ± 0.00 | 0.02 ± 0.00 |
| Kaempferol 3-O-glucuronide p | 36.2 | 272, 368 | 461 | - | 0.26 ± 0.01 | 0.19 ± 0.01 * |
| Vitisin A n | 36.6 | 256, 515 | - | 562 | 0.05 ± 0.00 | 0.07 ± 0.00 |
| Malvidin 3-O-glucoside c | 36.9 | 274, 527 | - | 494 | 0.01 ± 0.00 | 0.02 ± 0.00 |
| Kaempferol-3-O-rutinoside c | 39.2 | 266, 348 | 593 | - | 4.23 ± 0.21 | 1.91 ± 0.08 * |
| Cyanidin 3-O-sambubioside c | 39.6 | 280, 517 | - | 617 | 0.10 ± 0.01 | 2.22 ± 0.14 * |
| Cyanidin 3-O-(6″-dioxalyl-glucoside) q | 41.0 | 280, 526 | - | 594 | 0.02 ± 0.00 | 0.22 ± 0.01 * |
| Luteolin 7-O-diglucuronide r | 42.0 | 253, 267, 292, 348 | - | 639 | 0.01 ± 0.00 | 0.02 ± 0.00 |
| Cyanidin 3-O-(3″,6″-O-dimalonyl-glucoside) q | 43.9 | 280, 522 | - | 622 | 0.01 ± 0.00 | 0.11 ± 0.01 * |
| 3-Hydroxyphloretin 2′-O-xylosyl-glucoside q | 45.8 | 242, 289 | 583 | 585 | 3.46 ± 0.12 | 3.95 ± 0.20 * |
| Secoisolariciresinol c | 46.2 | 229, 281 | - | 363 | 0.02 ± 0.00 | 0.03 ± 0.00 |
| Quercetin 3′-sulfate s | 47.3 | 255, 270, 303, 370 | - | 383 | 0.43 ±0.01 | 0.10 ± 0.00 * |
| Phloretin c | 47.5 | 242, 289 | - | 275 | 0.16 ± 0.01 | 0.20 ± 0.01 |
| Kaempferol c | 50.2 | 266, 366 | 285 | - | 4.92 ± 0.22 | 2.35 ± 0.08 * |
| Procyanidin dimer B type h | 54.1 | 233, 279 | 577 | - | 0.01 ± 0.00 | 0.03 ± 0.00 * |
| Luteolin 7-O-(2-apiosyl-6-malonyl)-glucoside t | 61.8 | 227, 348 | - | 667 | 0.12 ± 0.01 | 0.19 ± 0.01 * |
| Kaempferol 3-O-(6″-acetyl-galactoside) 7-O-rhamnoside l | 64.2 | 245, 265, 315, 350 | - | 637 | 2.25 ± 0.15 | 0.95 ± 0.04 * |
| Vanillic acid 4-sulfate u | 64.4 | 259, 292 | 247 | - | 0.03 ± 0.00 | 0.01 ± 0.00 * |
| Quercetin-3-O-α-L-rhamnosyl (1,2)-β-D-glucoside-7-O-α-L-rhamnoside o | 65.3 | 256, 374 | - | 757 | 2.48 ± 0.17 | 0.75 ± 0.04 * |
| Epigallocatechin 3-O-gallate-7-O-glucoside-4″-O-glucuronide v | 66.3 | 272 | - | 797 | 0.34 ± 0.02 | 5.00 ± 0.28 * |
| Cyanidin 3-O-diglucoside-5-O-glucoside w | 67.6 | 280, 522 | - | 774 | 0.03 ± 0.00 | 0.01 ± 0.00 * |
| Delphinidin 3-O-glucoside c | 69 | 276, 344, 525 | - | 466 | 0.03 ± 0.00 | 0.04 ± 0.00 |
| Lariciresinol-sesquilignan x | 73.4 | 230, 280 | - | 557 | 0.15 ± 0.01 | 0.08 ± 0.00 * |
| Malvidin 3,5-O-diglucoside c | 75.2 | 273, 537 | - | 657 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Methyl 4,6-di-O-galloyl-beta-D-glucopyranoside y | 77.7 | 280, 369 | - | 499 | 0.21 ± 0.01 | 0.12 ± 0.01 * |
| Kaempferol 3-O-glucosyl-rhamnosyl-glucoside l | 78.4 | 265, 294, 342 | - | 757 | 2.04 ± 0.08 | 0.54 ± 0.02 * |
| Lariciresinol c | 82.0 | 230, 280 | 359 | - | 0.35 ± 0.02 | 0.13 ± 0.01 * |
| Test | EE (mg/mL) | HGE (mg/mL) | RS a (µg/mL) |
|---|---|---|---|
| FRAP | 5.15 (3.65–7.52) b | 0.19 (0.13–0.26) b,c | 4.86 (3.23–5.65) |
| DPPH | 9.92 (7.65–12.52) b | 1.31 (0.85–1.52) b,c | 13.57 (12.22–14.88) |
| TEAC | 4.38 (2.21–6.31) b | 0.91 (0.52–1.31) b,c | 5.83 (4.12–6.55) |
| ORAC | 0.42 (0.17–0.65) b | 0.007 (0.005–0.009) b,c | 0.83 (0.62–1.05) |
| ADA | 21.80 (17.48–26.33) b | 10.23 (7.48–13.89) b,c | 46.42 (38.55–51.44) |
| PIA | 4.97 (3.07–6.85) b | 0.47 (0.27–0.68) b,c | 39.87 (32.68–44.82) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smeriglio, A.; Ingegneri, M.; Germanò, M.P.; Miori, L.; Battistini, G.; Betuzzi, F.; Malaspina, P.; Trombetta, D.; Cornara, L. Pharmacognostic Evaluation of Monarda didyma L. Growing in Trentino (Northern Italy) for Cosmeceutical Applications. Plants 2024, 13, 112. https://doi.org/10.3390/plants13010112
Smeriglio A, Ingegneri M, Germanò MP, Miori L, Battistini G, Betuzzi F, Malaspina P, Trombetta D, Cornara L. Pharmacognostic Evaluation of Monarda didyma L. Growing in Trentino (Northern Italy) for Cosmeceutical Applications. Plants. 2024; 13(1):112. https://doi.org/10.3390/plants13010112
Chicago/Turabian StyleSmeriglio, Antonella, Mariarosaria Ingegneri, Maria Paola Germanò, Luigi Miori, Giulia Battistini, Federica Betuzzi, Paola Malaspina, Domenico Trombetta, and Laura Cornara. 2024. "Pharmacognostic Evaluation of Monarda didyma L. Growing in Trentino (Northern Italy) for Cosmeceutical Applications" Plants 13, no. 1: 112. https://doi.org/10.3390/plants13010112