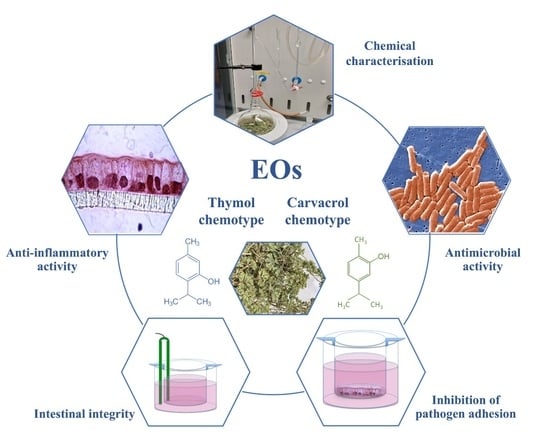
Chemical Composition and Biological Activities of Essential Oils from Origanum vulgare Genotypes Belonging to the Carvacrol and Thymol Chemotypes
Abstract
:1. Introduction
2. Results
2.1. Composition of EOs
2.2. Enantiomeric Distribution of Chiral Compounds as Determined after Isolation from Oregano Dried Leaves and Flowers and in Distilled Oregano EOs
2.3. Antimicrobial Activity of EOs by Agar Spot Test against Pathogen Indicator Strains
2.4. In Vitro Antimicrobial Activity of CAR1 and THY5 against L. monocytogenes OH and S. Typhimurium LT2 by Direct Contact Test
2.5. Antimicrobial Effects of CAR1 and THY5 during a Challenge Test of Beef Minced Meat
2.6. Effects of CAR1 and THY5 on Caco-2 Cell Permeability
2.7. Reduction in Pathogen Adhesion to Caco-2 Cells by CAR1 and THY5
2.8. Effects of CAR1 and THY5 on the NF-kB Pathway in Caco-2 Cells
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Isolation of the EOs
4.3. GC–MS Analyses of EO Compositions
4.4. Enantioselective GC–MS Analysis of Volatiles in Dried Leaves and Flowers and EO Constituents
4.5. Indicator Microorganism Strains
4.6. Agar Spot Test
4.7. Direct Contact Test
4.8. Challenge Test
4.9. Intestinal Caco-2 Cell Culture
4.10. Cell Monolayer Permeability Assessments
4.11. Impact of Oregano EOs (CAR1 and THY5) on Intestinal Barrier Integrity
4.12. Pathogen Adhesion Assay to Caco-2 Cells
4.13. Inflammation Induction by TNF-α and EO Treatment for Gene Expression Analysis of NF-kB Pathway
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Franz, C.; Novak, J. Sources of Essential Oils. In Handbook of Essential Oils: Science, Technology, and Applications; Baser, K.H.C., Buchbauer, G., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2010; pp. 39–82. [Google Scholar]
- Kintzios, S.E. Oregano. In Handbook of Herbs and Spices, 2nd ed.; Peter, K.V., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2012; Volume 2, pp. 417–436. [Google Scholar]
- Rodriguez-Garcia, I.; Silva-Espinoza, B.A.; Ortega-Ramirez, L.A.; Leyva, J.M.; Siddiqui, M.W.; Cruz-Valenzuela, M.R.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Oregano Essential Oil as an Antimicrobial and Antioxidant Additive in Food Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 1717–1727. [Google Scholar] [CrossRef]
- The Rapid Alert System for Food and Feed—Annual Report 2020; Publications Office of the European Union: Luxembourg, 2021; Available online: https://food.ec.europa.eu/system/files/2021-08/rasff_pub_annual-report_2020.pdf (accessed on 16 February 2023).
- Dawood, M.A.O.; El Basuini, M.F.; Zaineldin, A.I.; Yilmaz, S.; Hasan, M.T.; Ahmadifar, E.; El Asely, A.M.; Abdel-Latif, H.M.R.; Alagawany, M.; Abu-Elala, N.M.; et al. Antiparasitic and Antibacterial Functionality of Essential Oils: An Alternative Approach for Sustainable Aquaculture. Pathogens 2021, 10, 185. [Google Scholar] [CrossRef]
- Burggraf, A.; Rienth, M. Origanum vulgare Essential Oil Vapour Impedes Botrytis Cinerea Development on Grapevine (Vitis vinifera) Fruit. Phytopathol. Mediterr. 2020, 59, 331–344. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.; Vazquez-Olivo, G.; Heredia, J. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [Green Version]
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.-M.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum vulgare L. Essential Oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef] [PubMed]
- Pezzani, R.; Vitalini, S.; Iriti, M. Bioactivities of Origanum vulgare L.: An Update. Phytochem. Rev. 2017, 16, 1253–1268. [Google Scholar] [CrossRef]
- Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Alqubaisy, M.; AlAli, M.; Molouki, A.; Ong-Abdullah, J.; Abushelaibi, A.; Lai, K.-S.; Lim, S.-H.E. An Overview of the Potential Therapeutic Applications of Essential Oils. Molecules 2021, 26, 628. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Meng, X.; Li, Y.; Zhao, C.-N.; Tang, G.-Y.; Li, H.-B. Antibacterial and Antifungal Activities of Spices. Int. J. Mol. Sci. 2017, 18, 1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do Nascimento, L.D.; de Moraes, A.A.B.; da Costa, K.S.; Pereira Galúcio, J.M.; Taube, P.S.; Costa, C.M.L.; Neves Cruz, J.; de Aguiar Andrade, E.H.; de Faria, L.J.G. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Plati, F.; Paraskevopoulou, A. Micro- and Nano-Encapsulation as Tools for Essential Oils Advantages’ Exploitation in Food Applications: The Case of Oregano Essential Oil. Food Bioprocess Technol. 2022, 15, 949–977. [Google Scholar] [CrossRef]
- Pontes-Quero, G.M.; Esteban-Rubio, S.; Pérez Cano, J.; Aguilar, M.R.; Vázquez-Lasa, B. Oregano Essential Oil Micro- and Nanoencapsulation With Bioactive Properties for Biotechnological and Biomedical Applications. Front. Bioeng. Biotechnol. 2021, 9, 703684. [Google Scholar] [CrossRef] [PubMed]
- Lukas, B.; Schmiderer, C.; Novak, J. Essential Oil Diversity of European Origanum vulgare L. (Lamiaceae). Phytochemistry 2015, 119, 32–40. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- De Falco, E.; Mancini, E.; Roscigno, G.; Mignola, E.; Taglialatela-Scafati, O.; Senatore, F. Chemical Composition and Biological Activity of Essential Oils of Origanum vulgare L. Subsp. Vulgare L. under Different Growth Conditions. Molecules 2013, 18, 14948–14960. [Google Scholar] [CrossRef] [Green Version]
- Ietswaart, J.H. A Taxonomic Revision of the Genus Origanum (Labiatae); Leiden University Press: The Hague, The Netherlands, 1980; 153p. [Google Scholar]
- Napoli, E.; Giovino, A.; Carrubba, A.; How Yuen Siong, V.; Rinoldo, C.; Nina, O.; Ruberto, G. Variations of Essential Oil Constituents in Oregano (Origanum vulgare Subsp. Viridulum (=O. Heracleoticum) over Cultivation Cycles. Plants 2020, 9, 1174. [Google Scholar] [CrossRef]
- Bonfanti, C.; Iannì, R.; Mazzaglia, A.; Lanza, C.M.; Napoli, E.M.; Ruberto, G. Emerging Cultivation of Oregano in Sicily: Sensory Evaluation of Plants and Chemical Composition of Essential Oils. Ind. Crop. Prod. 2012, 35, 160–165. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; Leto, C.; Leone, R.; Licata, M.; Virga, G.; Ruberto, G.; Napoli, E.M.; La Bella, S. Essential Oil Characteristics of Wild Sicilian Oregano Populations in Relation to Environmental Conditions. J. Essent. Oil Res. 2014, 26, 210–220. [Google Scholar] [CrossRef]
- Kokkini, S.; Karousou, R.; Dardioti, A.; Krigas, N.; Lanaras, T. Autumn Essential Oils of Greek Oregano. Phytochemistry 1997, 44, 883–886. [Google Scholar] [CrossRef]
- Giuliani, C.; Maggi, F.; Papa, F.; Maleci Bini, L. Congruence of Phytochemical and Morphological Profiles along an Altitudinal Gradient in Origanum vulgare ssp. Vulgare from Venetian Region (NE Italy). Chem. Biodivers. 2013, 10, 569–583. [Google Scholar] [CrossRef]
- Bisht, D.; Chanotiya, C.S.; Rana, M.; Semwal, M. Variability in Essential Oil and Bioactive Chiral Monoterpenoid Compositions of Indian Oregano (Origanum vulgare L.) Populations from Northwestern Himalaya and Their Chemotaxonomy. Ind. Crop. Prod. 2009, 30, 422–426. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B. Pharmacologically Active Compounds in the Environment and Their Chirality. Chem. Soc. Rev. 2010, 39, 4466. [Google Scholar] [CrossRef] [Green Version]
- Hubatsch, I.; Ragnarsson, E.G.E.; Artursson, P. Determination of Drug Permeability and Prediction of Drug Absorption in Caco-2 Monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef]
- Napoli, E.M.; Curcuruto, G.; Ruberto, G. Screening the Essential Oil Composition of Wild Sicilian Oregano. Biochem. Syst. Ecol. 2009, 37, 484–493. [Google Scholar] [CrossRef]
- Novak, J.; Lukas, B.; Franz, C. Temperature Influences Thymol and Carvacrol Differentially in Origanum spp. (Lamiaceae). J. Essent. Oil Res. 2010, 22, 412–415. [Google Scholar] [CrossRef]
- Rubiolo, P.; Sgorbini, B.; Liberto, E.; Cordero, C.; Bicchi, C. Essential Oils and Volatiles: Sample Preparation and Analysis. A Review. Flavour Fragr. J. 2010, 25, 282–290. [Google Scholar] [CrossRef]
- Di Vito, M.; Cacaci, M.; Barbanti, L.; Martini, C.; Sanguinetti, M.; Benvenuti, S.; Tosi, G.; Fiorentini, L.; Scozzoli, M.; Bugli, F.; et al. Origanum vulgare Essential Oil vs. a Commercial Mixture of Essential Oils: In Vitro Effectiveness on Salmonella spp. from Poultry and Swine Intensive Livestock. Antibiotics 2020, 9, 763. [Google Scholar] [CrossRef]
- Serio, A.; Chiarini, M.; Tettamanti, E.; Paparella, A. Electronic Paramagnetic Resonance Investigation of the Activity of Origanum vulgare L. Essential Oil on the Listeria Monocytogenes Membrane. Lett. Appl. Microbiol. 2010, 51, 149–157. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Holley, R.A.; Patel, D. Improvement in Shelf-Life and Safety of Perishable Foods by Plant Essential Oils and Smoke Antimicrobials. Food Microbiol. 2005, 22, 273–292. [Google Scholar] [CrossRef]
- Govaris, A.; Solomakos, N.; Pexara, A.; Chatzopoulou, P.S. The Antimicrobial Effect of Oregano Essential Oil, Nisin and Their Combination against Salmonella Enteritidis in Minced Sheep Meat during Refrigerated Storage. Int. J. Food Microbiol. 2010, 137, 175–180. [Google Scholar] [CrossRef]
- Sivropoulou, A.; Papanikolaou, E.; Nikolaou, C.; Kokkini, S.; Lanaras, T.; Arsenakis, M. Antimicrobial and Cytotoxic Activities of Origanum Essential Oils. J. Agric. Food Chem. 1996, 44, 1202–1205. [Google Scholar] [CrossRef]
- Cristani, M.; D’Arrigo, M.; Mandalari, G.; Castelli, F.; Sarpietro, M.G.; Micieli, D.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of Four Monoterpenes Contained in Essential Oils with Model Membranes: Implications for Their Antibacterial Activity. J. Agric. Food Chem. 2007, 55, 6300–6308. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The Phenolic Hydroxyl Group of Carvacrol Is Essential for Action against the Food-Borne Pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 Cell Line as a Model of the Intestinal Barrier: Infuence of Cell and Culture-Related Factors on Caco-2 Cell Functional Characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Lea, T. Caco-2 Cell Line. In The Impact of Food Bioactives on Health; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 103–111. ISBN 978-3-319-15791-7. [Google Scholar]
- Kaschubek, T.; Mayer, E.; Rzesnik, S.; Grenier, B.; Bachinger, D.; Schieder, C.; König, J.; Teichmann, K. Effects of Phytogenic Feed Additives on Cellular Oxidative Stress and Inflammatory Reactions in Intestinal Porcine Epithelial Cells. J. Anim. Sci. 2018, 96, 3657–3669. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, J.; Peng, J.; Wei, H. Oregano Essential Oil Induces SOD1 and GSH Expression through Nrf2 Activation and Alleviates Hydrogen Peroxide-Induced Oxidative Damage in IPEC-J2 Cells. Oxidative Med. Cell. Longev. 2016, 2016, 5987183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bimczok, D.; Rau, H.; Sewekow, E.; Janczyk, P.; Souffrant, W.B.; Rothkötter, H.-J. Influence of Carvacrol on Proliferation and Survival of Porcine Lymphocytes and Intestinal Epithelial Cells in Vitro. Toxicol. In Vitro 2008, 22, 652–658. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Pichardo, S.; Moreno, F.J.; Bermúdez, J.M.; Aucejo, S.; Cameán, A.M. Cytotoxicity and Morphological Effects Induced by Carvacrol and Thymol on the Human Cell Line Caco-2. Food Chem. Toxicol. 2014, 64, 281–290. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Dušan, F.; Marián, S.; Katarína, D.; Dobroslava, B. Essential Oils—Their Antimicrobial Activity against Escherichia coli and Effect on Intestinal Cell Viability. Toxicol. In Vitro 2006, 20, 1435–1445. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Maisanaba, S.; Puerto, M.; Pichardo, S.; Jos, A.; Moyano, R.; Cameán, A.M. A Subchronic 90-Day Oral Toxicity Study of Origanum vulgare Essential Oil in Rats. Food Chem. Toxicol. 2017, 101, 36–47. [Google Scholar] [CrossRef]
- Bukovská, A.; Cikoš, Š.; Juhás, Š.; Il’ková, G.; Rehák, P.; Koppel, J. Effects of a Combination of Thyme and Oregano Essential Oils on TNBS-Induced Colitis in Mice. Mediat. Inflamm. 2007, 2007, 23296. [Google Scholar] [CrossRef] [Green Version]
- Cappelli, K.; Sabino, M.; Trabalza-Marinucci, M.; Acuti, G.; Capomaccio, S.; Menghini, L.; Verini-Supplizi, A. Differential Effects of Dietary Oregano Essential Oil on the Inflammation Related Gene Expression in Peripheral Blood Mononuclear Cells From Outdoor and Indoor Reared Pigs. Front. Vet. Sci. 2021, 8, 602811. [Google Scholar] [CrossRef]
- Cheng, C.; Zou, Y.; Peng, J. Oregano Essential Oil Attenuates RAW264.7 Cells from Lipopolysaccharide-Induced Inflammatory Response through Regulating NADPH Oxidase Activation-Driven Oxidative Stress. Molecules 2018, 23, 1857. [Google Scholar] [CrossRef] [Green Version]
- Džamić, A.M.; Nikolić, B.J.; Giweli, A.A.; Mitić-Ćulafić, D.S.; Soković, M.D.; Ristić, M.S.; Knežević-Vukčević, J.B.; Marin, P.D. Libyan Thymus capitatus essential oil: Antioxidant, antimicrobial, cytotoxic and colon pathogen adhesion-inhibition properties. J. Appl. Microbiol. 2015, 119, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Van Alphen, L.B.; Burt, S.A.; Veenendaal, A.K.J.; Bleumink-Pluym, N.M.C.; van Putten, J.P.M. The Natural Antimicrobial Carvacrol Inhibits Campylobacter jejuni Motility and Infection of Epithelial Cells. PLoS ONE 2012, 7, e45343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dal Sasso, M.; Culici, M.; Braga, P.C.; Guffanti, E.E.; Mucci, M. Thymol: Inhibitory Activity on Escherichia coli and Staphylococcus aureus Adhesion to Human Vaginal Cells. J. Essent. Oil Res. 2006, 18, 455–461. [Google Scholar] [CrossRef]
- Upadhyay, A.; Johny, A.K.; Amalaradjou, M.A.R.; Ananda Baskaran, S.; Kim, K.S.; Venkitanarayanan, K. Plant-Derived Antimicrobials Reduce Listeria monocytogenes Virulence Factors In Vitro, and down-Regulate Expression of Virulence Genes. Int. J. Food Microbiol. 2012, 157, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef] [Green Version]
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/chemistry/ (accessed on 16 February 2023).
- Liberto, E.; Cagliero, C.; Sgorbini, B.; Bicchi, C.; Sciarrone, D.; Zellner, B.D.; Mondello, L.; Rubiolo, P. Enantiomer Identification in the Flavour and Fragrance Fields by “Interactive” Combination of Linear Retention Indices from Enantioselective Gas Chromatography and Mass Spectrometry. J. Chromatogr. A 2008, 1195, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Zinno, P.; Devirgiliis, C.; Ercolini, D.; Ongeng, D.; Mauriello, G. Bacteriophage P22 to Challenge Salmonella in Foods. Int. J. Food Microbiol. 2014, 191, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Natoli, M.; Leoni, B.D.; D’Agnano, I.; D’Onofrio, M.; Brandi, R.; Arisi, I.; Zucco, F.; Felsani, A. Cell Growing Density Affects the Structural and Functional Properties of Caco-2 Differentiated Monolayer. J. Cell. Physiol. 2011, 226, 1531–1543. [Google Scholar] [CrossRef]
- Monastra, G.; Sambuy, Y.; Ferruzza, S.; Ferrari, D.; Ranaldi, G. Alpha-Lactalbumin Effect on Myo-Inositol Intestinal Absorption: In Vivo and In Vitro. CDD 2018, 15, 1305–1311. [Google Scholar] [CrossRef]
- Guantario, B.; Zinno, P.; Schifano, E.; Roselli, M.; Perozzi, G.; Palleschi, C.; Uccelletti, D.; Devirgiliis, C. In Vitro and in Vivo Selection of Potentially Probiotic Lactobacilli From Nocellara Del Belice Table Olives. Front. Microbiol. 2018, 9, 595. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| No. | Compound Name | Class | Oregano Subspecies/Hybrid | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Origanum vulgare ssp. viridulum × Origanum vulgare ssp. hirtum | Origanum heracleoticum L. | |||||||||
| CAR1 | CAR2 | CAR3 | THY1 | THY2 | THY3 | THY4 | THY5 | |||
| 1 | α-thujene | MH | 0.74 | 0.75 | 0.78 | 1.04 | 0.72 | 1.19 | 0.83 | 0.35 |
| 2 | α-pinene | MH | 0.32 | 0.32 | 0.33 | 0.39 | 0.29 | 0.46 | 0.32 | 0.16 |
| 3 | 1-octen-3-ol | Ot | 0.15 | 0.15 | 0.14 | - | - | - | - | - |
| 4 | 3-octanone | Ot | 0.07 | 0.08 | 0.06 | - | - | - | - | - |
| 5 | sabinene | MH | - | - | - | - | - | 0.07 | - | - |
| 6 | β-pinene | MH | 0.05 | 0.06 | 0.06 | 0.07 | - | 0.08 | - | - |
| 7 | β-myrcene | MH | 1.15 | 1.18 | 1.19 | 1.63 | 1.22 | 1.84 | 1.41 | 1.01 |
| 8 | α-phellandrene | MH | 0.14 | 0.17 | 0.17 | 0.27 | 0.19 | 0.30 | 0.21 | 0.14 |
| 9 | α-terpinene | MH | 1.09 | 1.24 | 1.37 | 3.45 | 2.45 | 3.92 | 2.92 | 2.21 |
| 10 | p-cymene | MH | 2.64 | 2.73 | 2.59 | 4.79 | 4.11 | 5.25 | 4.63 | 3.60 |
| 11 | limonene | MH | 0.24 | 0.28 | 0.28 | 0.42 | 0.32 | 0.48 | 0.36 | 0.28 |
| 12 | cis-β-ocimene | MH | - | - | - | 1.44 | 1.30 | 1.87 | 1.36 | 1.64 |
| 13 | tr-β-ocimene | MH | - | - | - | 0.20 | 0.17 | 0.26 | 0.18 | 0.22 |
| 14 | γ-terpinene | MH | 5.33 | 6.30 | 7.11 | 17.96 | 13.16 | 22.13 | 15.90 | 16.95 |
| 15 | cis-sabinene hydrate | OM | 0.29 | 0.29 | 0.28 | 0.18 | 0.15 | 0.23 | 0.13 | 0.15 |
| 16 | terpinolene | MH | - | - | - | 0.07 | - | 0.08 | - | - |
| 17 | linalool | OM | - | - | - | 0.30 | 0.32 | 0.41 | 0.32 | 0.39 |
| 18 | borneol | OM | - | 0.08 | - | - | 0.44 | - | - | - |
| 19 | terpinen-4-ol | OM | 0.29 | 0.35 | 0.32 | 0.57 | - | 0.63 | 0.49 | 0.43 |
| 20 | thymol methyl ether | OM | - | - | - | 0.70 | 0.86 | 1.30 | 1.06 | 2.14 |
| 21 | carvacrol methyl ether | OM | - | - | - | 4.24 | 3.52 | 4.63 | 3.82 | 3.48 |
| 22 | thymol | OM | 0.14 | 3.19 | 1.68 | 56.22 | 65.47 | 47.32 | 61.35 | 59.36 |
| 23 | carvacrol | OM | 84.70 | 80.59 | 81.32 | 0.93 | 0.68 | 1.28 | 0.86 | 0.50 |
| 24 | β-caryophyllene | SH | 2.45 | 1.93 | 2.08 | 1.20 | 1.11 | 1.54 | 0.95 | 1.80 |
| 25 | α-humulene | SH | 0.06 | 0.08 | 0.09 | 0.08 | 0.08 | 0.11 | - | 0.13 |
| 26 | γ-muurolene | SH | - | - | - | 0.09 | 0.08 | - | - | 0.08 |
| 27 | germacrene D | SH | - | - | - | 1.52 | 1.36 | 2.25 | 1.23 | 2.22 |
| 28 | bicyclogermacrene | SH | - | - | - | 0.15 | 0.13 | 0.20 | 0.12 | 0.24 |
| 29 | β-bisabolene | SH | 0.15 | 0.24 | 0.16 | 1.28 | 1.17 | 1.52 | 1.02 | 1.56 |
| 30 | γ-cadinene | SH | - | - | - | 0.15 | 0.12 | 0.10 | 0.08 | 0.12 |
| 31 | δ-cadinene | SH | - | - | - | 0.47 | 0.39 | 0.39 | 0.33 | 0.47 |
| 32 | unknown | SH | - | - | - | 0.19 | 0.18 | 0.17 | 0.14 | 0.24 |
| Total content of compounds grouped in chemical classes | ||||||||||
| Monoterpene Hydrocarbons (MH) | 11.71 | 13.03 | 13.88 | 31.74 | 23.94 | 37.93 | 28.11 | 26.58 | ||
| Oxygenated Monoterpenes (OM) | 85.42 | 84.50 | 83.59 | 63.13 | 71.44 | 55.80 | 68.02 | 66.46 | ||
| Sesquiterpene Hydrocarbons (SH) | 2.66 | 2.25 | 2.33 | 5.13 | 4.61 | 6.27 | 3.87 | 6.85 | ||
| Others | 0.22 | 0.23 | 0.20 | - | - | - | - | - | ||
| Compound/ Enantiomer | LRI 1 | Oregano Subspecies/Hybrid | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Origanum vulgare ssp. viridulum × Origanum vulgare ssp. hirtum | Origanum heracleoticum L. | |||||||||
| CAR1 | CAR2 | CAR3 | THY1 | THY2 | THY3 | THY4 | THY5 | |||
| (a) | ||||||||||
| α-thujene | n.i. 2 | 950 | 65 | 64 | 64 | 65 | 65 | 65 | 65 | 64 |
| n.i. | 952 | 35 | 36 | 36 | 35 | 35 | 35 | 35 | 36 | |
| α-pinene | S/− | 984 | 12 | 12 | 12 | 9 | 9 | 9 | 9 | 10 |
| R/+ | 988 | 88 | 88 | 88 | 91 | 91 | 91 | 91 | 90 | |
| β-pinene | R/+ | 1026 | 73 | 73 | 72 | 73 | 74 | 74 | 74 | 73 |
| S/− | 1032 | 27 | 27 | 28 | 27 | 26 | 26 | 26 | 27 | |
| α-phellandrene | R/− | 1036 | 5 | 5 | 5 | 4 | 4 | 3 | 3 | 4 |
| S/+ | 1039 | 95 | 95 | 95 | 96 | 96 | 97 | 97 | 96 | |
| linalool | R/− | 1217 | 89 | 90 | 83 | 90 | 92 | 91 | 92 | 90 |
| S/+ | 1224 | 11 | 10 | 17 | 10 | 8 | 9 | 8 | 10 | |
| terpinene-4-ol | S/+ | 1300 | 54 | 54 | 54 | 52 | 50 | 49 | 48 | 49 |
| R/− | 1304 | 46 | 46 | 46 | 48 | 50 | 51 | 52 | 51 | |
| α-terpineol | R/+ | 1350 | 11 | 9 | 10 | 8 | 7 | 8 | 8 | 10 |
| S/− | 1360 | 89 | 91 | 90 | 92 | 93 | 92 | 92 | 90 | |
| (b) | ||||||||||
| α-thujene | n.i. 2 | 950 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| n.i. | 952 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| α-pinene | S/− | 984 | 6 | 7 | 6 | 3 | 5 | 4 | 4 | 3 |
| R/+ | 988 | 94 | 93 | 94 | 97 | 95 | 96 | 96 | 97 | |
| β-pinene | R/+ | 1026 | 79 | 79 | 81 | 79 | 79 | 84 | 84 | n.d. |
| S/− | 1032 | 21 | 21 | 19 | 21 | 21 | 16 | 16 | n.d. | |
| α-phellandrene | R/− | 1036 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| S/+ | 1039 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| linalool | R/− | 1217 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| S/+ | 1224 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| terpinene-4-ol | S/+ | 1300 | 58 | 59 | 58 | 63 | 62 | 61 | 63 | 62 |
| R/− | 1304 | 42 | 41 | 42 | 37 | 38 | 39 | 37 | 38 | |
| α-terpineol | R/+ | 1350 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | n.d. |
| S/− | 1360 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | n.d. | |
| Pathogen Indicator | Inhibition Halo Radius (mm) | ||||
|---|---|---|---|---|---|
| CAR1 | CAR2 | CAR3 | THY3 | THY5 | |
| Listeria monocytogenes OH | +++ | +++ | +++ | +++ | +++ |
| Listeria monocytogenes SA | + | + | + | +++ | +++ |
| Listeria monocytogenes CAL | +++ | +++ | +++ | +++ | +++ |
| Listeria innocua 1770 | ++ | ++ | ++ | +++ | +++ |
| Salmonella enterica Typhimurium LT2 | +++ | ++ | +++ | +++ | +++ |
| Salmonella enterica Give | +++ | +++ | +++ | +++ | +++ |
| Salmonella enterica Derby | +++ | +++ | +++ | +++ | +++ |
| Enterotoxigenic E. coli (ETEC) K88 | +++ | +++ | +++ | +++ | ++ |
| Pseudomonas putida WSC358 | +++ | ++ | +++ | +++ | ++ |
| Pseudomonas putida KT2240 | +++ | ++ | ++ | ++ | ++ |
| Pseudomonas fluorescens B13 | + | + | + | ++ | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zinno, P.; Guantario, B.; Lombardi, G.; Ranaldi, G.; Finamore, A.; Allegra, S.; Mammano, M.M.; Fascella, G.; Raffo, A.; Roselli, M. Chemical Composition and Biological Activities of Essential Oils from Origanum vulgare Genotypes Belonging to the Carvacrol and Thymol Chemotypes. Plants 2023, 12, 1344. https://doi.org/10.3390/plants12061344
Zinno P, Guantario B, Lombardi G, Ranaldi G, Finamore A, Allegra S, Mammano MM, Fascella G, Raffo A, Roselli M. Chemical Composition and Biological Activities of Essential Oils from Origanum vulgare Genotypes Belonging to the Carvacrol and Thymol Chemotypes. Plants. 2023; 12(6):1344. https://doi.org/10.3390/plants12061344
Chicago/Turabian StyleZinno, Paola, Barbara Guantario, Gabriele Lombardi, Giulia Ranaldi, Alberto Finamore, Sofia Allegra, Michele Massimo Mammano, Giancarlo Fascella, Antonio Raffo, and Marianna Roselli. 2023. "Chemical Composition and Biological Activities of Essential Oils from Origanum vulgare Genotypes Belonging to the Carvacrol and Thymol Chemotypes" Plants 12, no. 6: 1344. https://doi.org/10.3390/plants12061344