Accumulation of Toxic Arsenic by Cherry Radish Tuber (Raphanus sativus var. sativus Pers.) and Its Physiological, Metabolic and Anatomical Stress Responses
Abstract
:1. Introduction
2. Results
2.1. Arsenic Content and Effect on Biomass of Cherry Radish
2.2. Phosphorus and Sulphur Content under Arsenic Exposure
2.3. Phytohormone Content under Arsenic Exposure
2.4. Free Amino Acid Content under Arsenic Exposure
2.5. Total Anthocyanin Content and Ascorbic Acid under Arsenic Exposure
2.6. Anatomy of Cherry Radish under Arsenic Exposure
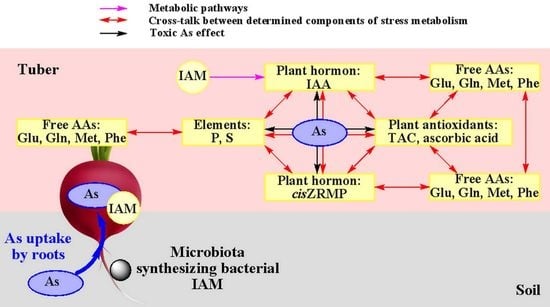
3. Discussion
4. Materials and Methods
4.1. Pot Experiment
4.2. Microscopic Observations
4.3. Determination of Elements
4.4. Determination of Phytohormones
4.5. Determination of Free Amino Acids
4.6. Determination of Ascorbic Acid
4.7. Determination of Total Anthocyanin Content
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eisler, R. Eisler’s Encyclopedia of Environmentally Hazardous Priority Chemicals; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 978-0-444-53105-6. [Google Scholar]
- Paltseva, A.; Cheng, Z.; Deeb, M.; Groffman, P.M.; Shaw, R.K.; Maddaloni, M. Accumulation of arsenic and lead in garden-grown vegetables: Factors and mitigation strategies. Sci. Total Environ. 2018, 640, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M. Arsenic uptake, toxicity, detoxification, and speciation in plants: Physiological, biochemical, and molecular aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef] [Green Version]
- Warming, M.; Hansen, M.G.; Holm, P.E.; Magid, J.; Hansen, T.H.; Trapp, S. Does intake of trace elements through urban gardening in Copenhagen pose a risk to human health? Environ. Pollut. 2015, 202, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Tremlová, J.; Sehnal, M.; Száková, J.; Goessler, W.; Steiner, O.; Najmanová, J.; Horáková, T.; Tlustoš, P. A profile of arsenic species in different vegetables growing in arsenic-contaminated soils. Arch. Agron. Soil Sci. 2017, 63, 918–927. [Google Scholar] [CrossRef]
- Armendariz, A.L.; Talano, M.A.; Travaglia, C.; Reinoso, H.; Oller, A.L.W.; Agostini, E. Arsenic toxicity in soybean seedlings and their attenuation mechanisms. Plant Physiol. Biochem. 2016, 98, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Riyazuddin, R.; Nisha, N.; Ejaz, B.; Khan, M.I.R.; Kumar, M.; Ramteke, P.W.; Gupta, R. A Comprehensive review on the heavy metal toxicity and sequestration in plants. Biomolecules 2022, 12, 43. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M. Antioxidants as modulators of arsenic-induced oxidative stress tolerance in plants: An overview. J. Hazard. Mater. 2022, 427, 127891. [Google Scholar] [CrossRef]
- Ranjan, A.; Gautam, S.; Michael, R.; Shukla, T.; Trivedi, P.K. Arsenic-induced galactinol synthase1 gene, AtGolS1, provides arsenic stress tolerance in Arabidopsis thaliana. Environ. Exp. Bot. 2023, 207, 105217. [Google Scholar] [CrossRef]
- Pickering, I.J.; Prince, R.C.; George, M.J.; Smith, R.D.; George, G.N.; Salt, D.E. Reduction and coordination of arsenic in Indian mustard. Plant Physiol. 2000, 122, 1171–1177. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.Q. Effects of compost and phosphate on plant arsenic accumulation from soils near pressure-treated wood. Environ. Pollut. 2004, 132, 435–442. [Google Scholar] [CrossRef]
- Liu, W.-J.; Wood, B.A.; Raab, A.; McGrath, S.P.; Zhao, F.-J.; Feldmann, J. Complexation of arsenite with phytochelatins reduces arsenite efflux and translocation from roots to shoots in Arabidopsis. Plant Physiol. 2010, 152, 2211–2221. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.; Juhasz, A.L.; Weber, J. Arsenic uptake and speciation in vegetables grown under greenhouse conditions. Environ. Geochem. Health. 2009, 31, 125–132. [Google Scholar] [CrossRef]
- Zemanová, V.; Pavlíková, D.; Hnilička, F.; Pavlík, M. Arsenic toxicity-induced physiological and metabolic changes in the shoots of Pteris cretica and Spinacia oleracea. Plants 2021, 10, 2009. [Google Scholar] [CrossRef]
- Martínez-Castillo, J.I.; Saldana-Robles, A.; Ozuna, C. Arsenic stress in plants: A metabolomic perspective. Plant Stress 2022, 3, 100055. [Google Scholar] [CrossRef]
- Farooq, M.A.; Islam, F.; Ali, B.; Najeeb, U.; Mao, B.; Gill, R.A.; Yan, G.; Siddique, K.H.M.; Zhou, W. Arsenic toxicity in plants: Cellular and molecular mechanisms of its transport and metabolism. Environ. Exp. Bot. 2016, 132, 42–52. [Google Scholar] [CrossRef]
- Zhang, M.; Lv, D.; Ge, P.; Bian, Y.; Chen, G.; Zhu, G.; Li, X.; Yan, Y. Phosphoproteome analysis reveals new drought response and defense mechanisms of seedling leaves in bread wheat (Triticum aestivum L.). J. Proteom. 2014, 109, 290–308. [Google Scholar] [CrossRef]
- Zemanová, V.; Pavlík, M.; Kyjaková, P.; Pavlíková, D. Fatty acid profiles of ecotypes of hyperaccumulator Noccaea caerulescens growing under cadmium stress. J. Plant Physiol. 2015, 180, 27–34. [Google Scholar] [CrossRef]
- Pavlíková, D.; Zemanová, V.; Pavlík, M.; Dobrev, P.I.; Hnilička, F.; Motyka, V. Response of cytokinins and nitrogen metabolism in the fronds of Pteris sp. under arsenic stress. PLoS ONE 2020, 15, e0233055. [Google Scholar] [CrossRef]
- Piacentini, D.; Rovere, F.D.; Sofo, A.; Fattorini, L.; Falasca, G.; Altamura, M.M. Nitric oxide cooperates with auxin to mitigate the alterations in the root system caused by cadmium and arsenic. Front. Plant Sci. 2020, 11, 1182. [Google Scholar] [CrossRef]
- Ćosić, T.; Motyka, V.; Raspor, M.; Sajid, S.; Devrnja, N.; Dobrev, P.I.; Ninković, S. Comprehensive phytohormone profiling of kohlrabi during in vitro growth and regeneration: The interplay with cytokinin and sucrose. Life 2022, 12, 1585. [Google Scholar] [CrossRef]
- Islam, E.; Khan, M.T.; Irem, S. Biochemical mechanisms of signaling: Perspectives in plants under arsenic stress. Ecotox. Environ. Safe 2015, 114, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Bano, K.; Kumar, B.; Alyemeni, M.N.; Ahmad, P. Protective mechanisms of sulfur against arsenic phytotoxicity in Brassica napus by regulating thiol biosynthesis, sulfur-assimilation, photosynthesis, and antioxidant response. Plant Physiol. Biochem. 2022, 188, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Liu, H.; Zhou, D.; Zhou, H.; Fan, G.; Chen, W.; Li, J.; Lou, L.; Gao, Y. Sulfur reduces the root-to-shoot translocation of arsenic and cadmium by regulating their vacuolar sequestration in wheat (Triticum aestivum L.). Front. Plant Sci. 2022, 13, 1032681. [Google Scholar] [CrossRef]
- Pavlík, M.; Pavlíková, D.; Staszková, L.; Neuberg, M.; Kaliszová, R.; Száková, J.; Tlustoš, P. The effect of arsenic contamination on amino acids metabolism in Spinacia oleracea L. Ecotoxicol. Environ. Saf. 2010, 73, 1309–1313. [Google Scholar] [CrossRef]
- Pathare, V.; Srivastava, S.; Suprasanna, P. Evaluation of effects of arsenic on carbon, nitrogen, and sulfur metabolism in two contrasting varieties of Brassica juncea. Acta Physiol. Plant. 2013, 35, 3377–3389. [Google Scholar] [CrossRef]
- Okunev, R.V. Free amino acid accumulation in soil and tomato plants (Solanum lycopersicum L.) associated with arsenic stress. Water Air Soil Pollut. 2019, 230, 253. [Google Scholar] [CrossRef]
- Kumar, N.; Gautam, A.; Dubey, A.K.; Ranjan, R.; Pandey, A.; Kumari, B.; Singh, G.; Mandotra, S.; Chauhan, P.S.; Srikrishna, S.; et al. GABA mediated reduction of arsenite toxicity in rice seedling through modulation of fatty acids, stress responsive amino acids and polyamines biosynthesis. Ecotox. Environ. Safe 2019, 173, 15–27. [Google Scholar] [CrossRef]
- Praveen, A.; Pandey, A.; Gupta, M. Protective role of nitric oxide on nitrogen-thiol metabolism and amino acids profiling during arsenic exposure in Oryza sativa L. Ecotoxicology 2020, 29, 825–836. [Google Scholar] [CrossRef]
- Finnegan, P.M.; Chen, W. Arsenic toxicity: The effects on plant metabolism. Front. Physiol. 2012, 3, 182. [Google Scholar] [CrossRef] [Green Version]
- Kruse, J.; Hansch, R.; Mendel, R.R.; Rennenberg, H. The role of root nitrate reduction in the systemic control of biomass partitioning between leaves and roots in accordance to the C/N-status of tobacco plants. Plant Soil 2010, 32, 387–403. [Google Scholar] [CrossRef]
- Zemanová, V.; Pavlík, M.; Pavlíková, D.; Tlustoš, P. The significance of methionine, histidine and tryptophan in plant responses and adaptation to cadmium stress. Plant Soil Environ. 2014, 60, 426–432. [Google Scholar] [CrossRef] [Green Version]
- Zemanová, V.; Popov, M.; Pavlíková, D.; Kotrba, P.; Hnilička, F.; Česká, J.; Pavlík, M. Effect of arsenic stress on 5-methylcytosine, photosynthetic parameters and nutrient content in arsenic hyperaccumulator Pteris cretica (L.) var. Albo-lineata. BMC Plant Biol. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Kofroňová, M.; Hrdinová, A.; Mašková, P.; Soudek, P.; Tremlová, J.; Pinkas, D.; Lipavská, H. Strong antioxidant capacity of horseradish hairy root cultures under arsenic stress indicates the possible use of Armoracia rusticana plants for phytoremediation. Ecotox. Environ. Safe 2019, 174, 295–304. [Google Scholar] [CrossRef]
- Hu, L.; Fan, H.; Wu, D.; Liao, Y.; Shen, F.; Liu, W.; Huang, R.; Zhang, B.; Wang, X. Effects of selenium on antioxidant enzyme activity and bioaccessibility of arsenic in arsenic-stressed radish. Ecotox. Environ. Safe 2020, 200, 110768. [Google Scholar] [CrossRef]
- Smirnoff, N. The function and metabolism of ascorbic acid in plants. Ann. Bot. 1996, 78, 661–669. [Google Scholar] [CrossRef] [Green Version]
- Saleem, M.H.; Mfarrej, M.F.B.; Alatawi, A.; Mumtaz, S.; Imran, M.; Ashraf, M.A.; Rizwan, M.; Usman, K.; Ahmad, P.; Ali, S. Silicon enhances morpho-physio-biochemical responses in arsenic stressed spinach (Spinacia oleracea L.) by minimizing its uptake. J. Plant Growth Regul. 1–20. [CrossRef]
- Horbowicz, M.; Kosson, R.; Sempruch, C.; Debski, H.; Koczkodaj, D. Effect of methyl jasmonate vapors on level of anthocyanins, biogenic amines and decarboxylases activity in seedlings of chosen vegetable species. Acta Sci. Pol.-Hortorum. Cult. 2014, 13, 3–15. [Google Scholar]
- Marconi, S.; Beni, C.; Ciampa, A.; Diana, G.; Neri, U.; Aromolo, R.; Sequi, P.; Valentini, M. Arsenic contamination in radish tuber investigated by means of MRI and ICO OES. J. Food Qual. 2010, 33, 529–543. [Google Scholar] [CrossRef]
- Nazir, A.; Rafique, F.; Ahmed, K.; Khan, S.A.; Khan, N.; Akbar, M.; Zafar, M. Evaluation of heavy metals effects on morpho-anatomical alterations of wheat (Triticum aestivum L.) seedlings. Microsc. Res. Tech. 2021, 84, 2517–2529. [Google Scholar] [CrossRef]
- Prerostova, S.; Dobrev, P.I.; Knirsch, V.; Jarosova, J.; Gaudinova, A.; Zupkova, B.; Prášil, I.T.; Janda, T.; Brzobohatý, B.; Skalák, J.; et al. Light quality and intensity modulate cold acclimation in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 2736. [Google Scholar] [CrossRef]
- Zemanová, V.; Pavlík, M.; Pavlíková, D. Cadmium toxicity induced contrasting patterns of concentrations of free sarcosine, specific amino acids and selected microelements in two Noccaea species. PLoS ONE 2017, 12, e0177963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlík, M.; Pavlíková, D.; Zemanová, V.; Hnilička, F.; Urbanová, V.; Száková, J. Trace elements present in airborne particulate matter–Stressors of plant metabolism. Ecotox. Environ. Safe 2012, 79, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Kofroňová, M.; Hrdinová, A.; Mašková, P.; Tremlová, J.; Soudek, P.; Petrová, P.; Pinkas, D.; Lipavská, H. Multi-component antioxidative system and robust carbohydrate status, the essence of plant arsenic tolerance. Antioxidants 2020, 9, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Aal, E.-S.M.; Hucl, P. A rapid method for quantifying total anthocyanins in blue aleurone and purple pericarp wheats. Cereal Chem. 1999, 76, 350–354. [Google Scholar] [CrossRef]




| As Content in Plant Parts (mg/kg DM) | |||
|---|---|---|---|
| leaves | tubers | root | |
| As0 | nd | 3.6 ± 0.2 a | 12.3 ± 2.8 a |
| As20 | nd | 4.7 ± 0.2 b | 25.5 ± 6.7 a |
| As100 | 6.6 ± 0.9 | 31.1 ± 0.5 c | 353.0 ± 55.8 b |
| As0 | As20 | As100 | |
|---|---|---|---|
| Total AAs content (μmol/kg FM) | 3676.1 ± 443.2 b | 4291.6 ± 376.8 c | 3020.9 ± 376.9 a |
| Transport AAs (μmol/kg FM) | 2081.7 ± 210.7 a | 2416.9 ± 285.9 b | 2223.1 ± 289.7 ab |
| Aromatic AAs (μmol/kg FM) | 56.7 ± 18.4 b | 32.2 ± 9.8 a | 19.2 ± 1.2 a |
| Gln (μmol/kg FM) | 1127.2 ± 122.5 a | 1306.4 ± 189.8 ab | 1508.0 ± 214.4 b |
| Met (μmol/kg FM) | 2.7 ± 1.4 a | 2.6 ± 1.2 a | 5.7 ± 0.2 b |
| Glu/Gln (−) | 0.4 ± 0.03 a | 0.4 ± 0.06 a | 0.2 ± 0.02 b |
| Location | Suchdol, Prague (50°8′8″ N, 14°22′43″ E) |
|---|---|
| Soil type and subtype | Haplic Chernozem |
| pHKCl (−) | 7.1 |
| Cation exchange capacity (mmol(+)/kg) | 258 |
| Organic carbon (%) | 1.83 |
| Pseudo-total content of As (mg/kg) | 16 ± 1.7 |
| Water-soluble fraction of As (mg/kg) | 0.10 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlíková, D.; Pavlík, M.; Zemanová, V.; Novák, M.; Doležal, P.; Dobrev, P.I.; Motyka, V.; Kraus, K. Accumulation of Toxic Arsenic by Cherry Radish Tuber (Raphanus sativus var. sativus Pers.) and Its Physiological, Metabolic and Anatomical Stress Responses. Plants 2023, 12, 1257. https://doi.org/10.3390/plants12061257
Pavlíková D, Pavlík M, Zemanová V, Novák M, Doležal P, Dobrev PI, Motyka V, Kraus K. Accumulation of Toxic Arsenic by Cherry Radish Tuber (Raphanus sativus var. sativus Pers.) and Its Physiological, Metabolic and Anatomical Stress Responses. Plants. 2023; 12(6):1257. https://doi.org/10.3390/plants12061257
Chicago/Turabian StylePavlíková, Daniela, Milan Pavlík, Veronika Zemanová, Milan Novák, Petr Doležal, Petre I. Dobrev, Václav Motyka, and Kamil Kraus. 2023. "Accumulation of Toxic Arsenic by Cherry Radish Tuber (Raphanus sativus var. sativus Pers.) and Its Physiological, Metabolic and Anatomical Stress Responses" Plants 12, no. 6: 1257. https://doi.org/10.3390/plants12061257