Water Content of Plant Tissues: So Simple That Almost Forgotten?
Abstract
:1. Introduction
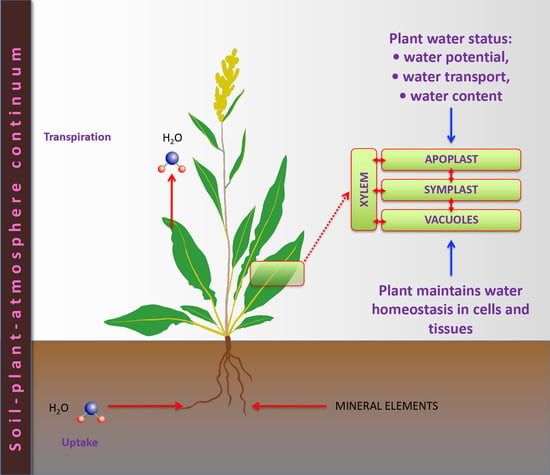
2. Structural Organization of Water in Plant Tissues
3. Water Content of Different Plant Parts
3.1. Water in Leaves
3.2. Water in Fruits
3.3. Water in Seeds
3.4. Water in Vegetative Propagules
3.5. Water in Vegetables
3.6. Water Storage in Woody Plants
4. Effect of Environmental Factors on Water Content
4.1. Effect of Air Humidity on Tissue Water Content
4.2. Effect of Mineral Nutrition on Water Content
4.3. Effect of Biotic Interactions on Water Content
4.4. Water Relationships in Clonal Plants as a Part of Physiological Integration
4.5. Succulence as Drought Avoidance
4.6. Effect of Salinity on Water Content
5. Conclusions
- (i)
- The phenomena of water storage and water content of tissues, apart from drought response studies, has been mostly assessed in the context of succulence syndrome either in classical succulents or succulent halophytes;
- (ii)
- High organ- and species-specific variability can be found with respect to water content in plants;
- (iii)
- The presence of “succulence” and other forms of water storage in particular plant species is not necessarily associated with relatively high water content;
- (iv)
- Salinity can have different effects on water content, even among salt-tolerant and salt-accumulating species;
- (v)
- The expression of absolute water content on a dry biomass basis makes easily noticeable functional sense.
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chavarria, G.; dos Santos, H.P. Plant water relations: Absorption, transport and control mechanisms. In Advances in Selected Plant Physiology Aspects; Montanaro, G., Dischio, B., Eds.; IntechOpen: Rijeka, Croatia, 2012; pp. 105–132. [Google Scholar]
- Vadez, V.; Kholova, J.; Medina, S.; Kakkera, A.; Anderberg, H. Transpiration efficiency: New insights into an old story. J. Exp. Bot. 2014, 65, 6141–6153. [Google Scholar] [CrossRef] [Green Version]
- Privalov, P.L.; Crane-Robinson, C. Role of water in the formation of macromolecular structures. Eur. Biophys. J. 2017, 46, 203–224. [Google Scholar] [CrossRef] [Green Version]
- Filipović, A. Water plant and soil relation under stress situations. In Soil Moisture Importance; Meena, R.S., Datta, R., Eds.; IntechOpen: London, UK, 2021; pp. 73–103. [Google Scholar]
- Spomer, L.A. Techniques for measuring plant water. HortScience 1985, 20, 1021–1028. [Google Scholar] [CrossRef]
- De Swaef, T.; Pieters, O.; Appeltans, S.; Borra-Serrano, I.; Coudron, W.; Couvreur, V.; Garré, S.; Lootens, P.; Nicolai, B.; Pols, L.; et al. On the pivotal role of water potential to model plant physiological processes. Silico Plants 2022, 4, 1–28. [Google Scholar] [CrossRef]
- Turner, N.C. Techniques and experimental approaches for the measurement of plant water status. Plant Soil 1981, 58, 339–366. [Google Scholar] [CrossRef]
- Matin, M.A.; Brown, J.H.; Ferguson, H. Leaf water potential, relative water content, and diffusive resistance as screening techniques for drought resistance in barley. Agron. J. 1989, 81, 100–105. [Google Scholar] [CrossRef]
- Boyer, J.S.; James, R.A.; Munns, R.; Condon, T.A.G.; Passioura, J.B. Osmotic adjustment leads to anomalously low estimates of relative water content in wheat and barley. Funct. Plant Biol. 2008, 35, 1172–1182. [Google Scholar] [CrossRef]
- Garnier, E.; Shipley, B.; Roumet, C.; Laurent, G. A standardized protocol for the determination of specific leaf area and leaf dry matter content. Funct. Ecol. 2001, 15, 688–695. [Google Scholar] [CrossRef]
- Pritchard, M.K.; Scanlon, M.G. Mapping dry matter and sugars in potato tubers for prediction of whole tuber process quality. Can. J. Plant Sci. 1997, 77, 461–467. [Google Scholar] [CrossRef]
- Al Hasan, M.; Estrelles, E.; Soriano, P.; López-Gresa, M.P.; Belles, J.M.; Boscaiu, M.; Vicente, O. Unraveling salt tolerance mechanisms in halophytes: A comparative study on four Mediterranean Limonium species with different geographic distribution patterns. Front. Plant Sci. 2017, 8, 1438. [Google Scholar] [CrossRef] [Green Version]
- Nisar, F.; Gul, B.; Aziz, I.; Hameed, A.; Egan, T. Increasing salinity leads to differential growth and H2O2 homeostasis in plants produced from heteromorphic seeds of the succulent halophyte Arthrocnemum indicum. Plant Physiol. Biochem. 2021, 166, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.-H.; Chen, M.; Song, J.; Wang, B.-S. Increase in aquaporin activity is involved in leaf succulence of the euhalophyte Suaeda salsa, under salinity. Plant Sci. 2009, 176, 200–205. [Google Scholar] [CrossRef]
- Pisis, P.; Anagnostopopou-Konsta, A.; Apekis, L. A dielectric study of the state of water in plant stems. J. Exp. Bot. 1987, 38, 1528–1540. [Google Scholar] [CrossRef]
- Steudle, E. Water transport across plant tissue: Role of water channels. Biol. Cell 1997, 89, 259–273. [Google Scholar] [CrossRef]
- Jensen, H.E.; Jensen, K.H.; Rosbjerg, D. Plant water relationships and evapotranspiration. In Hydrological Interactions Between Atmosphere, Soil and Vegetation; Kienitz, G., Milly, P.C.D., Van Genuchten, M.T., Rosjberg, D., Shuttleworth, W.J., Eds.; International Association of Hydrological Sciences: Wallingford, UK, 1991; pp. 295–307. [Google Scholar]
- Maylani, E.D.; Yuniati, R.; Wardhana, W. The effect of leaf surface character on the ability of water hyacinth, Eichornia crassipes (Mart.) Solms. to transpire water. IOP Conf. Ser. Mater. Sci. Eng. 2020, 902, 012070. [Google Scholar] [CrossRef]
- Niklas, K.J. A mechanical perspective on foliage leaf form and function. New Phytol. 1999, 143, 19–31. [Google Scholar] [CrossRef]
- Vincent, J.F.V. The influence of water content on the stiffness and fracture properties of grass leaves. Grass Forage Sci. 1983, 38, 107–114. [Google Scholar] [CrossRef]
- Vertucci, C.W.; Leopold, A.C. The relationship between water binding and desiccation tolerance in tissues. Plant Physiol. 1987, 85, 232–238. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.Q. Dielectric relaxation of water and water-plasticized biomolecules in relation to cellular water organization, cytoplasmic viscosity, and desiccation tolerance in recalcitrant seed tissues. Plant Physiol. 2000, 124, 1203–1215. [Google Scholar] [CrossRef] [Green Version]
- Malavasi, U.C.; Davis, A.S.; de Matos Malavasi, M. Estimating water in living woody stems—A review. Cerne 2016, 22, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Fiorani, F.; Rascher, U.; Jahnke, S.; Schurr, U. Imaging plants dynamics in heterogenic environments. Curr. Opin. Biotechnol. 2012, 23, 227–235. [Google Scholar] [CrossRef]
- Windt, C.W.; Nabel, M.; Kochs, J.; Jahnke, S.; Schurr, U. A mobile NMR sensor and relaxometric method to non-destructively monitor water and dry matter content in plants. Front. Plant Sci. 2021, 12, 617768. [Google Scholar] [CrossRef] [PubMed]
- Goss, J.A. Physiology of Plants and Their Cells; Pergamon Press: New York, NY, USA, 2013. [Google Scholar]
- Garnier, E.; Laurent, G. Leaf anatomy, specific mass and water content in congeneric annual and perennial grass species. New Phytol. 1994, 128, 725–736. [Google Scholar] [CrossRef]
- Lambers, H.; Poorter, H. Inherent variation in growth rate between higher plants: A search for physiological causes and ecological consequences. In Advances in Ecological Research; Caswell, H., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 34, pp. 283–362. [Google Scholar]
- Poorter, H.; Bergkotte, M. Chemical composition of 24 wild species differing in relative growth rate. Plant Cell Environ. 1992, 15, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Dijkstra, P.; Lambers, H. A physiological analysis of genetic variation in relative growth rate within Plantago major L. Funct. Ecol. 1989, 3, 577–587. [Google Scholar] [CrossRef]
- A’yuni, D.Q.; Djaeni, M.; Asiah, N.; Subagio, A. Enhancement of onion bulb drying with air dehumidification assisted dryer. AIMS Agric. Food 2022, 7, 168–183. [Google Scholar] [CrossRef]
- Snellgrove, R.C.; Splittstoesser, W.E.; Stribley, D.P.; Tinker, P.B. The distribution of carbon and the demand of the fungal symbiont in leek plants with vesicular-arbuscular mycorrhizas. New Phytol. 1982, 92, 75–87. [Google Scholar] [CrossRef]
- Bayat, F.; Rezvani, S. Effect of harvesting time and moisture on mechanical properties of garlic (Allium sativum L.) skin. Agric. Eng. Int. CIGGR J. 2012, 14, 161–167. [Google Scholar]
- Santoso, U.; Kubo, K.; Ota, T.; Tadokoro, T.; Maekawa, A. Nutrient composition of kopyor coconuts (Cocos nucifera L.). Food Chem. 1996, 57, 299–304. [Google Scholar] [CrossRef]
- Jain, R.; Singh, M.K.; Swaroop, K.; Reddy, M.V.; Janakiram, T.; Kumar, P.; Pinder, R. Optimization of spacing and nitrogen dose for growth and flowering of statice (Limonium sinuatum). Indian J. Agric. Sci. 2018, 88, 1108–1114. [Google Scholar] [CrossRef]
- Zia, S.; Egan, T.P.; Khan, M.A. Growth and selective ion transport of Limonium stocksii Plumbaginaceae under saline conditions. Pak. J. Bot. 2008, 40, 697–709. [Google Scholar]
- Jiang, F.; Timergalina, L.; Kudoyarova, G.; Jeschke, W.D.; Hartung, W. Growth and development of the facultative root hemiparasite Rhinanthus minor after removal of its host. Funct. Plant Biol. 2007, 34, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Klaren, C.H.; van de Dijk, S.J. Water relations of the hemiparasite Rhinanthus serotinus before and after attachment. Physiol. Plant. 1976, 38, 121–125. [Google Scholar] [CrossRef]
- Prokopoviča, V.; Ievinsh, G. Ranunculus sceleratus as a model species to decrypt the role of ethylene in plant adaptation to salinity. Plants 2023, 12, 370. [Google Scholar] [CrossRef] [PubMed]
- Vicente, A.R.; Powell, A.; Greve, C.L.; Labavitch, J.M. Cell wall disassembly events in boysenberry (Rubus idaeus L. × Rubus ursinus Cham, & Schldl.) fruit development. Funct. Plant Biol. 2007, 34, 614–623. [Google Scholar]
- Hajjar, G.; Quellec, S.; Challois, S.; Bousset-Vaslin, L.; Joly, G.; Langrume, C.; Deleu, C.; Leport, L.; Musse, M. Characterization of the water shortage effects on potato tuber tissues during growth using MRI relaxometry and biochemical parameters. Plants 2022, 11, 1918. [Google Scholar] [CrossRef]
- Solaiman, A.H.M.; Nishizawa, T.; Roy, T.S.; Rahnam, M.; Chakraborty, R.; Choudhary, J.; Sarkar, M.D.; Hasanuzzaman, M. Yield, dry matter, specific gravity and color of three Bangladeshi local potato cultivars as influenced by stage of maturity. J. Plant Sci. 2015, 19, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Ievinsh, G.; Andersone-Ozola, U.; Sieriņa, A. Development and physiological performance of hydroponically-grown ornamental indoor plants in relation to their potential use in botanical biofilters: Effect of mineral nutrient availability. Proc. Latv. Acad. Sci. B 2022, 76, 278–288. [Google Scholar] [CrossRef]
- Matse, D.T.; Huang, C.-H.; Huang, Y.-M.; Yen, M.-Y. Nitrogen uptake and growth of white clover inoculated with indigenous and exotic Rhizobium strains. J. Plant Nutr. 2020, 43, 2013–2027. [Google Scholar] [CrossRef]
- Saura-Mas, S.; Lloret, F. Leaf and shoot water content and leaf dry matter content of Mediterranean woody species with different post-fire regenerative strategies. Ann. Bot. 2007, 99, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Schieving, F.; Stuefer, J.F.; Anten, N.P.R. The effects of mechanical stress and spectral shading on the growth and allocation of ten genotypes of a stoloniferous plant. Ann. Bot. 2007, 99, 121–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, X.; Zhang, W.; Du, T.; Kang, S.; Davies, W.J. Responses of water accumulation and solute metabolism in tomato fruit to water scarcity and implications for main fruit quality variables. J. Exp. Bot. 2020, 71, 1249–1264. [Google Scholar] [CrossRef]
- Schreiber, L.; Riederer, M. Ecophysiology of cuticular transpiration: Comparative investigation of cuticular water permeability of plant species from different habitats. Oecologia 1996, 107, 426–432. [Google Scholar] [CrossRef]
- Matthews, M.A.; Shackel, K.A. Growth and water transport in fleshy fruit. In Vascular Transport in Plants; Holbrook, N.M., Zwieniecki, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 181–197. [Google Scholar]
- Brummell, D.A.; Cin, V.D.; Crisosto, C.H.; Labavitch, J.M. Cell wall metabolism during maturation, ripening and senescence of peach fruit. J. Exp. Bot. 2004, 55, 2029–2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, X.; Li, H.; Zhang, W.; Yao, Z.; Wang, Y.; Du, T. Water transport in fleshy fruits: Research advances, methodologies, and future directions. Physiol. Plant. 2021, 172, 2203–2216. [Google Scholar] [CrossRef] [PubMed]
- Roch, L.; Prigent, S.; Klose, H.; Cakpo, C.-B.; Beauvoit, B.; Deborde, C.; Foullen, L.; van Delft, P.; Jacob, D.; Usadel, B.; et al. Biomass composition explains fruit relative growth rate and discriminates climacteric from non-climacteric species. J. Exp. Bot. 2020, 71, 5823–5836. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreno, C.; De Pascual-Teresa, S.; De Ancos, B.; Cano, M.P. Nutritional value of fruits. In Handbook of Fruits and Fruit Processing; Hui, Y.H., Barta, J., Cano, M.P., Gusek, T., Sidhu, J.S., Sinha, N., Eds.; Blackwell Science: Ames, IA, USA, 2006; pp. 29–43. [Google Scholar]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Bonafe, E.G.; Suzuki, R.M.; Souza, N.E.; Matsushita, M.; Visentainer, J.V. Proximate composition, mineral contents and fatty acid composition of the different parts and dried peels of tropical fruits cultivated in Brazil. J. Braz. Chem. Soc. 2017, 28, 308–318. [Google Scholar] [CrossRef]
- Saputri, A.; Aminy, N.; Rahmadi, I.; Nasution, S.; Mareta, D.T.; Permana, L.; Nurdin, S.U. Comparison of proximate analysis value of fresh fruits and vacuum fried fruit chips. E3S Web Confer. 2022, 344, 04001. [Google Scholar] [CrossRef]
- Untalan, M.K.C.; Perez, I.F.R.; Reyes, G.H.; Escalona, K.M.H.; De Guzman, L.D.; Lummangles, R.F.L. Proximate analysis and antioxidant properties of selected fruits in Batangas. Asia Pacific J. Multidisc. Res. 2015, 3, 41–45. [Google Scholar]
- Yong, J.W.H.; Ge, L.; Ng, Y.F.; Tan, S.N. The chemical composition and biological properties of coconut (Cocos nucifera L.) water. Molecules 2009, 14, 5144–5164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, R.H. Temporal patterns of seed quality development, decline, and timing of maximum quality during seed development and maturation. Seed Sci. Res. 2019, 29, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Carrera-Castaño, G.; Calleja-Cabrera, J.; Pernas, M.; Gómez, L.; Oñate-Sánchez, L. An updated overview on the regulation of seed germination. Plants 2020, 9, 703. [Google Scholar] [CrossRef] [PubMed]
- Steinbrecher, T.; Leubner-Metzger, G. The biomechanics of seed germination. J. Exp. Bot. 2017, 68, 765–783. [Google Scholar] [CrossRef] [Green Version]
- Bradford, K.J. Seed storage and longevity. In Seed Production and Quality; Seed Biotechnology Center: Davis, CA, USA, 2004; pp. 76–84. [Google Scholar]
- De Vitis, M.; Hay, F.R.; Dickie, J.B.; Trivedi, C.; Choi, J.; Fiegener, R. Seed storage: Maintaining seed viability and vigor for restoration use. Restor. Ecol. 2020, 28, S249–S255. [Google Scholar] [CrossRef]
- Ekin, Z. Some analytical quality characteristics for evaluating the utilization and consumption of potato (Solanum tuberosum L.) tubers. Afr. J. Biotechnol. 2011, 10, 6001–6010. [Google Scholar]
- Goffart, J.P.; Olivier, M.; Frankinet, M. Potato crop nitrogen status assessment to improve N fertilization management and efficiency: Past–present–future. Potato Res. 2008, 51, 355–383. [Google Scholar] [CrossRef]
- Fatideh, M.M.; Asil, M.H. Onion yield, quality and storability as affected with different soil moisture and nitrogen regimes. South West. J. Hortic. Biol. Environ. 2012, 3, 146–165. [Google Scholar]
- Kiura, I.N.; Gichimu, B.M.; Rotich, F. Proximate and nutritional composition of stored bulb onions as affected by harvest and postharvest treatments. Int. J. Agron. 2021, 2021, 5532349. [Google Scholar] [CrossRef]
- Utama-ang, N.; Cheewinworasak, T.; Simawonthamgul, N.; Samakradhamrongthai, R.S. Effect of drying condition of Thai garlic (Allium sativum L.) on physicochemical and sensory properties. Int. Food Res. J. 2018, 25, 1365–1372. [Google Scholar]
- Duke, J.A.; Atchley, A.A. Handbook on Proximate Analysis Tables of Higher Plants; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Hanif, R.; Iqbal, Z.; Iqbal, M.; Hanif, S.; Rasheed, M. Use of vegetables as nutritional food: Role in human health. J. Agric. Biol. Sci. 2006, 1, 22–26. [Google Scholar]
- Toivonen, P.M.A. Postharvest physiology of vegetables. In Handbook of Vegetables and Vegetable Processing; Sinha, N.K., Ed.; Blackwell Publishing: Ames, IA, USA, 2011; pp. 199–220. [Google Scholar]
- Holbrook, N.M. Stem water storage. In Plant Stem: Physiology and Functional Morphology; Gartner, B.L., Ed.; Academic Press: San Diego, CA, USA, 1995; pp. 157–174. [Google Scholar]
- Skelton, R. Of storage and stems: Examining the role of stem water storage in plant water balance. Plant Physiol. 2019, 179, 1433–1434. [Google Scholar] [CrossRef] [Green Version]
- Scholz, F.G.; Phillips, N.G.; Bucci, S.J.; Meinzer, F.C.; Goldstein, G. Hydraulic capacitance: Biohysics and functional significance of internal water sources in relation to tree size. In Size- and Age-Related Changes in Tree Structure and Function; Meinzer, F.C., Lachenbruch, B., Dawson, T.E., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 341–362. [Google Scholar]
- Jupa, R.; Plavcová, L.; Gloser, V.; Jansen, S. Linking xylem water storage with anatomical parameters in five temperate tree species. Tree Physiol. 2016, 36, 756–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, G.; Andrade, J.L.; Meinzer, F.C.; Holbrook, N.M.; Cavelier, J.; Jackson, P.; Celis, A. Stem water storage and diurnal patterns of water use in tropical forest canopy trees. Plant Cell Environ. 1998, 21, 397–406. [Google Scholar] [CrossRef]
- Knipfer, T.; Reyes, C.; Earles, J.M.; Berry, Z.C.; Johnson, D.M.; Brodersen, C.R.; McElrone, A.J. Spatiotemporal coupling of vessel cavitation and discharge of stored xylem water in a tree sapling. Plant Physiol. 2019, 179, 1658–1668. [Google Scholar] [CrossRef] [Green Version]
- Kevers, C.; Franck, T.; Strasser, R.J.; Dommes, J.; Gaspar, T. Hyperhydricity of micropropagated shoots: A typically stress-induced change of physiological state. Plant Cell Tissue Organ Cult. 2004, 77, 181–191. [Google Scholar] [CrossRef]
- Polivanova, O.B.; Bedarev, V.A. Hyperhdricity in plant tissue culture. Plants 2022, 11, 3313. [Google Scholar] [CrossRef]
- Rojas-Martinez, L.; Visser, R.G.F.; de Klerk, G.-J. The hyperhydricity syndrome: Waterlogging of plant tissues as a major cause. Propag. Ornam. Plants 2010, 10, 169–175. [Google Scholar]
- van den Dries, N.; Gianni, S.; Czerednik, A.; Krens, F.A.; de Klerk, G.-J.M. Flooding of the apoplast is a key factor in the development of hyperhydricity. J. Exp. Bot. 2013, 84, 5221–5230. [Google Scholar] [CrossRef] [PubMed]
- Purmale, L.; Jēkabsone, A.; Andersone-Ozola, U.; Karlsons, A.; Osvalde, A.; Ievinsh, G. Comparison of in vitro and in planta heavy metal tolerance and accumulation potential of different Armeria maritima accessions from a dry coastal meadow. Plants 2022, 11, 2014. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. Humidity in plant tissue culture vessels. Biosyst. Eng. 2004, 88, 231–241. [Google Scholar] [CrossRef]
- Apostolo, N.M.; Llorente, B.E. Anatomy of normal and hyperhydric leaves and shoots of in vitro grown Simmondsia chinensis (Link) Schn. In Vitro Cell. Dev. Biol. Plant 2000, 36, 243–249. [Google Scholar] [CrossRef]
- Kevers, C.; Gaspar, T. Vitrification of carnation in vitro: Changes in water content extracellular space, air volume, and ion levels. Physiol. Végét. 1986, 24, 647–653. [Google Scholar]
- Gribble, K.; Tingle, J.; Sarafis, V.; Heaton, A.; Holford, P. Position of water in vitrified plants visualized by NMR imaging. Protoplasma 1998, 201, 110–114. [Google Scholar] [CrossRef]
- Ievinsh, G.; Andersone-Ozola, U.; Karlsons, A.; Osvalde, A. Tripleurospermum maritimum from a coastal shingle beach: Nitrophilic status, tolerance to salinity and heavy metals. Environ. Exp. Biol. 2021, 19, 265–273. [Google Scholar]
- Ievinsh, G.; Andersone-Ozola, U.; Zeipiņa, S. Comparison of the effects of compost and vermicompost soil amendments in organic production of four herb species. Biol. Agric. Hortic. 2020, 36, 267–282. [Google Scholar] [CrossRef]
- Aslam, Z.; Ahmad, A.; Idrees, M.; Iqbal, N.; Akbar, G.; Ali, U.; Ibrahim, M.U.; Bellitürk, K.; Naeem, S.; Nawaz, M.; et al. Comparative analysis of nutritional sources on the morpho-physiological characteristics of mung bean (Vigna radiata). Int. J. Agric. Food Sci. 2020, 4, 314–322. [Google Scholar] [CrossRef]
- Kováčik, P.; Šimanský, V.; Smoleń, S.; Neupauer, J.; Olšovská, K. The effect of vermicompost and earthworms (Eisenia fetida) application on phytomass and macroelement concentration and tetanic ratio in carrot. Agronomy 2022, 12, 2770. [Google Scholar] [CrossRef]
- Hussain, N.; Abbasi, S.A. Efficacy of the vermicomposts of different organic wastes as “clean” fertilizers: State-of-the-art. Sustainability 2018, 10, 1205. [Google Scholar] [CrossRef] [Green Version]
- Piccolo, A.; Celano, G.; Pietramellara, G. Effects of fractions of coal-derived humic substances on seed germination and growth of seedlings (Lactuca sativa and Lycopersicon esculentum). Biol. Fertil. Soils 1993, 16, 11–15. [Google Scholar] [CrossRef]
- Ogburn, R.M.; Edwards, E.J. The ecological water-use strategies of succulent plants. In Advances in Botanical Research; Kader, J.-C., Delseny, M., Eds.; Academic Press: Burlington, MA, USA, 2010; Volume 55, pp. 179–225. [Google Scholar]
- Aung, K.; Jiang, Y.; He, S.Y. The role of water in plant-microbe interactions. Plant J. 2018, 93, 771–780. [Google Scholar] [CrossRef] [Green Version]
- Xin, X.-F.; Nomura, K.; Aung, K.; Velásquez, A.C.; Yao, J.; Boutrot, F.; Chang, J.H.; Zipfel, C.; He, S.Y. Bacteria establish an aqueous living space in plants crucial for virulence. Nature 2016, 539, 524–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegenauer, V.; Körner, M.; Albert, M. Plants under stress by parasitic plants. Curr. Opin. Plant Biol. 2017, 38, 34–41. [Google Scholar] [CrossRef]
- Jiang, F.; Jeschke, W.D.; Hartung, W. Water flows in the parasitic association Rhinanthus minor/Horedum vulgare. J. Exp. Bot. 2003, 54, 1985–1993. [Google Scholar] [CrossRef]
- Jiang, F.; Veselova, S.; Veselov, D.; Kudoyarova, G.; Leschke, W.D.; Hartung, W. Cytokinin flows from Hordeum vulgare to the hemiparasite Rhinanthus minor and the influence of infection on host and parasite cytokinins relations. Funct. Plant Biol. 2005, 32, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.C.; Watkinson, A.R. The host range and selectivity of a parasitic plant: Rhinanthus minor. Oecologia 1989, 78, 401–406. [Google Scholar] [CrossRef]
- Matthies, D. Closely related parasitic plants have similar host requirements and related effects on hosts. Ecol. Evol. 2021, 11, 12011–12024. [Google Scholar] [CrossRef] [PubMed]
- Hibberd, J.M.; Quick, W.P.; Press, M.C.; Scholes, J.D.; Jeschke, W.D. Solute fluxes from tobacco to the parasitic angiosperm Orobanche cernua and the influence of infection on host carbon and nitrogen relations. Plant Cell Environ. 1999, 22, 937–947. [Google Scholar] [CrossRef]
- Furuhashi, T.; Furuhashi, K.; Weckwerth, W. The parasitic mechanism of the holostemparasitic plant Cuscuta. J. Plant Interact. 2011, 6, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Jeschke, W.D.; Baig, A.; Hilpert, A. Sink-stimulated photosynthesis, increased transpiration and increased demand-dependent stimulation of nitrate uptake: Nitrogen and carbon relations in the parasitic association Cuscuta reflexa–Coleus blumei. J. Exp. Bot. 1997, 48, 915–925. [Google Scholar] [CrossRef] [Green Version]
- Inoue, T.; Yamauchi, Y.; Eltayeb, A.H.; Sanejima, H.; Babiker, A.G.T.; Sugimoto, Y. Gas exchange of root hemi-parasite Striga hermonthica and its host Sorghum bicolor under short-term soil water stress. Biol. Plant. 2013, 57, 773–777. [Google Scholar] [CrossRef]
- Augé, R.M. Water relations, drought and vesicular-arbuscular symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Fernández-Lizarazo, J.C.; Moreno-Fonseca, L.P. Mechanisms for tolerance to water-deficit stress in plants inoculated with arbuscular mycorrhizal fungi. A review. Agron. Colomb. 2016, 34, 179–189. [Google Scholar] [CrossRef]
- Püschel, D.; Bitterlich, M.; Rydlová, J.; Jansa, J. Facilitation of plant water uptake by an arbuscular mycorrhizal fungus: A Gordian knot of roots and hyphae. Mycorrhiza 2020, 30, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.-S.; Xia, R.-X. Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J. Plant Physiol. 2006, 163, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, N.; Armanda, E.; Duque, E.; Roldán, A.; Azcón, R. Contribution of arbuscular mycorrhizal fungi and/or bacteria to enhancing plant drought tolerance under natural soil conditions: Effectiveness of autochtonous or allochtonous strains. J. Plant Physiol. 2015, 174, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Zarik, L.; Meddich, A.; Hijri, M.; Hafidi, M.; Ouhammou, A.; Ouhamane, L.; Duponnois, R.; Boumezzaogh, A. Use of arbuscular mycorrhizal fungi to improve the drought tolerance of Cupressus atlantica G. Comptes Rendus Biol. 2016, 339, 185–195. [Google Scholar] [CrossRef]
- Duc, N.H.; Csintalan, Z.; Posta, K. Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants. Plant Physiol. Biochem. 2018, 132, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Kumar, A.; Al-Dbass, A.M.; Alqarawi, A.A.; Al-Arjani, A.-B.F.; Singh, G.; Farooq, M.; Abd_Allah, E.F. Arbuscular mycorrhizal fungi and biochar improves drought tolerance in chickpea. Saudi J. Biol. Sci. 2019, 26, 614–624. [Google Scholar] [CrossRef]
- Augé, R.M.; Schekel, K.A.; Wample, R.L. Leaf water and carbohydrate status of VA mycorrhizal rose exposed to drought stress. Plant Soil 1987, 99, 291–302. [Google Scholar] [CrossRef]
- Delavaux, C.S.; Smith-Ramesh, L.M.; Kuebbing, S.E. Beyond nutrients: A meta-analysis of the diverse effects of arbuscular mycorrhizal fungi on plants and soils. Ecology 2017, 98, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.V.B.; Vilar, J.J.; Burity, H.A.; de França, F.P. Alleviation of water stress effects in cowpea by Bradyrhizobium spp. inoculation. Plant Soil 1999, 207, 67–75. [Google Scholar] [CrossRef]
- Zacarías, J.J.J.; Altamirano-Hernández, J.; Cabriales, J.J.P. Nitrogenase activity and trhelose content in nodules of drought-stressed common beans infected with effective (Fix+) and ineffective (Fix–) rhizobia. Soil Biol. Biochem. 2004, 36, 1975–1981. [Google Scholar] [CrossRef]
- Irshad, A.; Rehman, R.N.U.; Abrar, M.M.; Saeed, Q.; Sharif, R.; Hu, T. Contribution of rhizobium-legume symbiosis in salt stress tolerance in Medicago truncatula evaluated through photosynthesis, antioxidant enzymes, and compatible solutes accumulation. Sustainability 2021, 13, 3369. [Google Scholar] [CrossRef]
- Vannier, N.; Mony, C.; Bittebiere, A.-K.; Theis, K.R.; Rosenberg, E.; Vandenkoornhuyse, P. Clonal plants as meta-holobionts. mSystems 2019, 4, e00213–e00218. [Google Scholar] [CrossRef] [Green Version]
- Franklin, S.; Alpert, P.; Salguero-Gómez, R.; Janovský, Z.; Herben, T.; Klimešová, J.; Douhovnikoff, V. Next-gen plant clonal ecology. Perspect. Plant Ecol. Evol. Syst. 2021, 49, 125601. [Google Scholar] [CrossRef]
- Magyar, G.; Kun, A.; Oborny, B.; Stuefer, J.F. Importance of plasticity and decision-making strategies for plant resource acquisition in spatio-temporally variable environments. New Phytol. 2007, 174, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Huang, L.; Yu, F.-H.; Bezemer, T.M. Intraspecific aggregation and soil heterogeneity: Competitive interactions of two clonal plants with contrasting spatial architecture. Plant Soil 2018, 425, 231–240. [Google Scholar] [CrossRef]
- Yu, H.; Wang, L.; Liu, C.; Yu, D.; Qu, J. Effects of a spatially heterogeneous nutrient distribution on the growth of clonal wetland plants. BMC Ecology 2020, 20, 59. [Google Scholar] [CrossRef] [PubMed]
- de Kroon, H.; Fransen, B.; van Rheenen, J.W.A.; van Dijk, A.; Kreulen, R. High levels of inter-ramet water translocation in two rhizomatous Carex species, as quantified by deuterium labelling. Oecologia 1996, 106, 73–84. [Google Scholar] [CrossRef]
- Ikegami, M.; Whigham, D.E.; Werger, M.J.A. Optimal biomass allocation in heterogeneous environments in a clonal plant—Spatial division of labor. Ecol. Model. 2008, 213, 156–164. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; During, H.J.; Li, L. Do clonal plants show greater division of labour morphologically and physiologically at higher patch contrasts? PLoS ONE 2011, 6, e25401. [Google Scholar] [CrossRef] [PubMed]
- Baret, M.; DesRochers, A. Root connections can trigger physiological responses to defoliation in nondefoliated aspen suckers. Botany 2011, 89, 753–761. [Google Scholar] [CrossRef]
- Fang, D.; Mei, T.; Röll, A.; Hölscher, D. Water transfer between bamboo culms in the period of sprouting. Front. Plant Sci. 2019, 10, 786. [Google Scholar] [CrossRef] [Green Version]
- Jónsdóttir, I.S.; Callaghan, T.V.; Headley, A.D. Resource dynamics within arctic clonal plants. Ecol. Bull. 1996, 45, 53–64. [Google Scholar]
- Suzuki, J.-I.; Stuefer, J.F. On the ecological and evolutionary significance of storage in clonal plants. Plant Species Biol. 1999, 14, 11–17. [Google Scholar] [CrossRef]
- Eggli, U.; Nyffeler, R. Living under temporarily arid conditions—Succulence as an adaptive strategy. Bradleya 2009, 27, 13–36. [Google Scholar] [CrossRef]
- Griffiths, H.; Males, J. Succulent plants. Curr. Biol. 2017, 27, R890–R896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Males, J. Secrets of succulence. J. Exp. Bot. 2017, 68, 2121–2134. [Google Scholar] [CrossRef] [Green Version]
- Grace, O.M. Succulent plant diversity as natural capital. Plants People Planet 2019, 1, 336–345. [Google Scholar] [CrossRef] [Green Version]
- Winter, K. Ecophysiology of constitutive and facultative CAM photosynthesis. J. Exp. Bot. 2019, 70, 6495–6508. [Google Scholar] [CrossRef] [PubMed]
- Heyduk, K. The genetic control of succulent leaf development. Curr. Opin. Plant Biol. 2021, 59, 101978. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.E.; Herppich, W.B.; Roscher, Y.; Burkart, M. Relationships between leaf succulence and Crassulacean acid metabolism in the genus Sansevieria (Asparagaceae). Flora 2019, 261, 151489. [Google Scholar] [CrossRef]
- Veste, M.; Herppich, W.B. Cpmparative ecophysiology of the leaf-succulents Augea capensis (C3) and Malephora pupureo-crocea (CAM) in the Knersvlakte, Succulent Karoo, South Africa. Flora 2021, 278, 151807. [Google Scholar] [CrossRef]
- Chiang, J.-M.; Lin, T.-C.; Luo, Y.-C.; Chang, C.-T.; Cheng, J.-Y.; Martin, C.E. Relationships among rainfall, leaf hydrenchyma, and Crassulacean acid metabolism in Pyrrosia lanceolata (L.) Fraw. (Polypodiaceae) in central Taiwan. Flora 2013, 208, 343–350. [Google Scholar] [CrossRef]
- Fradera-Soler, M.; Rudall, P.J.; Prychid, C.J.; Grace, O.M. Evolutionary success in arid habitats: Morpho-anatomy of succulent leaves of Crassula species from southern Africa. J. Arid Environ. 2021, 185, 104319. [Google Scholar] [CrossRef]
- Ogburn, R.M.; Edwards, E.J. Repeated origin of three-dimensional leaf venation releases constraints on the evolution of succulence in plants. Curr. Biol. 2013, 23, 722–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, J.E.; Kaiser, W.M. Response of the succulent leaves of Peperomia magnoliaefolia to dehydration. Plant Physiol. 1987, 83, 190–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauber, F.; Konrad, W.; Roth-Nebelsick, A. Aerial roots of orchids: The velamen radicum as a porous material for efficient imbibition of water. Appl. Phys. A 2020, 126, 885. [Google Scholar] [CrossRef]
- Herppich, W.B.; Martin, C.E.; Tötzke, C.; Manke, I.; Kardjilov, N. External water transport is more important than vascular transport in extreme atmospheric epiphyte Tillandsia usneoides (Spanish moss). Plant Cell Environ. 2019, 42, 1645–1656. [Google Scholar] [CrossRef]
- Ievinsh, G.; Ieviņa, S.; Andersone-Ozola, U.; Samsone, I. Leaf sodium, potassium and electrolyte accumulation capacity of plant species from salt-affected coastal habitats of the Baltic Sea: Towards a definition of Na hyperaccumulation. Flora 2021, 274, 151748. [Google Scholar] [CrossRef]
- Rozema, J.; Schat, H. Salt tolerance of halophytes, research questions reviewed in the perspective of saline agriculture. Environ. Exp. Bot. 2013, 92, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Piirainen, M.; Liebisch, O.; Kadereit, G. Phylogeny, biogeography, systematics and taxonomy of Salicornioideae (Amaranthaceae/Chenopodiaceae)—A cosmopolitan, highly specilaized hygrohalophyte lineage dating back to the Oligocene. Taxon 2017, 66, 109–132. [Google Scholar] [CrossRef] [Green Version]
- Kadereit, G.; Mucina, L.; Freitag, H. Phylogeny of Salicornioideae (Chenopodiaceae): Diversification, biogeography, and evolutionary trends in leaf and flower morphology. Taxon 2006, 55, 617–642. [Google Scholar] [CrossRef]
- Saadeddin, R.; Doddema, H. Anatomy of the ‘extreme’ halophyte Arthrocnemum fruticosum (L.) Moq. in relation to its physiology. Ann. Bot. 1986, 57, 531–544. [Google Scholar] [CrossRef] [Green Version]
- Redondo-Gómez, S.; Wharmby, C.; Moreno, F.J.; De Cires, A.; Castillo, J.M.; Luque, T.; Davy, A.J.; Figueroa, M.E. Presence of internal photosynthetic cylinder surrounding the stele in stems of the tribe Salicornieae (Chemopodiaceae) from SW Iberian Peninsula. Photosynthetica 2005, 43, 157–159. [Google Scholar] [CrossRef]
- Ahmed, H.A.I.; Shabala, L.; Shabala, S. Understanding the mechanistic basis of adaptation of perennial Sarcocornia quinqueflora species to soil salinity. Physiol. Plant. 2021, 172, 1997–2010. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.A.I.; Shabala, S.; Goemann, K.; Shabala, L. Development of suberized barrier is critical for ion partitioning between senescent and non-senescent tissues in a succulent halophyte Sarcocornia quinqueflora. Environ. Exp. Bot. 2022, 194, 104692. [Google Scholar] [CrossRef]
- Lv, S.; Jiang, P.; Chen, X.; Fan, P.; Wang, X.; Li, Y. Multiple compartmentalization of sodium conferred salt tolerance in Salicornia europaea. Plant Physiol. Biochem. 2012, 51, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Pérez, S.; Dehnavi, A.R.; Leszczyński, K.; Luińska-Mielińska, S.; Ludwiczak, A.; Peirnik, A. Salicornia europaea L. functional traits indicate its optimum growth. Plants 2022, 11, 1051. [Google Scholar] [CrossRef]
- Pan, Y.-Q.; Guo, H.; Wang, S.-M.; Zhao, B.; Zhang, J.-L.; Ma, Q.; Yin, H.-J.; Bao, A.-K. The photosynthesis, Na+/K+ homeostasis and osmotic adjustment of Atriplex canescens in response to salinity. Front. Plant Sci. 2016, 7, 848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.A.; Ungar, I.A.; Showalters, A.M. Effects of salinity on growth, water relations and ion accumulation of the subtropical perennial halophyte, Atriplex griffithii var. stocksii. Ann. Bot. 2000, 85, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Gul, B.; Weber, D.J. Effect of salinity on the growth and ion content of Salicornia rubra. Commun. Soil Sci. Plant Anal. 2001, 32, 2965–2977. [Google Scholar] [CrossRef]
- Benzarti, M.; Rejeb, K.B.; Messedi, D.; Mna, A.B.; Hessini, K.; Ksontini, M.; Abdelly, C.; Debez, A. Effect of high salinity on Atriplex portulacoides: Growth, leaf water relations and solute accumulation in relation with osmotic adjustment. S. Afr. J. Bot. 2014, 95, 70–77. [Google Scholar] [CrossRef]
- Hamouda, I.; Badri, M.; Mejri, M.; Cruz, C.; Siddique, K.H.M.; Hessini, K. Salt tolerance of Beta macrocarpa is associated with efficient osmotic adjustment and increased apoplastic water content. Plant Biol. 2016, 18, 369–375. [Google Scholar] [CrossRef]
- He, H.; Zhou, W.; Lü, H.; Liang, B. Growth, leaf morphological and physiological adaptability of leaf beet (Beta vulgaris var. cicla) to salt stress: A soil culture experiment. Agronomy 2022, 12, 1393. [Google Scholar] [CrossRef]
- Hajiboland, R.; Bahrami-Rad, S.; Zeinalzade, N.; Atazadeh, E.; Akhani, H.; Poschenrieder, C. Differential functional traits underlying the contrasting salt tolerance in Lepidium species. Plant Soil 2020, 448, 315–334. [Google Scholar] [CrossRef]
- Akat, H.; Altunlu, H. The effects of sewage sludge applications on growth, yield and flower quality of Limonium sinuatum (statice) under salinity conditions. Ege Üniv. Ziraat Fak. Derg. 2019, 56, 103–108. [Google Scholar]
- Akat, H.; Saracoglu, O.A.; Cakar, H. Yield responses of Limonium sinuatum cultivars under salinity stress. J. Environ. Biol. 2020, 41, 302–309. [Google Scholar] [CrossRef]
- He, J.; Koh, D.J.Q.; Qin, L. LED spectrum quality and NaCl salinity interact to affect growth, photosynthesis and phytochemical production of Mesembryanthemum crystallinum. Funct. Plant Biol. 2022, 49, 483–495. [Google Scholar] [CrossRef]
- Agarie, S.; Shimoda, T.; Shimizu, Y.; Baumann, K.; Sunagawa, H.; Kondo, A.; Ueno, O.; Nakahara, T.; Nose, A.; Cushman, J.C. Salt tolerance, salt accumulation, and ionic homeostasis in an epidermal bladder-cell-less mutant of the common ice plant Mesembryanthemum crystallinum. J. Exp. Bot. 2007, 58, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.Q.; Konishi, A.; Cushman, J.C.; Morokuma, M.; Toyota, M.; Agarie, S. Ion accumulation and expression of ion homeostasis-related genes associated with halophilism, NaCl-promoted growth in a halophyte Messembryanthemum crystallinum L. Plant Product. Sci. 2020, 23, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Sleimi, N.; Guerfali, S.; Bankaji, I. Biochemical indicators of salt stress in Plantago maritima: Implications for environmental stress assessment. Ecol. Indic. 2015, 48, 570–577. [Google Scholar] [CrossRef]
- Ahmed, H.A.I.; Shabala, L.; Shabala, S. Tissue-specificity of ROS-induced K+ and Ca2+ fluxes in succulent stems of the perennial halophyte Sarcocornia quinqueflora in the context of salinity stress tolerance. Plant Physiol. Biochem. 2021, 166, 1022–1031. [Google Scholar] [CrossRef]
- Slama, I.; Ghnaya, T.; Hessini, K.; Messedi, D.; Savouré, A.; Abdelly, C. Comparative study of the effects of mannitol and PEG osmotic stress on growth and solute accumulation in Sesuvium portulacastrum. Environ. Exp. Bot. 2007, 61, 10–17. [Google Scholar] [CrossRef]
- Slama, I.; Ghnaya, T.; Savouré, A.; Abdelly, C. Combined effects of long-term salinity and soil drying on growth, water relations, nutrient status and proline accumulation of Sesuvium portulacastrum. Comptes Rendus Biol. 2008, 331, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Rabhi, M.; Castagna, A.; Remorini, D.; Scattino, C.; Smaoui, A.; Ranieri, A.; Abdelly, C. Photosynthetic responses to salinity in two obligate halophyttes: Sesuvium portulacastrum and Tecticornia indica. S. Afr. J. Bot. 2012, 79, 39–47. [Google Scholar] [CrossRef]
- Lokhande, V.H.; Nikam, T.D.; Patade, V.Y.; Ahire, M.L.; Suprassana, P. Effects of optimal and supra-optimal salinity stress on antioxidative defence, osmolytes and in vitro growth responses in Sesuvium portulacastrum L. Plant Cell Tissue Organ Cult. 2011, 104, 41–49. [Google Scholar] [CrossRef]
- Geissler, N.; Hussin, S.; Kpyro, H.-W. Interactive effects of NaCl salinity and elevated atmospheric CO2 concentration on growth, photosynthesis, water relations and chemical composition of the potential cash crop halophyte Aster tripolium L. Envrion. Exp. Bot. 2009, 65, 220–231. [Google Scholar] [CrossRef]
- Wiszniewska, A.; Koźmińska, A.; Hanus-Fajerska, E.; Dziurka, M. Insight into mechanisms of multiple stresses tolerance in a halophyte Aster tripolium subjected to salinity and heavy metal stress. Ecotoxicol. Environ. Saf. 2019, 180, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Shabala, S.; Dragišić Maksimović, J.; Maksimović, V.; Bonales-Alatorre, E.; Shabala, L.; Yu, M.; Zhang, G.; Živanović, B.D. Revealing mechanisms of salinity tissue tolerance in succulent halophytes: A case study for Carpobrotus rossi. Plant Cell Environ. 2018, 41, 2654–2667. [Google Scholar] [CrossRef]
- Lokhande, V.H.; Gor, B.K.; Desai, N.S.; Nikam, T.D.; Suprasanna, P. Sesuvium portulacastrum, a plant for drought, salt stress, sand fixation and phytoremediation. Agron. Sustain. Dev. 2013, 33, 329–348. [Google Scholar] [CrossRef] [Green Version]
- Sukhorukov, A.P.; Nilova, M.V.; Erst, A.S.; Kushunina, M.; Baider, C.; Verloove, F.; Salas-Pascual, M.; Belyaeva, I.V.; Krinitsina, A.A.; Bruyns, P.V.; et al. Diagnostics, taxonomy, nomenclature and distribution of perennial Sesuvium (Aizoaceae) in Africa. PhytoKeys 2018, 92, 45–88. [Google Scholar] [CrossRef] [Green Version]
- Adams, P.; Nelson, D.E.; Yamada, S.; Chmara, W.; Jensen, R.G.; Bohnert, H.J.; Griffiths, H. Growth and development of Mesembryanthemum crystallinum (Aizoaceae). New Phytol. 1998, 138, 171–190. [Google Scholar] [CrossRef]
- Loconsole, D.; Murillo-Amador, B.; Cristiano, G.; De Lucia, B. Halophyte common ice plants: A future solution to arable land salinization. Sustainability 2019, 11, 6076. [Google Scholar] [CrossRef] [Green Version]
- Adams, P.; Thomas, J.C.; Vernon, D.M.; Bohnert, H.J.; Jensen, R.G. Distinct cellular and organismic responses to salt stress. Plant Cell Physiol. 1992, 33, 1215–1223. [Google Scholar]
- Ievinsh, G. Coastal plant species as electrolytophytes: Effect of NaCl and light intensity on accumulation characteristics of Atriplex glabriuscula from coastal drift lines. Environ. Exp. Biol. 2020, 18, 95–105. [Google Scholar]
- Khedr, A.H.A.; Serag, M.S.; Nemat-Alla, M.M.; Abo-Elnaga, A.Z.; Nada, R.M.; Quick, W.P.; Abogadallah, G.M. A DREB gene from the xero-halophyte Atriplex halimus is induced by osmotic but not ionic stress and shows distinct differences from glycophytic homologues. Plant Cell Tissue Organ Cult. 2011, 106, 191–206. [Google Scholar] [CrossRef]
- Li, J.; Hussain, T.; Feng, X.; Guo, K.; Chen, H.; Yang, C.; Liu, X. Comparative study on the resistance of Suaeda glauca and Suaeda salsa to drought, salt, and alkali stresses. Ecol. Eng. 2019, 140, 105593. [Google Scholar] [CrossRef]
- Rozema, J.; Gude, H.; Bijl, F.; Wesselman, H. Sodium concentration in xylem sap in relation to ion exclusion, accumulation and secretion in halophytes. Acta Bot. Neerl. 1981, 30, 309–311. [Google Scholar] [CrossRef]
- Kaburagi, E.; Morikawa, Y.; Yamada, M.; Fujiyama, H. Sodium enhances nitrate uptake in Swiss chard (Beta vulgaris var. cicla L.). Soil Sci. Plant Nutr. 2014, 60, 561–658. [Google Scholar] [CrossRef] [Green Version]
- Ievinsh, G.; Andersone-Ozola, U.; Jēkabsone, A. Similar responses of relatively salt-tolerant plants to Na and K during chloride salinity: Comparison of growth, water content and ion accumulation. Life 2022, 12, 1577. [Google Scholar] [CrossRef] [PubMed]
- Al Hassan, M.; Pacurar, A.; López-Gresa, M.P.; Donat-Torres, M.P.; Llinares, J.V.; Boscaiu, M.; Vicente, O. Effects of salt stress on three ecologically distinct Plantago species. PLoS ONE 2016, 11, e0160236. [Google Scholar] [CrossRef] [Green Version]
- Koźmińska, A.; Wiszniewska, A.; Hanus-Fajerska, E.; Boscaiu, M.; Al Hasan, M.; Halecki, W.; Vicente, O. Identification of salt and drought biochemical stress markers in several Silene vulgaris populations. Sustainability 2019, 11, 800. [Google Scholar] [CrossRef] [Green Version]
- Kozminska, A.; Al Hassan, M.; Hanus-Fajerska, E.; Naranjo, M.A.; Boscaiu, M.; Vicente, O. Comparative analysis of water deficit and salt tolerance mechanisms in Silene. S. Afr. J. Bot. 2018, 117, 193–206. [Google Scholar] [CrossRef]
- Izadi-Darbandi, E.; Mehdikhani, H. Salinity effect on some of the morphophysiological traits of three plantago species (Plantago spp.). Sci. Hortic. 2018, 236, 43–51. [Google Scholar] [CrossRef]
- Landorfa-Svalbe, Z.; Andersone-Ozola, U.; Ievinsh, G. Type of anion largely determines salinity tolerance in four Rumex species. Plants 2023, 12, 92. [Google Scholar] [CrossRef]
- Poorter, H.; Fiorani, F.; Stitt, M.; Schurr, U.; Finck, A.; Gibon, Y.; Usadel, B.; Munns, R.; Atkin, O.K.; Tardieu, F.; et al. The art of growing plants for experimental purposes: A practical guide for the plant biologist. Funct. Plant Biol. 2012, 39, 821–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soltabayeva, A.; Ongaltay, A.; Omondi, J.O.; Srivastava, S. Morphological, physiological and molecular markers for salt-stressed plants. Plants 2021, 10, 243. [Google Scholar] [CrossRef] [PubMed]










| Species | Plant Part | Treatment or Conditions | H2O Content (g g−1 DM) | Reference |
|---|---|---|---|---|
| Allium cepa | Bulbs | After harvest | 5.09 | A’yuni et al., 2022 [31] |
| After drying, total | 4.26 | |||
| After drying, outer layer | 0.3 | |||
| Allium porrum | Leaves | Non-mycorrhizal, 48 h | 5.98 | Snellgrove et al., 1982 [32] |
| Mycorrhizal, 48 h | 6.52 | |||
| Non-mycorrhizal, 214 h | 4.48 | |||
| Mycorrhizal, 214 h | 5.32 | |||
| Roots | Non-mycorrhizal, 48 h | 9.00 | ||
| Mycorrhizal, 48 h | 8.75 | |||
| Non-mycorrhizal, 214 h | 6.89 | |||
| Mycorrhizal, 214 h | 5.80 | |||
| Allium sativum | Peel of bulb | Drying 2 days | 3.75 | Bayat, Rezvani 2012 [33] |
| Drying 9 days | 1.15 | |||
| Drying 16 days | 0.12 | |||
| Drying 23 days | 0.07 | |||
| Cocos nucifera | Fruit endosperm | 6 months | 15.2 | Santoso et al., 1996 [34] |
| 12 months | 16.0 | |||
| Limonium sinuatum | Shoots | N 0 | 2.72 | Jain et al., 2018 [35] |
| N 10 g m−2 | 3.04 | |||
| N 20 g m−2 | 3.48 | |||
| N 30 g m−2 | 3.19 | |||
| Roots | N 0 | 1.05 | ||
| N 10 g m−2 | 1.87 | |||
| N 20 g m−2 | 2.02 | |||
| N 30 g m−2 | 1.55 | |||
| Limonium stocksii | Leaves | Greenhouse conditions | 4.3–3.7 | Zia et al., 2008 [36] |
| Stems | 2.6–2.8 | |||
| Roots | 1.6–2.3 | |||
| Plantago major | Leaves | A4 faster growing | 9.3 | Dijkstra, Lambers 1989 [30] |
| W9 slower growing | 7.2 | |||
| Roots | A4 faster growing | 15.1 | ||
| W9 slower growing | 11.2 | |||
| Rhinanthus minor | Shoot | Non-parasitic | 4.82 | Jiang et al., 2007 [37] |
| Host-free attached | 4.05 | |||
| Root | Non-parasitic | 8.43 | ||
| Host-free attached | 6.67 | |||
| Rhinanthus serotinus | Leaves | Unattached | 4.88 | Klaren, van de Dijk 1976 [38] |
| Attached | 8.52 | |||
| Ranunculus sceleratus | Leaf petioles | High air humidity | 18.4 | Prokopoviča, Ievinsh 2023 [39] |
| Normal air humidity | 16.2 | |||
| Leaf blades | High air humidity | 11.3 | ||
| Normal air humidity | 10.0 | |||
| Stems | High air humidity | 13.7 | ||
| Normal air humidity | 11.8 | |||
| Rubus idaeus × Rubus ursinus | Fruits | Green | 3.35 | Vicente et al., 2007 [40] |
| Red 25% | 4.88 | |||
| Red 75% | 5.06 | |||
| Red 100% | 5.90 | |||
| Purple | 5.33 | |||
| Solanum tuberosum | Tubers | 32 days after emergence | 5.4 | Hajjar et al., 2022 [41] |
| 46 days after emergence | 3.4 | |||
| 60 days after emergence | 2.6 | |||
| Solanum tuberosum | Tubers | 80 days after planting | 4.26 d | Solaiman et al., 2015 [42] |
| 90 days after planting | 3.41 c | |||
| 100 days after planting | 2.93 b | |||
| 110 days after planting | 2.60 a | |||
| Solanum tuberosum | Tubers | Outside | 2.4 | Pritchard, Scanlon 1997 [11] |
| Inside | 3.5 | |||
| Apical end | 2.8 | |||
| Stem end | 3.0 | |||
| Tradescantia pallida | Leaves | Low fertilizer level | 19.1 | Ievinsh et al., 2022 [43] |
| High fertilizer level | 27.0 | |||
| Stems | Low fertilizer level | 15.1 | ||
| High fertilizer level | 30.1 | |||
| Trifolium pratense | Shoots | Control | 7.50 | Matse et al., 2020 [44] |
| Inoculated CHB 1120 | 5.41 | |||
| Inoculated CHB 1121 | 5.53 | |||
| Inoculated BCRC 14266 | 6.65 | |||
| Roots | Control | 14.71 | ||
| Inoculated CHB 1120 | 7.75 | |||
| Inoculated CHB 1121 | 9.80 | |||
| Inoculated BCRC 14266 | 6.41 |
| Species | Plant Part | H2O Content (g g−1 DM) | Reference |
|---|---|---|---|
| Actinidia deliciosa | Peeled fruit | 5.25 | Sánchez-Moreno et al., 2006 [53] |
| Ananas comosus | Peeled fruit | 5.25 | Sánchez-Moreno et al., 2006 [53] |
| Ananas comosus | Pulp Peel | 6.63 4.78 | Morais et al., 2017 [54] |
| Ananas comosus | Peeled fruit | 5.03 | Saputri et al., 2022 [55] |
| Artocarpus heterophyllus | Peeled fruit | 3.44 | Saputri et al., 2022 [55] |
| Carica papaya | Peeled fruit | 8.09 | Sánchez-Moreno et al., 2006 [53] |
| Carica papaya | Peeled fruit | 6.91 | Untalan et al., 2015 [56] |
| Carica papaya | Pulp Peel | 7.20 6.58 | Morais et al., 2017 [54] |
| Citrullus lanatus | Peeled fruit | 13.29 | Sánchez-Moreno et al., 2006 [53] |
| Citrullus lanatus | Pulp Peel | 11.99 12.51 | Morais et al., 2017 [54] |
| Citrus garndis | Peeled fruit | 11.11 | Untalan et al., 2015 [56] |
| Citrus × sinnensis | Peeled fruit | 6.69 | Sánchez-Moreno et al., 2006 [53] |
| Cucumis melo | Pulp Peel | 13.93 11.66 | Morais et al., 2017 [54] |
| Cucumis melo | Peeled fruit | 11.50 | Sánchez-Moreno et al., 2006 [53] |
| Fragaria × ananasa | Whole fruit | 10.11 | Sánchez-Moreno et al., 2006 [53] |
| Malus domestica | Peeled fruit | 6.14 | Sánchez-Moreno et al., 2006 [53] |
| Mangifera indica | Peeled fruit | 5.25 | Sánchez-Moreno et al., 2006 [53] |
| Manilkara sapota | Peeled fruit | 2.78 | Untalan et al., 2015 [56] |
| Musa lacatan | Peeled fruit | 2.65 | Untalan et al., 2015 [56] |
| Musa spp. | Peeled fruit | 3.00 | Sánchez-Moreno et al., 2006 [53] |
| Musa spp. | Pulp Peel | 3.04 8.80 | Morais et al., 2017 [54] |
| Musa spp. | Peeled fruit | 3.00 | Saputri et al., 2022 [55] |
| Passiflora edulis | Pulp Peel | 7.40 6.19 | Morais et al., 2017 [54] |
| Persea americana | Pulp Peel | 6.52 1.92 | Morais et al., 2017 [54] |
| Persea americana | Peeled fruit | 3.76 | Sánchez-Moreno et al., 2006 [53] |
| Prunus armeniaca | Peeled fruit | 7.33 | Sánchez-Moreno et al., 2006 [53] |
| Prunus avium | Peeled fruit | 4.00 | Sánchez-Moreno et al., 2006 [53] |
| Prunus domestica | Peeled fruit | 5.25 | Sánchez-Moreno et al., 2006 [53] |
| Prunus persica | Peeled fruit | 8.09 | Sánchez-Moreno et al., 2006 [53] |
| Psidium guajava | Peeled fruit | 4.56 | Sánchez-Moreno et al., 2006 [53] |
| Pyrus communis | Peeled fruit | 6.14 | Sánchez-Moreno et al., 2006 [53] |
| Rubus idaeus | Whole fruit | 6.14 | Sánchez-Moreno et al., 2006 [53] |
| Tamarindus indica | Peeled fruit | 0.50 | Untalan et al., 2015 [56] |
| Vitis vinifera | Peeled fruit | 4.56 | Sánchez-Moreno et al., 2006 [53] |
| Species | Plant Part | H2O Content (g g−1 DM) | Reference |
|---|---|---|---|
| Asparagus officinalis | Shoot | 11.85 | Duke, Atchley 1986 [68] |
| Beta vulgaris | Leaves | 9.99 | Duke, Atchley 1986 [68] |
| Beta vulgaris | Root | 6.87 | Duke, Atchley 1986 [68] |
| Beta vulgaris var. cicla | Leaves | 10.24 | Duke, Atchley 1986 [68] |
| Brassica oleracea var. acephala | Leaves | 6.87 | Duke, Atchley 1986 [68] |
| Brassica oleracea var. botrytis | Inflorescence | 10.24 | Hanif et al., 2006 [69] |
| Brassica oleracea var. capitata | Leaves | 11.50 | Hanif et al., 2006 [69] |
| Brassica oleracea var. capitata | Leaves | 12.16 | Duke, Atchley 1986 [68] |
| Brassica rapa var. rapa | Root | 10.77 | Duke, Atchley 1986 [68] |
| Colocasia esculenta | Root | 2.70 | Duke, Atchley 1986 [68] |
| Dioscorea spp. | Root | 2.77 | Duke, Atchley 1986 [68] |
| Daucus carota | Root | 7.48 | Duke, Atchley 1986 [68] |
| Daucus carota | Root | 8.09 | Hanif et al., 2006 [69] |
| Glycine max | Shoot | 6.30 | Duke, Atchley 1986 [68] |
| Ipomoea batatas | Root | 2.40 | Duke, Atchley 1986 [68] |
| Lactuca sativa | Leaves | 15.12 | Hanif et al., 2006 [69] |
| Pastinaca sativa | Root | 3.79 | Duke, Atchley 1986 [68] |
| Raphanus sativus | Root | 17.18 | Duke, Atchley 1986 [68] |
| Raphanus sativus | Root | 12.89 | Hanif et al., 2006 [69] |
| Spinacia oleracea | Leaves | 9.76 | Duke, Atchley 1986 [68] |
| Spinacia oleracea | Leaves | 24.00 | Hanif et al., 2006 [69] |
| Vigna radiata | Shoot | 7.93 | Duke, Atchley 1986 [68] |
| Species | Conditions | Part, Characteristics | H2O Content (g g−1 DM) | Reference |
|---|---|---|---|---|
| Augea capensis | Field conditions, winter Field conditions, summer | Leaves | 9.8 6.8 | Veste, Herpicch 2021 [136] |
| Malephora purpureo-crocea | Field conditions, winter Field conditions, summer | Leaves | 13.0 9.8 | Veste, Herpicch 2021 [136] |
| Crassula brevifolia | Greenhouse conditions | Leaves | 11.66 | Fradera-Soler et al., 2021 [138] |
| Crassula multicava | Greenhouse conditions | Leaves | 9.59 | Fradera-Soler et al., 2021 [138] |
| Crassula nudicaulis | Greenhouse conditions | Leaves | 14.72 | Fradera-Soler et al., 2021 [138] |
| Crassula tecta | Greenhouse conditions | Leaves | 10.78 | Fradera-Soler et al., 2021 [138] |
| Sansevieria ballyi | Greenhouse conditions | Leaves, C3 | 12.29 | Martin et al., 2019 [135] |
| Sansevieria cylindrica | Greenhouse conditions | Leaves, CAM | 11.33 | Martin et al., 2019 [135] |
| Sansevieria ehrenbergii | Greenhouse conditions | Leaves, CAM | 1.92 | Martin et al., 2019 [135] |
| Sansevieria fischeri | Greenhouse conditions | Leaves, CAM | 7.52 | Martin et al., 2019 [135] |
| Sansevieria gracilis | Greenhouse conditions | Leaves, C3 | 11.33 | Martin et al., 2019 [135] |
| Sansevieria parva | Greenhouse conditions | Leaves, CAM | 14.67 | Martin et al., 2019 [135] |
| Sansevieria raffillii | Greenhouse conditions | Leaves, CAM | 10.50 | Martin et al., 2019 [135] |
| Sansevieria senegambica | Greenhouse conditions | Leaves, C3 | 13.39 | Martin et al., 2019 [135] |
| Sansevieria suffruticosa | Greenhouse conditions | Leaves, CAM | 6.00 | Martin et al., 2019 [135] |
| Sansevieria volkensii | Greenhouse conditions | Leaves, CAM | 16.18 | Martin et al., 2019 [135] |
| Species | Characteristic | Part | Salinity | H2O Content (g g−1 DM) | Reference |
|---|---|---|---|---|---|
| Atriplex canescens | Xerohalophyte C4 | Whole plant | 0 | 9.0 | Pan et al., 2016 [153] |
| 100 mM NaCl | 9.3 | ||||
| 200 mM NaCl | 6.7 | ||||
| 400 mM NaCl | 6.1 | ||||
| Atriplex griffithii | Halophyte | Leaves | 0 | 8.7 | Khan et al., 2000 [154] |
| 90 mM NaCl | 3.7 | ||||
| 180 mM NaCl | 3.7 | ||||
| 360 mM NaCl | 3.7 | ||||
| Atriplex halimus | Xerohalophyte C4 | Shoot | 0 | 8.0 a | Khedr et al., 2001 [155] |
| 50 mM NaCl | 7.5 a | ||||
| 200 mM NaCl | 7.5 a | ||||
| 500 mM NaCl | 5.7 b | ||||
| Atriplex portulacoides | Euhalophyte | Leaves | 0 | 7.9 a | Benzarti et al., 2014 [156] |
| 200 mM NaCl | 9.9 b | ||||
| 400 mM NaCl | 7.8 a | ||||
| 800 mM NaCl | 7.8 a | ||||
| 1000 mM NaCl | 6.3 a | ||||
| Beta macrocarpa | Halophyte | Leaves | 0 | 10.2 a | Hamouda et al., 2016 [157] |
| 100 mM NaCl | 8.9 a | ||||
| 200 mM NaCl | 7.7 a | ||||
| Beta vulgaris var. cicla | Leaf beet crop | Leaves | 0 | 8.0 | He et al., 2022 [158] |
| 0.3% NaCl | 9.6 | ||||
| 0.5% NaCl | 6.7 | ||||
| 0.7% NaCl | 6.1 | ||||
| Lepidium latifolium | Halophyte | Leaves | 0 | 5.63 | Hajiboland et al., 2020 [159] |
| 300 mM NaCl | 10.53 | ||||
| Lepidium sativum | Glycophyte | Leaves | 0 | 7.95 | Hajiboland et al., 2020 [159] |
| 200 mM NaCl | 8.96 | ||||
| Limonium sinuatum | Recretohalophyte | Shoots | 0 | 4.2 | Akat, Altunlu 2019 [160] |
| 50 mM NaCl | 3.9 | ||||
| 100 mM NaCl | 3.9 | ||||
| Roots | 0 | 4.9 | |||
| 50 mM NaCl | 4.8 | ||||
| 100 mM NaCl | 5.1 | ||||
| Limonium sinuatum ‘Compindi White’ | Recretohalophyte | Shoots | 1 dS m−1 | 1.56 | Akat et al., 2020 [161] |
| 5 dS m−1 | 1.89 | ||||
| 10 dS m−1 | 1.76 | ||||
| 20 dS m−1 | 1.36 | ||||
| Roots | 1 dS m−1 | 0.46 | |||
| 5 dS m−1 | 0.48 | ||||
| 10 dS m−1 | 0.43 | ||||
| 20 dS m−1 | 0.33 | ||||
| Limonium sinuatum ‘Compindi Deep Blue’ | Recretohalophyte | Shoots | 1 dS m−1 | 1.55 | Akat et al., 2020 [161] |
| 5 dS m−1 | 1.66 | ||||
| 10 dS m−1 | 1.83 | ||||
| 20 dS m−1 | 1.37 | ||||
| Roots | 1 dS m−1 | 1.14 | |||
| 5 dS m−1 | 1.11 | ||||
| 10 dS m−11 | 0.93 | ||||
| 20 dS m−1 | 0.43 | ||||
| Mesembryanthemum crystallinum | Succulent C3/CAM euhalophyte | Leaves | 100 mM NaCl | 49.0 | He et al., 2022 [162] |
| 500 mM NaCl | 15.7 | ||||
| Mesembryanthemum crystallinum | Succulent C3/CAM euhalophyte | Leaves | 0 | 32.3 | Agarie et al., 2007 [163] |
| 100 mM NaCl | 39.0 | ||||
| 400 mM NaCl | 21.2 | ||||
| Stems | 0 | 21.2 | |||
| 100 mM NaCl | 32.3 | ||||
| 400 mM NaCl | 19.0 | ||||
| Mesembryanthemum crystallinum | Succulent C3/CAM euhalophyte | Cell suspension culture | 0 | 27.0 | Tran et al., 2020 [164] |
| 50 mM NaCl | 31.0 | ||||
| 100 mM NaCl | 36.1 | ||||
| 200 mM NaCl | 35.7 | ||||
| 400 mM NaCl | 32.3 | ||||
| Plantago maritima | Hygrohalophyte | Shoots | 0 | 17.2 | Sleimi et al., 2015 [165] |
| 200 mM NaCl | 13.9 * | ||||
| 500 mM NaCl | 7.5 * | ||||
| Roots | 0 | 9.8 | |||
| 300 mM NaCl | 9.1 * | ||||
| 500 mM NaCl | 5.3 * | ||||
| Salicornia europaea | Succulent euhalophyte | Shoots | 0 | 5.7 | Cárdenas-Pérez et al., 2022 [152] |
| 200 mM NaCl | 4.1 | ||||
| 800 mM NaCl | 6.3 | ||||
| Roots | 0 | 3.7 | |||
| 200 mM NaCl | 2.5 | ||||
| 800 mM NaCl | 3.0 | ||||
| Salicornia europaea | Succulent euhalophyte | Shoots | 0 | 8.1 | Lv et al., 2012 [151] |
| 200–300 mM | 13.3 | ||||
| 800–1000 mM | 3.0 | ||||
| Salicornia rubra | Succulent euhalophyte | Shoots | 0 | 7.9 | Khan et al., 2001 [155] |
| 200 mM NaCl | 7.8 | ||||
| 400 mM NaCl | 12.1 | ||||
| 600 mM NaCl | 9.0 | ||||
| 800 mM NaCl | 8.4 | ||||
| Roots | 0 | 6.3 | |||
| 200 mM NaCl | 2.3 | ||||
| 400 mM NaCl | 6.4 | ||||
| 600 mM NaCl | 15.0 | ||||
| 800 mM NaCl | 8.3 | ||||
| Sarcocornia quinqueflora | Succulent euhalophyte | Roots Leaves | 0 | 1.5 | Ahmed et al., 2021 [166] |
| 400–600 mM | 3.0 | ||||
| 0 | 9.3 | ||||
| 600 mM | 7.5 | ||||
| 1000 mM | 4.5 | ||||
| Sesuvium portulacastrum | Succulent halophyte | Leaves | 0 | 15.5 | Slama et al., 2007 [167] |
| Roots | 0 | 12.0 | |||
| Sesuvium portulacastrum | Succulent halophyte | Leaves | 0 | 11.3 | Slama et al., 2008 [168] |
| 100 mM NaCl | 9.1 | ||||
| Sesuvium portulacastrum | Succulent halophyte | Shoots | 0 | 5.88 | Rabhi et al., 2012 [169] |
| 200 mM NaCl | 11.28 | ||||
| 400 mM NaCl | 11.80 | ||||
| Roots | 0 | 4.26 | |||
| 200 mM NaCl | 7.63 | ||||
| 400 mM NaCl | 5.55 | ||||
| Sesuvium portulacastrum | Succulent halophyte | Shoots in vitro | 0 | 13.0 | Lokhande et al., 2011 [170] |
| 200 mM NaCl | 18.0 | ||||
| 400 mM NaCl | 14.7 | ||||
| 600 mM NaCl | 8.0 | ||||
| Suaeda salsa | Succulent euhalophyte | Leaves | 0 | 11.0 | Qi et al., 2009 [14] |
| 100 mM NaCl | 14.0 | ||||
| Tripleurospermum maritimum | Coastal salt-tolerant species | Roots | 0 | 6.2 | Ievinsh et al., 2021 [86] |
| 2 g Na+ L−1 | 8.7 | ||||
| Leaves | 0 | 3.5 | |||
| 2 g Na+ L−1 | 3.7 | ||||
| Stems | 0 | 2.7 | |||
| 2 g Na+ L−1 | 3.5 | ||||
| Flowers | 0 | 4.0 | |||
| 2 g Na+ L−1 | 3.9 | ||||
| Ranunculus sceleratus | Coastal salt-tolerant species | Leaf petioles | 0 | 18.4 | Prokopoviča, Ievinsh 2023 [39] |
| 4 g Na+ L−1 | 14.7 | ||||
| Leaf blades | 0 | 11.3 | |||
| 4 g Na+ L−1 | 5.1 | ||||
| Stems | 0 | 13.7 | |||
| 4 g Na+ L−1 | 8.0 | ||||
| Tripolium pannonicum (Aster tripolium) | Hygrohalophyte | Leaves | 0–100% seawater | 5.3–6.6 | Geissler et al., 2009 [171] |
| Tripolium pannonicum (Aster tripolium) | Hygrohalophyte | Shoots | 0 | 6.8 | Wiszniewska et al., 2019 [172] |
| 150 mM NaCl | 3.9 | ||||
| 300 mM NaCl | 3.3 | ||||
| Roots | 0 | 6.6 | |||
| 150 mM NaCl | 5.5 | ||||
| 300 mM NaCl | 4.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ievinsh, G. Water Content of Plant Tissues: So Simple That Almost Forgotten? Plants 2023, 12, 1238. https://doi.org/10.3390/plants12061238
Ievinsh G. Water Content of Plant Tissues: So Simple That Almost Forgotten? Plants. 2023; 12(6):1238. https://doi.org/10.3390/plants12061238
Chicago/Turabian StyleIevinsh, Gederts. 2023. "Water Content of Plant Tissues: So Simple That Almost Forgotten?" Plants 12, no. 6: 1238. https://doi.org/10.3390/plants12061238