Antibacterial, Antifungal and Algicidal Activity of Phlorotannins, as Principal Biologically Active Components of Ten Species of Brown Algae
Abstract
:1. Introduction
2. Results
2.1. Phlorotannin Content and Chemical Characterization
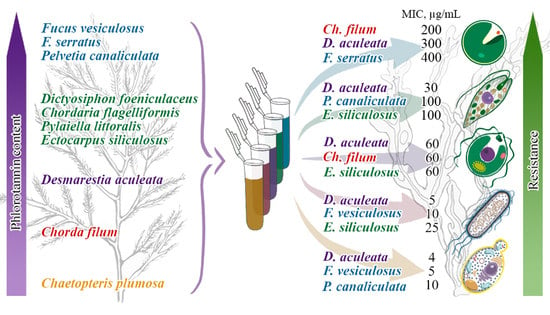
2.2. Minimum Inhibitory Concentrations of Phlorotannin Preparations
2.3. Antibiotic Activity of Phlorotannin extracts against E. coli
3. Discussion
4. Materials and Methods
4.1. Algal Material Collection
4.2. Phlorotannin Extraction
4.3. Analysis of Phlorotannin Content
4.4. Test Organisms and Growth Media
4.5. Measurement of Minimum Inhibitory Concentrations
4.6. Antibiotic Activity Assay
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K. Antimicrobial bioactive compounds from marine algae: A mini review. Ind. J. Geo-Mar. Sci. 2016, 45, 1076–1085. [Google Scholar]
- Martinez, J.H.; Castaneda, H.G. Preparation and chromatographic analysis of phlorotannins. J. Chromatogr. Sci. 2013, 51, 825–838. [Google Scholar] [CrossRef]
- Shrestha, S.; Zhang, W.; Smid, S.D. Phlorotannins: A review on biosynthesis, chemistry and bioactivity. Food Biosci. 2021, 39, 100832. [Google Scholar] [CrossRef]
- Steevensz, A.J.; Mackinnon, S.L.; Hankinson, R.; Craft, C.; Connan, S.; Stengel, D.B.; Melanson, J.E. Profiling phlorotannins in brown macroalgae by liquid chromatography-high resolution mass spectrometry. Phytochem. Anal. 2012, 23, 547–553. [Google Scholar] [CrossRef]
- Birkemeyer, C.; Lemesheva, V.; Billig, S.; Tarakhovskaya, E. Composition of intracellular and cell wall-bound phlorotannin fractions in fucoid algae indicates specific functions of these metabolites dependent on the chemical structure. Metabolites 2020, 10, 369. [Google Scholar] [CrossRef] [PubMed]
- Glombitza, K.W.; Rauwald, H.W.; Eckhardt, G. Fucole, Polyhydroxyoligophenyle aus Fucus vesiculosus. Phytochemistry 1975, 14, 1403–1405. [Google Scholar] [CrossRef]
- Grosse-Damhues, J.; Glombitza, K.W. Isofuhalols, a type of phlorotannin from the brown alga Chorda filum. Phytochemistry 1984, 23, 2639–2642. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Popović Perković, Z. Phenolic content of brown algae (Pheophyceae) species: Extraction, identification, and quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef]
- Steinberg, P.D. Biogeographical variation in brown algal polyphenolics and other secondary metabolites: Comparison between temperate Australasia and North America. Oecologia 1989, 78, 373–382. [Google Scholar] [CrossRef]
- Van Alstyne, K.L.; Paul, V.J. The biogeography of polyphenolic compounds in marine macroalgae: Temperate brown algal defenses deter feeding by tropical herbivorous fishes. Oecologia 1990, 84, 158–163. [Google Scholar] [CrossRef]
- Targett, N.M.; Arnold, T.M. Predicting the effects of brown algal phlorotannins on marine herbivores in tropical and temperate oceans. J. Phycol. 1998, 34, 195–205. [Google Scholar] [CrossRef]
- Pavia, H.; Toth, G.B. Inducible chemical resistance to herbivory in the brown seaweed Ascophyllum nodosum. Ecology 2000, 81, 3212–3225. [Google Scholar] [CrossRef]
- Van Alstyne, K.L.; Pelletreau, K.N. Effects of nutrient enrichment on growth and phlorotannin production in Fucus gardneri embryos. Mar. Ecol. Prog. Ser. 2000, 206, 33–43. [Google Scholar] [CrossRef]
- Connan, S.; Delisle, F.; Deslandes, E.; Ar Gall, E. Intra-thallus phlorotannin content and antioxidant activity in Phaeophyceae of temperate waters. Bot. Mar. 2006, 49, 39–46. [Google Scholar] [CrossRef]
- Iken, K.; Amsler, C.D.; Hubbard, J.M.; McClintock, J.B.; Baker, B.J. Allocation patterns of phlorotannins in Antarctic brown algae. Phycologia 2007, 46, 386–395. [Google Scholar] [CrossRef]
- Kamiya, M.; Nishio, T.; Yokoyama, A.; Yatsuya, K.; Nishigaki, T.; Yoshikawa, S.; Ohki, K. Seasonal variation of phlorotannin in sargassacean species from the coast of the Sea of Japan. Phycol. Res. 2010, 58, 53–61. [Google Scholar] [CrossRef]
- Ford, L.; Stratakos, A.C.; Theodoridou, K.; Dick, J.T.; Sheldrake, G.N.; Linton, M.; Corcionivoschi, N.; Walsh, P.J. Polyphenols from brown seaweeds as a potential antimicrobial agent in animal feeds. ACS Omega 2020, 5, 9093–9103. [Google Scholar] [CrossRef] [PubMed]
- Cassani, L.; Gomez-Zavaglia, A.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Seaweed-based natural ingredients: Stability of phlorotannins during extraction, storage, passage through the gastrointestinal tract and potential incorporation into functional foods. Food Res. Int. 2020, 137, 109676. [Google Scholar] [CrossRef]
- Koivikko, R.; Loponen, J.; Honkanen, T.; Jormalainen, V. Contents of cytoplasmic, cell-wall-bound and exudes phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. J. Chem. Ecol. 2005, 31, 195–209. [Google Scholar] [CrossRef]
- Koivikko, R.; Loponen, J.; Pihlaja, K.; Jormalainen, V. High-performance liquid chromatographic analysis of phlorotannins from the brown alga Fucus vesiculosus. Phytochem. Anal. 2007, 18, 326–332. [Google Scholar] [CrossRef]
- Amsler, C.D.; Fairhead, V.A. Defensive and sensory chemical ecology of brown algae. Adv. Bot. Res. 2006, 43, 1–91. [Google Scholar] [CrossRef]
- Lemesheva, V.; Tarakhovskaya, E. Physiological functions of phlorotannins. Biol. Comm. 2018, 63, 70–76. [Google Scholar] [CrossRef]
- Creis, E.; Ar Gall, E.; Potin, P. Ubiquitous phlorotannins prospects and perspectives. In Blue Biotechnology: Production and Use of Marine Molecules, 1st ed.; La Barre, S., Bates, S.S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; pp. 67–116. [Google Scholar] [CrossRef]
- Deniaud-Bouët, E.; Hardouin, K.; Potin, P.; Kloareg, B.; Hervé, C. A review about brown algal cell walls and fucose-containing sulfated polysaccharides: Cell wall context, biomedical properties and key research challenges. Carbohydr. Polym. 2017, 175, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Lemesheva, V.; Birkemeyer, C.; Garbary, D.; Tarakhovskaya, E. Vanadium-dependent haloperoxidase activity and phlorotannin incorporation into the cell wall during early embryogenesis of Fucus vesiculosus (Phaeophyceae). Eur. J. Phycol. 2020, 55, 275–284. [Google Scholar] [CrossRef]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin extracts from Fucales characterized by HPLC-DAD-ESI-MSn: Approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, N.; Smyth, T.J.; Soler-Villa, A.; Fitzgerald, R.J.; Brunton, N.P. Phenolic content and antioxidant activity of fractions obtained from selected Irish macroalgae species (Laminaria digitata, Fucus serratus, Gracilaria gracilis and Codium fragile). J. Appl. Phycol. 2015, 27, 519–530. [Google Scholar] [CrossRef]
- Hermund, D.B.; Plaza, M.; Turner, C.; Jónsdóttir, R.; Kristinsson, H.G.; Jacobsen, C.; Nielsen, K.F. Structure dependent antioxidant capacity of phlorotannins from Icelandic Fucus vesiculosus by UHPLC-DAD-ECD-QTOFMS. Food Chem. 2018, 240, 904–909. [Google Scholar] [CrossRef]
- Toth, G.; Pavia, H. Lack of phlorotannin induction in the brown seaweed Ascophyllum nodosum in response to increased copper concentrations. Mar. Ecol. Prog. Ser. 2000, 192, 119–126. [Google Scholar] [CrossRef]
- Connan, S.; Stengel, D.B. Impacts of ambient salinity and copper on brown algae: 2. Interactive effects on phenolic pool and assessment of metal binding capacity of phlorotannin. Aquat. Toxicol. 2011, 104, 1–13. [Google Scholar] [CrossRef]
- Lüder, U.H.; Clayton, M.N. Induction of phlorotannins in the brown macroalga Ecklonia radiata (Laminariales, Phaeophyta) in response to simulated herbivory—The first microscopic study. Planta 2004, 218, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Wikström, S.A.; Pavia, H. Chemical settlement inhibition versus post-settlement mortality as an explanation for differential fouling of two congeneric seaweeds. Oecologia 2004, 138, 223–230. [Google Scholar] [CrossRef]
- Kubanek, J.; Lester, S.E.; Fenical, W.; Hay, M.E. Ambiguous role of phlorotannins as chemical defenses in the brown alga Fucus vesiculosus. Mar. Ecol. Prog. Ser. 2004, 277, 79–93. [Google Scholar] [CrossRef]
- Honkanen, T.; Jormalainen, V. Genotypic variation in tolerance and resistance to fouling in the brown alga Fucus vesiculosus. Oecologia 2005, 144, 196–205. [Google Scholar] [CrossRef]
- Negara, B.F.S.P.; Sohn, J.H.; Kim, J.S.; Choi, J.S. Antifungal and larvicidal activities of phlorotannins from brown seaweeds. Mar. Drugs 2021, 19, 223. [Google Scholar] [CrossRef]
- Nagayama, K.; Shibata, T.; Fujimoto, K.; Honjo, T.; Nakamura, T. Algicidal effect of phlorotannins from the brown alga Ecklonia kurome on red tide microalgae. Aquaculture 2003, 218, 601–611. [Google Scholar] [CrossRef]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS ONE 2012, 7, e31145. [Google Scholar] [CrossRef]
- Lopes, G.; Pinto, E.; Andrade, P.B.; Valentaõ, P. Antifungal activity of phlorotannins against dermatophytes and yeasts: Approaches to the mechanism of action and influence on Candida albicans virulence factor. PLoS ONE 2013, 8, e72203. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.H.; Kim, Y.M.; Kim, S.K. Antimicrobial effect of phlorotannins from marine brown algae. Food Chem. Toxicol. 2012, 50, 3251–3255. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.H.; Kim, D.H.; Lee, S.H.; Yoon, N.Y.; Kim, J.H.; Kim, T.H.; Chung, Y.H.; Kim, S.B.; Kim, Y.M.; Kim, H.W.; et al. In vitro antibacterial activity and synergistic antibiotic effects of phlorotannins isolated from Eisenia bicyclis against methicillin-resistant Staphylococcus aureus. Phytother. Res. 2013, 27, 1260–1264. [Google Scholar] [CrossRef]
- Eom, S.H.; Kang, M.S.; Kim, Y.M. Antibacterial activity of the Phaeophyta Ecklonia stolonifera on methicillin-resistant Staphylococcus aureus. Fish. Aquatic Sci. 2008, 11, 1–6. [Google Scholar] [CrossRef]
- Lee, J.H.; Eom, S.H.; Lee, E.H.; Jung, Y.J.; Kim, H.J.; Jo, M.R.; Son, K.T.; Lee, H.J.; Kim, J.H.; Lee, M.S. In vitro antibacterial and synergistic effect of phlorotannins isolated from edible brown seaweed Eisenia bicyclis against acne-related bacteria. Algae 2014, 29, 47–55. [Google Scholar] [CrossRef]
- Kim, H.J.; Dasagrandhi, C.; Kim, S.H.; Kim, B.G.; Eom, S.H.; Kim, Y.M. In vitro antibacterial activity of phlorotannins from edible brown algae, Eisenia bicyclis against streptomycin-resistant Listeria monocytogenes. Ind. J. Microbiol. 2018, 58, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, K.; Iwamura, Y.; Shibata, T.; Hirayama, I.; Nakamura, T. Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J. Antimicrob. Chemother. 2002, 50, 889–893. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef]
- Tan, J.B.L.; Lim, Y.Y. Critical analysis of current methods for assessing the in vitro antioxidant and antibacterial activity of plant extracts. Food Chem. 2015, 172, 814–822. [Google Scholar] [CrossRef]
- Stern, J.; Hagerman, A.; Steinberg, P.; Mason, P. Phlorotannin–protein interactions. J. Chem. Ecol. 1996, 22, 1877–1899. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins medical/pharmacological and related applications: A critical review. Sustain. Chem. Pharm. 2021, 22, 100481. [Google Scholar] [CrossRef]
- Kim, K.H.; Eom, S.H.; Kim, H.J.; Lee, D.S.; Nshimiyumukiza, O.; Kim, D.; Kim, Y.M.; Lee, M.S. Antifungal and synergistic effects of an ethyl acetate extract of the edible brown seaweed Eisenia bicyclis against Candida species. Fish. Aquat. Sci. 2014, 17, 209–214. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Bach, S.J.; McAllister, T.A. Sensitivity of Escherichia coli to seaweed (Ascophyllum nodosum) phlorotannins and terrestrial tannins. Asian-Australas. J. Anim. Sci. 2009, 22, 238–245. [Google Scholar] [CrossRef]
- Pradhan, B.; Nayak, R.; Bhuyan, P.P.; Patra, S.; Behera, C.; Sahoo, S.; Ki, J.-S.; Quarta, A.; Ragusa, A.; Jena, M. Algal phlorotannins as novel antibacterial agents with reference to the antioxidant modulation: Current advances and future directions. Mar. Drugs 2022, 20, 403. [Google Scholar] [CrossRef]
- Van Alstyne, K.L. Comparison of three methods for quantifying brown algal polyphenolic compounds. J. Chem. Ecol. 1995, 21, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Ragan, M.A.; Jensen, A. Quantitative studies on brown algal phenols. II. Seasonal variation in polyphenol content of Ascophyllum nodosum (L.) Le Jol. and Fucus vesiculosus (L.). J. Exp. Mar. Biol. Ecol. 1978, 34, 245–258. [Google Scholar] [CrossRef]
- Pavia, H.; Toth, G.B.; Lindgren, A.; Åberg, P. Intraspecific variation in the phlorotannin content of the brown alga Ascophyllum nodosum. Phycologia 2003, 42, 378–383. [Google Scholar] [CrossRef]
- Connan, S.; Goulard, F.; Stiger, V.; Deslandes, E.; Ar Gall, E. Interspecific and temporal variation in phlorotannin levels in an assemblage of brown algae. Bot. Mar. 2004, 47, 410–416. [Google Scholar] [CrossRef]
- Fairhead, V.A.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Variation in phlorotannin content within two species of brown macroalgae (Desmarestia anceps and D. menziesii) from the Western Antarctic Peninsula. Polar Biol. 2005, 28, 680–686. [Google Scholar] [CrossRef]
- Zubia, M.; Fabre, M.S.; Kerjean, V.; Le Lann, K.; Stiger-Pouvreau, V.; Fauchon, M.; Deslandes, E. Antioxidant and antitumoural activities of some Phaeophyta from Brittany coasts. Food Chem. 2009, 116, 693–701. [Google Scholar] [CrossRef]
- Marambio, J.; Bischof, K. Differential acclimation responses to irradiance and temperature in two co-occurring seaweed species in Arctic fjords. Polar Res. 2021, 40, 5702. [Google Scholar] [CrossRef]
- Pereira, R.C.; Valentin, Y.Y.; Teixeira, V.L.; Kelecom, A. Phlorotannins in Brazilian brown algae: Quantitative study and ecological implications. Planta Med. 1990, 56, 557–558. [Google Scholar] [CrossRef]
- Ye, B.R.; Jang, J.; Kwon, Y.K.; Jeon, S.M.; Jeong, J.Y.; Kang, D.H.; Oh, C.H.; Kim, J.H.; Affan, A.; Hyun, J.H. Antioxidant effect of tropical seaweed Pylaiella littoralis extracts collected from Chuuk lagoon in Federated States of Micronesia. Ocean Polar. Res. 2012, 34, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Magnusson, M.; Yuen, A.K.; Zhang, R.; Wright, J.T.; Taylor, R.B.; Maschmeyer, T.; de Nys, R. A comparative assessment of microwave assisted (MAE) and conventional solid-liquid (SLE) techniques for the extraction of phloroglucinol from brown seaweed. Algal Res. 2017, 23, 28–36. [Google Scholar] [CrossRef]
- Dörschmann, P.; Schmitt, C.; Bittkau, K.S.; Neupane, S.; Synowitz, M.; Roider, J.; Alban, S.; Held-Feindt, J.; Klettner, A. Evaluation of a brown seaweed extract from Dictyosiphon foeniculaceus as a potential therapeutic agent for the treatment of glioblastoma and uveal melanoma. Mar. Drugs 2020, 18, 625. [Google Scholar] [CrossRef] [PubMed]
- Chkhikvishvili, I.D.; Ramazanov, Z.M. Phenolic substances of brown algae and their antioxidant activity. Appl. Biochem. Microbiol. 2000, 36, 289–291. [Google Scholar] [CrossRef]
- Starko, S.; Soto Gomez, M.; Darby, H.; Demes, K.W.; Kawai, H.; Yotsukura, N.; Lindstrom, S.C.; Keeling, P.J.; Graham, S.W.; Martone, P.T. A comprehensive kelp phylogeny sheds light on the evolution of an ecosystem. Mol. Phylogenet. Evol. 2019, 136, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Van Alstyne, K.L.; McCarthy III, J.J.; Hustead, C.L.; Duggins, D.O. Geographic variation in polyphenolic levels of Northeastern Pacific kelps and rockweeds. Mar. Biol. 1999, 133, 371–379. [Google Scholar] [CrossRef]
- Dubois, A.; Iken, K. Seasonal variation in kelp phlorotannins in relation to grazer abundance and environmental variables in the Alaskan sublittoral zone. Algae 2012, 27, 9–19. [Google Scholar] [CrossRef]
- Lee, D.S.; Kang, M.S.; Hwang, H.J.; Eom, S.H.; Yang, J.Y.; Lee, M.S.; Lee, W.J.; Jeon, Y.J.; Choi, J.S.; Kim, Y.M. Synergistic effect between dieckol from Ecklonia stolonifera and β-lactams against methicillin-resistant Staphylococcus aureus. Biotechnol. Bioprocess Eng. 2008, 13, 758–764. [Google Scholar] [CrossRef]
- AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: https://www.algaebase.org (accessed on 27 October 2022).
- Choi, J.-S.; Lee, K.; Lee, B.-B.; Kim, Y.-C.; Kim, Y.D.; Hong, Y.-K.; Cho, K.K.; Choi, I.S. Antibacterial activity of the phlorotannins dieckol and phlorofucofuroeckol-A from Ecklonia cava against Propionibacterium acnes. Bot. Sci. 2014, 92, 425–431. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, Y.-M.; Kim, E.; Lee, M.-S. Synergistic antibacterial activity of Ecklonia cava extract against antibiotic resistant Enterococcus faecalis. Fish. Aquat. Sci. 2015, 48, 51–57. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, K.B.; Oh, S.M.; Lee, B.H.; Chee, H.Y. Antifungal activities of dieckol isolated from the marine brown alga Ecklonia cava against Trichophyton rubrum. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 504–507. [Google Scholar] [CrossRef]
- Kim, K.H.; Yu, D.; Eom, S.H.; Kim, H.J.; Kim, D.H.; Song, H.S.; Kim, D.M.; Kim, Y.M. Fucofuroeckol-A from edible marine alga Eisenia bicyclis to restore antifungal activity of fluconazole against fluconazole-resistant Candida albicans. J. Appl. Phycol. 2018, 30, 605–609. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Q.; Xu, C.; Yu, J.; Zhao, L.; Guo, Q. Damage to the membrane permeability and cell death of Vibrio parahaemolyticus caused by phlorotannins with low molecular weight from Sargassum thunbergii. J. Aquat. Food Prod. Technol. 2016, 25, 323–333. [Google Scholar] [CrossRef]
- Iken, K.; Amsler, C.D.; Amsler, M.O.; McClintock, J.B.; Baker, B.J. Field studies on deterrent properties of phlorotannins in Antarctic brown algae. Bot. Mar. 2009, 52, 547–557. [Google Scholar] [CrossRef]
- Draisma, S.G.A.; Prud’homme van Reine, W.F.; Stam, W.T.; Olsen, J.L. A reassessment of phylogenetic relationships within the Phaeophyceae based on RUBISCO large subunit and ribosomal DNA sequences. J. Phycol. 2001, 37, 586–603. [Google Scholar] [CrossRef]
- McInnes, A.G.; Ragan, M.A.; Smith, D.G.; Walter, J.A. High-molecular-weight phloroglucinol-based tannins from brown algae: Structural variants. Hydrobiologia 1984, 116, 597–602. [Google Scholar] [CrossRef]
- Haslam, E. Polyphenol-protein interactions. Biochem. J. 1974, 139, 285–288. [Google Scholar] [CrossRef]
- Schofield, P.; Mbugua, D.M.; Pell, A.N. Analysis of condensed tannins: A review. Anim. Feed Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]
- Pohl, A.; Lübke-Becker, A.; Heuwieser, W. Minimum inhibitory concentrations of frequently used antibiotics against Escherichia coli and Trueperella pyogenes isolated from uteri of postpartum dairy cows. J. Dairy Sci. 2018, 101, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Kidsley, A.K.; Abraham, S.; Bell, J.M.; O’Dea, M.; Laird, T.J.; Jordan, D.; Mitchell, P.; McDevitt, C.A.; Trott, D.J. Antimicrobial susceptibility of Escherichia coli and Salmonella spp. isolates from healthy pigs in Australia: Results of a pilot national survey. Front. Microbiol. 2018, 9, 1207. [Google Scholar] [CrossRef]
- Borman, A.M.; Muller, J.; Walsh-Quantick, J.; Szekely, A.; Patterson, Z.; Palmer, M.D.; Fraser, M.; Johnson, E.M. MIC distributions for amphotericin B, fluconazole, itraconazole, voriconazole, flucytosine and anidulafungin and 35 uncommon pathogenic yeast species from the UK determined using the CLSI broth microdilution method. J. Antimicrob. Chemother. 2020, 75, 1194–1205. [Google Scholar] [CrossRef]
- Paterson, D.M.; Wright, S.J.L. Diffusion gradient plates for herbicide toxicity tests on micro-algae and cyanobacteria. Lett. Appl. Microbiol. 1988, 7, 87–90. [Google Scholar] [CrossRef]
- Cérantola, S.; Breton, F.; Ar Gall, E.; Deslandes, E. Co-occurrence and antioxidant activities of fucol and fucophlorethol classes of polymeric phenols in Fucus spiralis. Bot. Mar. 2006, 49, 347–351. [Google Scholar] [CrossRef]
- Audibert, L.; Fauchon, M.; Blanc, N.; Hauchard, D.; Gall, E.A. Phenolic compounds in the brown seaweed Ascophyllum nodosum: Distribution and radical-scavenging activities. Phytochem. Anal. 2010, 21, 399–405. [Google Scholar] [CrossRef]
- Lalegerie, F.; Stengel, D.B. Concise review of the macroalgal species Pelvetia canaliculata (Linnaeus) Decaisne & Thuret. J. Appl. Phycol. 2022, 34, 2807–2825. [Google Scholar] [CrossRef]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta 1993, 1147, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Koebnik, R.; Locher, K.P.; Van Gelder, P. Structure and function of bacterial outer membrane proteins: Barrels in a nutshell. Mol. Microbiol. 2000, 37, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, W.; Wang, X. Insights into the structure of Escherichia coli outer membrane as the target for engineering microbial cell factories. Microb. Cell Fact. 2021, 20, 73. [Google Scholar] [CrossRef]
- Osumi, M. The ultrastructure of yeast: Cell wall structure and formation. Micron 1998, 29, 207–233. [Google Scholar] [CrossRef]
- Lesage, G.; Bussey, H. Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 317–343. [Google Scholar] [CrossRef] [Green Version]
- Guadalupe, Z.; Ayestarán, B. Effect of commercial mannoprotein addition on polysaccharide, polyphenolic, and color composition in red wines. J. Agric. Food Chem. 2008, 56, 9022–9029. [Google Scholar] [CrossRef]
- Dunker, S.; Wilhelm, C. Cell wall structure of coccoid green algae as an important trade-off between biotic interference mechanisms and multidimensional cell growth. Front. Microbiol. 2018, 9, 719. [Google Scholar] [CrossRef] [PubMed]
- Blumreisinger, M.; Meindl, D.; Loos, E. Cell wall composition of chlorococcal algae. Phytochemistry 1983, 22, 1603–1604. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin–Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Schaaper, R.M.; Danforth, B.N.; Glickman, B.W. Rapid repeated cloning of mutant lac repressor genes. Gene 1985, 39, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Stepchenkova, E.I.; Tarakhovskaya, E.R.; Siebler, H.M.; Pavlov, Y.I. Defect of Fe-S cluster binding by DNA polymerase delta in yeast suppresses UV-induced mutagenesis but enhances DNA polymerase zeta-dependent spontaneous mutagenesis. DNA Repair 2017, 49, 60–69. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001; Volume 1, 999p. [Google Scholar]
- Vogel, H.J.; Bonner, D.M. Acetylornithinase of Escherichia coli: Partial purification and some properties. J. Biol. Chem. 1956, 218, 97–106. [Google Scholar] [CrossRef]
- Kaiser, C.; Michaelis, S.; Mitchel, A. Methods in Yeast Genetics, a Cold Spring Harbor Laboratory Course Manual, 1994 ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1994; 202p. [Google Scholar]
- Cramer, M.; Myers, J. Growth and photosynthetic characteristics of Euglena gracilis. Arch. Mikrobiol. 1952, 17, 384–402. [Google Scholar] [CrossRef]
- Gorman, D.S.; Levine, R.P. Cytochrome f and plastocyanin: Their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardii. Proc. Natl. Acad. Sci. USA 1965, 54, 1665–1669. [Google Scholar] [CrossRef]
- Andersen, R.A.; Berges, J.A.; Harrison, P.J.; Watanabe, M.M. Recipes for freshwater and seawater media. In Algal Culture Techniques, 1st ed.; Andersen, R.A., Ed.; Elsevier Academic Press: London, UK, 2005; pp. 429–538. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [Green Version]




| Tested Phlorotannin Extracts/Compounds | Test Organisms | ||||
|---|---|---|---|---|---|
| E. coli | S. cerevisiae | E. gracilis | Ch. reinhardtii | Ch. vulgaris | |
| Chorda filum | 100 | 40 | 250 | 60 | 200 |
| Fucus vesiculosus | 10 | 5 | 100 | 150 | 500 |
| Fucus serratus | 20 | 10 | 150 | 100 | 400 |
| Pelvetia canaliculata | 20 | 10 | 100 | 130 | 400 |
| Desmarestia aculeata | 5 | 4 | 30 | 60 | 300 |
| Ectocarpus siliculosus | 25 | 25 | 100 | 60 | 500 |
| Chordaria flagelliformis | 70 | 150 | 400 | 300 | 900 |
| Dictyosiphon foeniculaceus | 300 | 300 | 400 | 150 | 800 |
| Pylaiella littoralis | >1000 | 400 | 800 | 400 | >1000 |
| Chaetopteris plumosa | 300 | 200 | 200 | 150 | 400 |
| Phloroglucinol | >1000 | >1000 | >1000 | >1000 | >1000 |
| Test Organisms | Brown Algae, Source of Phlorotannins | MIC Values | References | |
|---|---|---|---|---|
| Laminariales | ||||
| Gram-positive bacteria | Staphylococcus aureus (methicillin-resistant strain) | Ecklonia cava ssp. stolonifera | 500–600 | [42] |
| E. cava ssp. kurome | 64–128 | [68] | ||
| Eisenia bicyclis | 100–200 | [45] | ||
| E. bicyclis | 128–256 | [43] | ||
| E. bicyclis | 32–64 | [41] | ||
| S. epidermidis | E. bicyclis | 64–128 | [43] | |
| Streptococcus pyogenes | E. cava ssp. kurome | 400 | [45] | |
| Bacillus cereus | E. cava ssp. kurome | 200–400 | ||
| B. subtilis | E. cava ssp. stolonifera | 600 | [42] | |
| E. cava ssp. stolonifera | 64 | [68] | ||
| Listeria monocytogenes (streptomycin-resistant strain) | E. bicyclis | 16–256 | [44] | |
| Propionibacterium acnes | E. bicyclis | 32–256 | [43] | |
| E. cava | 39–312 | [70] | ||
| Enterococcus faecalis | E. cava | 128 | [71] | |
| Gram-negative bacteria | Acinetobacter sp. | E. cava ssp. stolonifera | 128 | [68] |
| Escherichia coli | E. cava ssp. kurome | 200–400 | [45] | |
| E. cava ssp. stolonifera | 256 | [68] | ||
| E. cava ssp. stolonifera | 500–900 | [42] | ||
| Vibrio parahaemolyticus | E. cava ssp. stolonifera | 600 | ||
| E. cava ssp. kurome | 200 | [45] | ||
| Campylobacter jejuni | 50 | |||
| C. fetus | 50 | |||
| Salmonella enteritidis | 200–800 | |||
| S. typhimurium | 200 | |||
| E. cava ssp. stolonifera | 500 | [42] | ||
| 256 | [68] | |||
| Pseudomonas aeruginosa | E. bicyclis | >1024 | [43] | |
| Klebsiella pneumoniae | E. cava ssp. stolonifera | 600 | [42] | |
| 256 | [68] | |||
| Algae | Karenia mikimotoi | E. cava ssp. kurome | 100 | [37] |
| Cochlodinium polykrikoides | ||||
| Chattonella antiqua | ||||
| Fungi | Trichophyton rubrum | Ecklonia cava | 148 * | [72] |
| Candida albicans | E. bicyclis | 4000–8000 512–2048 | [50,73] | |
| Candida glabrata | E. bicyclis | 4000–8000 | [50] | |
| Fucales | ||||
| Gram-positive bacteria | Streptococcus suis | Ascophyllum nodosum | 781 | [18] |
| Fucus serratus | 3125 | |||
| Staphylococcus aureus | Fucus spiralis | 7800 | [38] | |
| Gongolaria nodicaulis | 7800 | |||
| Gongolaria usneoides | 15,600 | |||
| Sargassum vulgare | 31,300 | |||
| S. epidermidis | Fucus spiralis | 3900 | ||
| Gongolaria nodicaulis | 3900 | |||
| Gongolaria usneoides | 7800 | |||
| Sargassum vulgare | 7800 | |||
| Micrococcus luteus | Fucus spiralis | 2000 | ||
| Gongolaria nodicaulis | 15,600 | |||
| Gongolaria usneoides | 31,300 | |||
| Sargassum vulgare | >31,300 | |||
| Gram-negative bacteria | Escherichia coli | Ascophyllum nodosum | 781 | [18] |
| Ascophyllum nodosum | 25–50 | [51] | ||
| Fucus serratus | 3125 | [18] | ||
| Fucus spiralis | >31,300 | [38] | ||
| Gongolaria nodicaulis | >31,300 | |||
| Gongolaria usneoides | >31,300 | |||
| Sargassum vulgare | >31,300 | |||
| Salmonella typhimurium | Fucus spiralis | >31,300 | [38] | |
| Salmonella agona | Ascophyllum nodosum | 1560 | [18] | |
| Fucus serratus | 3125 | |||
| Vibrio parahaemolyticus | Sargassum thunbergii | 900 | [74] | |
| Pseudomonas aeruginosa | Fucus spiralis | 31,300 | [38] | |
| Gongolaria nodicaulis | 31,300 | |||
| Gongolaria usneoides | >31,300 | |||
| Sargassum vulgare | >31,300 | |||
| Fungi | Gongolaria usneoides | 31,300 | [39] | |
| Gongolaria nodicaulis | 15,600 | |||
| Gongolaria usneoides | 31,300 | |||
| Candida parapsilosis | Fucus spiralis | >62,500 | ||
| Gongolaria nodicaulis | 62,500 | |||
| Gongolaria usneoides | 62,500 | |||
| Aspergillus species | Fucus spiralis | >62,500 | ||
| Gongolaria nodicaulis | >62,500 | |||
| Gongolaria usneoides | >62,500 | |||
| Epidermophyton floccosum | Fucus spiralis | 7800 | ||
| Gongolaria nodicaulis | 3900 | |||
| Gongolaria usneoides | 15,600 | |||
| Trichophyton rubrum | Fucus spiralis | 3900 | ||
| Gongolaria nodicaulis | 3900 | |||
| Gongolaria usneoides | 7800 | |||
| T. mentagrophytes | Fucus spiralis | 15,600 | ||
| Gongolaria nodicaulis | 7800 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemesheva, V.; Islamova, R.; Stepchenkova, E.; Shenfeld, A.; Birkemeyer, C.; Tarakhovskaya, E. Antibacterial, Antifungal and Algicidal Activity of Phlorotannins, as Principal Biologically Active Components of Ten Species of Brown Algae. Plants 2023, 12, 821. https://doi.org/10.3390/plants12040821
Lemesheva V, Islamova R, Stepchenkova E, Shenfeld A, Birkemeyer C, Tarakhovskaya E. Antibacterial, Antifungal and Algicidal Activity of Phlorotannins, as Principal Biologically Active Components of Ten Species of Brown Algae. Plants. 2023; 12(4):821. https://doi.org/10.3390/plants12040821
Chicago/Turabian StyleLemesheva, Valeriya, Renata Islamova, Elena Stepchenkova, Aleksandr Shenfeld, Claudia Birkemeyer, and Elena Tarakhovskaya. 2023. "Antibacterial, Antifungal and Algicidal Activity of Phlorotannins, as Principal Biologically Active Components of Ten Species of Brown Algae" Plants 12, no. 4: 821. https://doi.org/10.3390/plants12040821