Effect of Ginseng Sapogenin Protopanaxadiol-Enriched Rice (DJ-PPD) on Immunomodulation
Abstract
:1. Introduction
2. Results
2.1. Cell Viability and NO Production in LPS-Stimulated RAW264.7 Cells
2.2. Cellular Viability and NO Production in RAW264.7 Cells
2.3. Immune-Related mRNA Expression in LPS-Stimulated RAW264.7 Cells
2.4. Immune-Related mRNA Expression in RAW264.7 Cells
2.5. PGE2 Production
2.6. Phagocytosis Activity
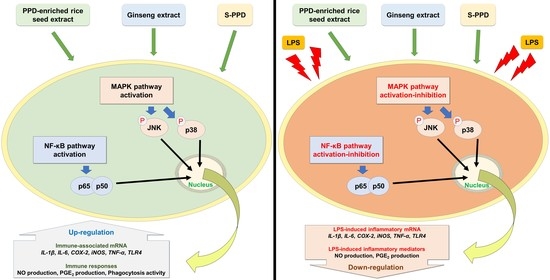
2.7. Effects of Various Treatments on NF-κB and MAPK Pathway Activation
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Sample Preparation
4.3. Cell Culture and Treatment
4.4. Cell Viability and NO Production Assays
4.5. RNA Isolation and cDNA Synthesis
4.6. Expression Analysis of Immune-Related Genes via Real-Time PCR
4.7. PGE2 Production
4.8. Phagocytosis Assay
4.9. Western Blot Assay
4.10. Statistical Analysis
5. Conclusions

Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varsha, K.K.; Narisetty, V.; Brar, K.K.; Madhavan, A.; Alphy, M.P.; Sindhu, R.; Awasthi, M.K.; Varjani, S.; Binod, P. Bioactive metabolites in functional and fermented foods and their role as immunity booster and anti-viral innate mechanisms. J. Food Sci. Technol. 2022, 1–10. [Google Scholar] [CrossRef]
- Al-Okbi, S.Y. Nutraceuticals of anti-inflammatory activity as complementary therapy for rheumatoid arthritis. Toxicol. Ind. Health 2014, 30, 738–749. [Google Scholar] [CrossRef]
- Lee, J.; Koo, N.; Min, D.B. Reactive Oxygen Species, Aging, and Antioxidative Nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2004, 3, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, G.N.K.; Ramachandran, A.; Suresh, V.; Senthil, V.; Priyadharshini, R.B. Nutraceuticals—A regulatory review. Int. J. Drug Regul. Aff. 2018, 3, 22–29. [Google Scholar]
- Dong, H.; Bai, L.-P.; Wong, V.K.W.; Zhou, H.; Wang, J.-R.; Liu, Y.; Jiang, Z.-H.; Liu, L. The in Vitro Structure-Related Anti-Cancer Activity of Ginsenosides and Their Derivatives. Molecules 2011, 16, 10619–10630. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Shahi, S.; Kang, H.K.; van Veen, H.W.; Fan, T.-P. Metabolites of ginsenosides as novel BCRP inhibitors. Biochem. Biophys. Res. Commun. 2006, 345, 1308–1314. [Google Scholar] [CrossRef]
- Lee, J.I.; Ha, Y.W.; Choi, T.W.; Kim, H.J.; Kim, S.-M.; Jang, H.-J.; Choi, J.-H.; Choi, M.H.; Chung, B.C.; Sethi, G.; et al. Cellular Uptake of Ginsenosides in Korean White Ginseng and Red Ginseng and Their Apoptotic Activities in Human Breast Cancer Cells. Planta Med. 2011, 77, 133–140. [Google Scholar] [CrossRef]
- Cho, J.Y.; Kim, A.R.; Yoo, E.S.; Baik, K.U.; Park, M.H. Ginsenosides from Panax ginseng Differentially Regulate Lymphocyte Proliferation. Planta Med. 2002, 68, 497–500. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Cho, J.Y. 20S-dihydroprotopanaxadiol, a ginsenoside derivative, boosts innate immune responses of monocytes and macrophages. J. Ginseng Res. 2013, 37, 293–299. [Google Scholar] [CrossRef]
- Wang, C.-Z.; Zhang, Z.; Wan, J.-Y.; Zhang, C.-F.; Anderson, S.; He, X.; Yu, C.; He, T.-C.; Qi, L.-W.; Yuan, C.-S. Protopanaxadiol, an Active Ginseng Metabolite, Significantly Enhances the Effects of Fluorouracil on Colon Cancer. Nutrients 2015, 7, 799–814. [Google Scholar] [CrossRef]
- Yang, Y.; Lee, J.; Rhee, M.H.; Yu, T.; Baek, K.-S.; Sung, N.Y.; Kim, Y.; Yoon, K.; Kim, J.H.; Kwak, Y.-S.; et al. Molecular mechanism of protopanaxadiol saponin fraction-mediated anti-inflammatory actions. J. Ginseng Res. 2015, 39, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.M.; Kim, S.D.; Kim, K.S.; Song, Y.B.; Kwak, Y.S.; Cho, J.Y.; Park, H.J.; Oh, J.W.; Rhee, M.H. Protopanaxadiol modulates LPS-induced inflammatory activity in murine macrophage RAW264.7 cells. J. Ginseng Res. 2006, 30, 181–187. [Google Scholar] [CrossRef]
- Oh, H.A.; Kim, D.-E.; Choi, H.J.; Kim, N.J.; Kim, D.-H. Anti-stress Effects of 20(S)-Protopanaxadiol and 20(S)-Protopanaxatriol in Immobilized Mice. Biol. Pharm. Bull. 2015, 38, 331–335. [Google Scholar] [CrossRef]
- Samukawa, K.; Suzuki, Y.; Ohkubo, N.; Aoto, M.; Sakanaka, M.; Mitsuda, N. Protective effect of ginsenosides Rg2 and Rh1 on oxidation-induced impairment of erythrocyte membrane properties. Biorheology 2008, 45, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Nah, S.-Y.; Kim, D.-H.; Rhim, H. Ginsenosides: Are Any of them Candidates for Drugs Acting on the Central Nervous System? CNS Drug Rev. 2007, 13, 381–404. [Google Scholar] [CrossRef]
- Han, J.Y.; Baek, S.-H.; Jo, H.J.; Yun, D.W.; Choi, Y.E. Genetically modified rice produces ginsenoside aglycone (protopanaxadiol). Planta 2019, 250, 1103–1110. [Google Scholar] [CrossRef]
- Monmai, C.; Kim, J.-S.; Baek, S.-H. Transgenic Rice Seed Extracts Exert Immunomodulatory Effects by Modulating Immune-Related Biomarkers in RAW264.7 Macrophage Cells. Nutrients 2022, 14, 4143. [Google Scholar] [CrossRef] [PubMed]
- Monmai, C.; Kim, J.-S.; Promyot, K.; Baek, S.-H. Protopanaxadiol-Enriched Rice Extracts Suppressed Oxidative and Melanogenic Activities in Melan-a Cells. Antioxidants 2023, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhang, F.; Shen, M.; Jia, S.; Xie, J. Phytosterols Suppress Phagocytosis and Inhibit Inflammatory Mediators via ERK Pathway on LPS-Triggered Inflammatory Responses in RAW264.7 Macrophages and the Correlation with Their Structure. Foods 2019, 8, 582. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF- B Family of Transcription Factors and Its Regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034–a000047. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Hayden, M.S.; Ghosh, S. NF-κB in immunobiology. Cell Res. 2011, 21, 223–244. [Google Scholar] [CrossRef]
- Arthur, J.S.C.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef]
- Cho, W.; Choe, J. Prostaglandin E2 stimulates COX-2 expression via mitogen-activated protein kinase p38 but not ERK in human follicular dendritic cell-like cells. BMC Immunol. 2020, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Takai, E.; Tsukimoto, M.; Kojima, S. TGF-β1 Downregulates COX-2 Expression Leading to Decrease of PGE2 Production in Human Lung Cancer A549 Cells, Which Is Involved in Fibrotic Response to TGF-β1. PLoS ONE 2013, 8, e76346. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular Mechanisms That Influence the Macrophage M1−M2 Polarization Balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Soares-Silva, M.; Diniz, F.F.; Gomes, G.N.; Bahia, D. The Mitogen-Activated Protein Kinase (MAPK) Pathway: Role in Immune Evasion by Trypanosomatids. Front. Microbiol. 2016, 7, 183. [Google Scholar] [CrossRef]
- Wu, J.; Li, L.; Sun, Y.; Huang, S.; Tang, J.; Yu, P.; Wang, G. Altered Molecular Expression of the TLR4/NF-κB Signaling Pathway in Mammary Tissue of Chinese Holstein Cattle with Mastitis. PLoS ONE 2015, 10, e0118458. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Jang, D.-I.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef] [PubMed]
- Geum, N.G.; Son, H.J.; Yu, J.H.; Yeo, J.H.; Choi, M.Y.; Lee, J.W.; Baek, J.K.; Eo, H.J.; Park, G.H.; Jeong, J.B. Kadsura japonica fruits exert immunostimulatory and anti-obesity activity in RAW264.7 and 3T3-L1 cells. Food Agric. Immunol. 2022, 33, 65–79. [Google Scholar] [CrossRef]
- Da Silva, D.M.; Langer, H.; Graf, T. Inflammatory and Molecular Pathways in Heart Failure—Ischemia, HFpEF and Transthyretin Cardiac Amyloidosis. Int. J. Mol. Sci. 2019, 20, 2322. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.P.; Cuong, N.X.; Luyen, B.T.T.; Quang, T.H.; Hanh, T.T.H.; Kim, S.; Koh, Y.-S.; Nam, N.H.; Van Kiem, P.; Van Minh, C.; et al. Anti-Inflammatory Components of the Starfish Astropecten polyacanthus. Mar. Drugs 2013, 11, 2917–2926. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Kang, M.-J.; Jo, M.J.; Seo, Y.B.; Park, N.G.; Kim, G.-D. Anti-Inflammatory Activity of β-thymosin Peptide Derived from Pacific Oyster (Crassostrea gigas) on NO and PGE2 Production by Down-Regulating NF-κB in LPS-Induced RAW264.7 Macrophage Cells. Mar. Drugs 2019, 17, 129. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Zhu, C.; Liang, Y.; Xing, Y.; Shi, C. MiR-424 overexpression protects alveolar epithelial cells from LPS-induced apoptosis and inflammation by targeting FGF2 via the NF-κB pathway. Life Sci. 2020, 242, 117213. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, X.-L.; Liu, Y.-F.; Gao, F.; Wei, D.; Li, X.-W.; Wang, H.-N.; Tan, Q.-R.; Jiang, W. LPS inhibits the effects of fluoxetine on depression-like behavior and hippocampal neurogenesis in rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1831–1835. [Google Scholar] [CrossRef]
- Ostos, M.A.; Recalde, D.; Zakin, M.M.; Scott-Algara, D. Implication of natural killer T cells in atherosclerosis development during a LPS-induced chronic inflammation. FEBS Lett. 2002, 519, 23–29. [Google Scholar] [CrossRef]
- Emaeshima, N.; Fernandez, R.C. Recognition of lipid A variants by the TLR4-MD-2 receptor complex. Front. Cell. Infect. Microbiol. 2013, 3, 3. [Google Scholar] [CrossRef]
- Zhang, M.; Tian, X.; Wang, Y.; Wang, D.; Li, W.; Chen, L.; Pan, W.; Mehmood, S.; Chen, Y. Immunomodulating activity of the polysaccharide TLH-3 from Tricholomalobayense in RAW264.7 macrophages. Int. J. Biol. Macromol. 2018, 107, 2679–2685. [Google Scholar] [CrossRef] [PubMed]







| Treatment | NO Production (µM) | |||
|---|---|---|---|---|
| 10 µg/mL (70 pg/mL of S-PPD) | 25 µg/mL (175 pg/mL of S-PPD) | 50 µg/mL (350 pg/mL of S-PPD) | 100 µg/mL (700 pg/mL of S-PPD) | |
| DJ + LPS | 31.03 ± 0.40 a,* | 29.21 ± 0.58 a,* | 26.36 ± 0.34 a,* | 24.25 ± 0.31 a,* |
| DJ−PPD + LPS | 29.47 ± 0.37 b,* | 24.80 ± 0.38 c,* | 21.89 ± 0.36 d,* | 16.81 ± 0.25 c,* |
| S−PPD + LPS | 29.60 ± 0.44 b,* | 27.65 ± 0.55 b,* | 22.94 ± 0.37 c,* | 19.41 ± 0.70 b,* |
| GE + LPS | 29.92 ± 0.15 b,* | 26.09 ± 0.43 c,* | 24.02 ± 0.07 b,* | 20.03 ± 0.83 b,* |
| RPMI | 0.00 ± 0.10 * | |||
| DMSO + LPS | 33.53 ± 0.43 | |||
| Aspirin + LPS | 6.32 ± 0.78 * | |||
| Treatment | NO Production (µM) | |||
|---|---|---|---|---|
| 10 µg/mL (70 pg/mL of S-PPD) | 25 µg/mL (175 pg/mL of S-PPD) | 50 µg/mL (350 pg/mL of S-PPD) | 100 µg/mL (700 pg/mL of S-PPD) | |
| DJ | 0.38 ± 0.40 c | 0.56 ± 0.09 c | 0.73 ± 0.07 b | 1.50 ± 0.06 b,* |
| DJ−PPD | 7.20 ± 0.18 a,* | 9.74 ± 0.16 a,* | 11.71 ± 0.25 a,* | 12.12 ± 0.09 a,* |
| S−PPD | 7.28 ± 0.16 a,* | 9.13 ± 0.11 b,* | 11.26 ± 0.23 a,* | 11.91 ± 0.19 a,* |
| GE | 7.12 ± 0.25 a,* | 9.68 ± 0.22 a,* | 11.46 ± 0.11 a,* | 12.05 ± 0.13 a,* |
| RPMI | 0.00 ± 0.10 | |||
| DMSO | 0.00 ± 0.05 | |||
| LPS | 34.35 ± 1.22 * | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monmai, C.; Kim, J.-S.; Baek, S.-H. Effect of Ginseng Sapogenin Protopanaxadiol-Enriched Rice (DJ-PPD) on Immunomodulation. Plants 2023, 12, 767. https://doi.org/10.3390/plants12040767
Monmai C, Kim J-S, Baek S-H. Effect of Ginseng Sapogenin Protopanaxadiol-Enriched Rice (DJ-PPD) on Immunomodulation. Plants. 2023; 12(4):767. https://doi.org/10.3390/plants12040767
Chicago/Turabian StyleMonmai, Chaiwat, Jin-Suk Kim, and So-Hyeon Baek. 2023. "Effect of Ginseng Sapogenin Protopanaxadiol-Enriched Rice (DJ-PPD) on Immunomodulation" Plants 12, no. 4: 767. https://doi.org/10.3390/plants12040767