Diversity of Citrullus colocynthis (L.) Schrad Seeds Extracts: Detailed Chemical Profiling and Evaluation of Their Medicinal Properties
Abstract
:1. Introduction
2. Results and Discussions
2.1. Chemo-Profiling of Different Extracts
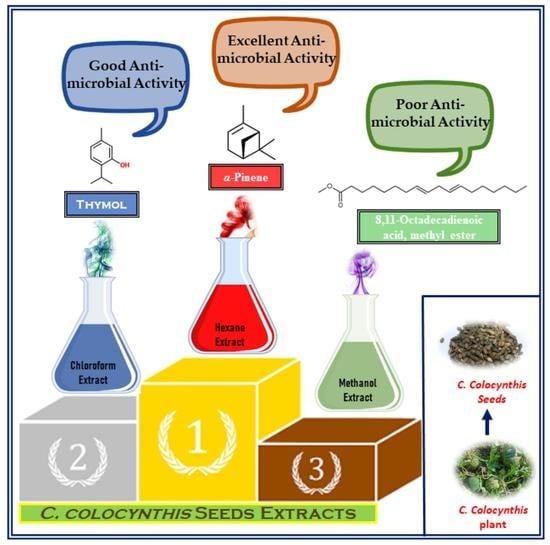
2.2. Antibacterial Properties
2.3. Anticancer Properties
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals
3.3. Preparation of C. colocynthis Seeds Extracts
3.4. GC and GC–MS Analysis of C. colocynthis Seeds Extracts
3.5. Calculation of Linear Retention Indices (LRIs)
3.6. Identification of Volatile Chemical Components
3.7. Evaluation of Antimicrobial and Anticancer Activity
3.7.1. Antimicrobial Activity
3.7.2. Anticancer Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makhuvele, R.; Naidu, K.; Gbashi, S.; Thipe, V.C.; Adebo, O.A.; Njobeh, P.B. The use of plant extracts and their phytochemicals for control of toxigenic fungi and mycotoxins. Heliyon 2020, 6, e05291. [Google Scholar] [CrossRef] [PubMed]
- Junsongduang, A.; Kasemwan, W.; Lumjoomjung, S.; Sabprachai, W.; Tanming, W.; Balslev, H. Ethnomedicinal knowledge of traditional healers in Roi Et, Thailand. Plants 2020, 9, 1177. [Google Scholar] [CrossRef] [PubMed]
- Uttra, A.M.; Ahsan, H.; Hasan, U.H.; Chaudhary, M.A. Traditional medicines of plant origin used for the treatment of inflammatory disorders in Pakistan: A review. J. Tradit. Chin. Med. 2018, 38, 636–656. [Google Scholar]
- Zhao, Z.; Li, Y.; Zhou, L.; Zhou, X.; Xie, B.; Zhang, W.; Sun, J. Prevention and treatment of COVID-19 using Traditional Chinese Medicine: A review. Phytomedicine 2021, 85, 153308. [Google Scholar] [CrossRef]
- Tesfaye, S.; Asres, K.; Lulekal, E.; Alebachew, Y.; Tewelde, E.; Kumarihamy, M.; Muhammad, I. Ethiopian medicinal plants traditionally used for the treatment of cancer, part 2: A review on cytotoxic, antiproliferative, and antitumor phytochemicals, and future perspective. Molecules 2020, 25, 4032. [Google Scholar] [CrossRef]
- Khan, M.; Adil, S.F.; Alkhathlan, H.Z.; Tahir, M.N.; Saif, S.; Khan, M.; Khan, S.T. COVID-19: A global challenge with old history, epidemiology and progress so far. Molecules 2020, 26, 39. [Google Scholar] [CrossRef]
- Pastor, N.; Collado, M.C.; Manzoni, P. Phytonutrient and nutraceutical action against COVID-19: Current review of characteristics and benefits. Nutrients 2021, 13, 464. [Google Scholar] [CrossRef]
- Alesci, A.; Aragona, M.; Cicero, N.; Lauriano, E.R. Can nutraceuticals assist treatment and improve COVID-19 symptoms? Nat. Prod. Res. 2022, 36, 2672–2691. [Google Scholar] [CrossRef]
- Houghton, P.J. Old yet new—Pharmaceuticals from plants. J. Chem. Educ. 2001, 78, 175. [Google Scholar] [CrossRef]
- Salehi, B.; Zucca, P.; Sharifi-Rad, M.; Pezzani, R.; Rajabi, S.; Setzer, W.N.; Varoni, E.M.; Iriti, M.; Kobarfard, F.; Sharifi-Rad, J. Phytotherapeutics in cancer invasion and metastasis. Phytother. Res. 2018, 32, 1425–1449. [Google Scholar] [CrossRef]
- Musthaba, S.M.; Baboota, S.; Ahmed, S.; Ahuja, A.; Ali, J. Status of novel drug delivery technology for phytotherapeutics. Expert Opin. Drug Deliv. 2009, 6, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, G.; Boffa, L.; Genzini, L.; Garella, D. Phytotherapeutics: An evaluation of the potential of 1000 plants. J. Clin. Pharm. Ther. 2010, 35, 11–48. [Google Scholar] [CrossRef]
- Dave, V.; Yadav, R.B.; Ahuja, R.; Yadav, S. Formulation design and optimization of novel fast dissolving tablet of chlorpheniramine maleate by using lyophilization techniques. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 31–39. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta BBA Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; Mandzhieva, S. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Gorlenko, C.L.; Kiselev, H.Y.; Budanova, E.V.; Zamyatnin Jr, A.A.; Ikryannikova, L.N. Plant secondary metabolites in the battle of drugs and drug-resistant bacteria: New heroes or worse clones of antibiotics? Antibiotics 2020, 9, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asimuddin, M.; Shaik, M.R.; Adil, S.F.; Siddiqui, M.R.H.; Alwarthan, A.; Jamil, K.; Khan, M. Azadirachta indica based biosynthesis of silver nanoparticles and evaluation of their antibacterial and cytotoxic effects. J. King Saud Univ. Sci. 2020, 32, 648–656. [Google Scholar] [CrossRef]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.-F. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, C.; Khatana, S.; Vijayvergia, R. Bioactivity of secondary metabolites of various plants: A review. Int. J. Pharm. Sci. Res 2019, 10, 494–504. [Google Scholar]
- Jones, W.P.; Kinghorn, A.D. Extraction of plant secondary metabolites. In Natural Products Isolation; Springer: Cham, Switzerland, 2006; pp. 323–351. [Google Scholar]
- De Silva, G.O.; Abeysundara, A.T.; Aponso, M.M.W. Extraction methods, qualitative and quantitative techniques for screening of phytochemicals from plants. Am. J. Essent. Oils Nat. Prod. 2017, 5, 29–32. [Google Scholar]
- Shaik, M.; Albalawi, G.; Khan, S.; Khan, M.; Adil, S.; Kuniyil, M.; Al-Warthan, A.; Siddiqui, M.; Alkhathlan, H.; Khan, M. “Miswak” based green synthesis of silver nanoparticles: Evaluation and comparison of their microbicidal activities with the chemical synthesis. Molecules 2016, 21, 1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matrose, N.A.; Obikeze, K.; Belay, Z.A.; Caleb, O.J. Impact of spatial variation and extraction solvents on bioactive compounds, secondary metabolites and antifungal efficacy of South African Impepho [Helichrysum odoratissimum (L.) Sweet]. Food Biosci. 2021, 42, 101139. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Ahmed, E.; Arshad, M.; Khan, M.Z.; Amjad, M.S.; Sadaf, H.M.; Riaz, I.; Sabir, S.; Ahmad, N. Secondary metabolites and their multidimensional prospective in plant life. J. Pharmacogn. Phytochem. 2017, 6, 205–214. [Google Scholar]
- Žlabur, J.Š.; Žutić, I.; Radman, S.; Pleša, M.; Brnčić, M.; Barba, F.J.; Rocchetti, G.; Lucini, L.; Lorenzo, J.M.; Domínguez, R. Effect of different green extraction methods and solvents on bioactive components of chamomile (Matricaria chamomilla L.) flowers. Molecules 2020, 25, 810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adnan, M.; Oh, K.K.; Azad, M.O.K.; Shin, M.H.; Wang, M.-H.; Cho, D.H. Kenaf (Hibiscus cannabinus L.) leaves and seed as a potential source of the bioactive compounds: Effects of various extraction solvents on biological properties. Life 2020, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, A.; Sinan, K.I.; Zengin, G.; Mahomoodally, M.F.; Bibi Sadeer, N.; Etienne, O.K.; Cziáky, Z.; Jekő, J.; Glamočlija, J.; Soković, M. Identification of chemical profiles and biological properties of Rhizophora racemosa G. Mey. extracts obtained by different methods and solvents. Antioxidants 2020, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Khan, M.; Al-Hamoud, K.; Adil, S.F.; Shaik, M.R.; Alkhathlan, H.Z. Comprehensive Phytochemical Analysis of Various Solvent Extracts of Artemisia judaica and Their Potential Anticancer and Antimicrobial Activities. Life 2022, 12, 1885. [Google Scholar] [CrossRef]
- Hussain, A.I.; Rathore, H.A.; Sattar, M.Z.; Chatha, S.A.; Sarker, S.D.; Gilani, A.H. Citrullus colocynthis (L.) Schrad (bitter apple fruit): A review of its phytochemistry, pharmacology, traditional uses and nutritional potential. J. Ethnopharmacol. 2014, 155, 54–66. [Google Scholar] [CrossRef]
- Li, Q.-Y.; Munawar, M.; Saeed, M.; Shen, J.-Q.; Khan, M.S.; Noreen, S.; Alagawany, M.; Naveed, M.; Madni, A.; Li, C.-X. Citrullus colocynthis (L.) Schrad (Bitter Apple Fruit): Promising traditional uses, pharmacological effects, aspects, and potential applications. Front. Pharmacol. 2021, 12, 791049. [Google Scholar] [CrossRef] [PubMed]
- Pravin, B.; Tushar, D.; Vijay, P.; Kishanchnad, K. Review on Citrullus colocynthis. Int. J. Res. Pharm. Chem 2013, 3, 46–53. [Google Scholar]
- Rahimi, R.; Amin, G.; Ardekani, M.R.S. A review on Citrullus colocynthis Schrad.: From traditional Iranian medicine to modern phytotherapy. J. Altern. Complement. Med. 2012, 18, 551–554. [Google Scholar] [CrossRef]
- Marzouk, B.; Marzouk, Z.; Haloui, E.; Fenina, N.; Bouraoui, A.; Aouni, M. Screening of analgesic and anti-inflammatory activities of Citrullus colocynthis from southern Tunisia. J. Ethnopharmacol. 2010, 128, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.; Ali, Q.; Hafeez, M.; Malik, A. Antibacterial and antifungal activity of fruit, seed and root extracts of Citrullus colocynthis plant. Biol. Clin. Sci. Res. J. 2020, 2020, 1. [Google Scholar] [CrossRef]
- Ostovar, M.; Akbari, A.; Anbardar, M.H.; Iraji, A.; Salmanpour, M.; Ghoran, S.H.; Heydari, M.; Shams, M. Effects of Citrullus colocynthis L. in a rat model of diabetic neuropathy. J. Integr. Med. 2020, 18, 59–67. [Google Scholar] [CrossRef]
- Cavazos, P.; Gonzalez, D.; Lanorio, J.; Ynalvez, R. Secondary metabolites, antibacterial and antioxidant properties of the leaf extracts of Acacia rigidula benth. and Acacia berlandieri benth. SN Appl. Sci. 2021, 3, 1–14. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.T.; Khan, M.; Mousa, A.A.; Mahmood, A.; Alkhathlan, H.Z. Chemical diversity in leaf and stem essential oils of Origanum vulgare L. and their effects on microbicidal activities. AMB Express 2019, 9, 176. [Google Scholar] [CrossRef]
- Kurnia, D.; Ajiati, D.; Heliawati, L.; Sumiarsa, D. Antioxidant properties and structure-antioxidant activity relationship of Allium species leaves. Molecules 2021, 26, 7175. [Google Scholar] [CrossRef]
- Bourhia, M.; Bouothmany, K.; Bakrim, H.; Hadrach, S.; Salamatullah, A.M.; Alzahrani, A.; Khalil Alyahya, H.; Albadr, N.A.; Gmouh, S.; Laglaoui, A. Chemical profiling, antioxidant, antiproliferative, and antibacterial potentials of chemically characterized extract of citrullus colocynthis L. seeds. Separations 2021, 8, 114. [Google Scholar] [CrossRef]
- Gurudeeban, S.; Ramanathan, T.; Satyavani, K. Characterization of volatile compounds from bitter apple (Citrullus colocynthis) using GC-MS. Int. J. Chem. Anal. Sci. 2011, 2, 108–110. [Google Scholar]
- Singh, S.; Devi, B. Estimation of phytoconstituents from Citrullus colocynthis (L.) schrad roots extract by GC-MS spectroscopy. Int. J. Sci. Res. 2016, 7, 648–652. [Google Scholar]
- El-Shazly, A.; Hafez, S.; Wink, M. Comparative study of the essential oils and extracts of Achillea fragrantissima (Forssk.) Sch. Bip. and Achillea santolina L.(Asteraceae) from Egypt. Die Pharm. Int. J. Pharm. Sci. 2004, 59, 226–230. [Google Scholar]
- Alsohaili, S.A.; Al-fawwaz, A.T. Composition and antimicrobial activity of Achillea fragrantissima essential oil using food model media. Eur. Sci. J. 2014, 10, 156–165. [Google Scholar]
- Alsohaili, S. Seasonal variation in the chemical composition and antimicrobial activity of essential oil extracted from Achillea fragrantissima grown in Northern-Eastern Jordanian desert. J. Essent. Oil-Bear. Plants 2018, 21, 139–145. [Google Scholar] [CrossRef]
- Allenspach, M.; Valder, C.; Flamm, D.; Grisoni, F.; Steuer, C. Verification of chromatographic profile of primary essential oil of Pinus sylvestris L. combined with chemometric analysis. Molecules 2020, 25, 2973. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Utegenova, G.A.; Pallister, K.B.; Kushnarenko, S.V.; Özek, G.; Özek, T.; Abidkulova, K.T.; Kirpotina, L.N.; Schepetkin, I.A.; Quinn, M.T.; Voyich, J.M. Chemical composition and antibacterial activity of essential oils from Ferula L. species against methicillin-resistant Staphylococcus aureus. Molecules 2018, 23, 1679. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Zhang, Y.; Zhu, Y.; Zhou, B.; Ren, C.; Liang, S.; Guo, Y. α-Pinene induces apoptotic cell death via caspase activation in human ovarian cancer cells. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 6631. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Abdelnour, S.A.; Elnesr, S.S. A review on the beneficial effect of thymol on health and production of fish. Rev. Aquac. 2021, 13, 632–641. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- Kokkini, S.; Karousou, R.; Vokou, D. Pattern of geographic variations of Origanum vulgare trichomes and essential oil content in Greece. Biochem. Syst. Ecol. 1994, 22, 517–528. [Google Scholar] [CrossRef]
- Chorianopoulos, N.; Kalpoutzakis, E.; Aligiannis, N.; Mitaku, S.; Nychas, G.-J.; Haroutounian, S.A. Essential oils of Satureja, Origanum, and Thymus species: Chemical composition and antibacterial activities against foodborne pathogens. J. Agric. Food. Chem. 2004, 52, 8261–8267. [Google Scholar] [CrossRef] [PubMed]
- Sousa Silveira, Z.d.; Macêdo, N.S.; Sampaio dos Santos, J.F.; Sampaio de Freitas, T.; Rodrigues dos Santos Barbosa, C.; Júnior, D.L.d.S.; Muniz, D.F.; Castro de Oliveira, L.C.; Júnior, J.P.S.; Cunha, F.A.B.d. Evaluation of the antibacterial activity and efflux pump reversal of thymol and carvacrol against Staphylococcus aureus and their toxicity in Drosophila melanogaster. Molecules 2020, 25, 2103. [Google Scholar] [CrossRef]
- Qoorchi Moheb Seraj, F.; Heravi-Faz, N.; Soltani, A.; Ahmadi, S.S.; Talebpour, A.; Afshari, A.R.; Ferns, G.A.; Bahrami, A. Thymol has anticancer effects in U-87 human malignant glioblastoma cells. Mol. Biol. Rep. 2022, 49, 9623–9632. [Google Scholar] [CrossRef] [PubMed]
- Mittermeier, V.K.; Dunkel, A.; Hofmann, T. Discovery of taste modulating octadecadien-12-ynoic acids in golden chanterelles (Cantharellus cibarius). Food Chem. 2018, 269, 53–62. [Google Scholar] [CrossRef]
- Shoge, M.; Amusan, T. Phytochemical, antidiarrhoeal activity, isolation and characterisation of 11-octadecenoic acid, methyl ester isolated from the seeds of Acacia nilotica Linn. J. Biotechnol. Immunol. 2020, 2, 1–12. [Google Scholar]
- Dhar, P.; Chan, P.; Cohen, D.T.; Khawam, F.; Gibbons, S.; Snyder-Leiby, T.; Dickstein, E.; Rai, P.K.; Watal, G. Synthesis, antimicrobial evaluation, and structure–activity relationship of α-pinene derivatives. J. Agric. Food. Chem. 2014, 62, 3548–3552. [Google Scholar] [CrossRef]
- Freitas, P.R.; de Araújo, A.C.J.; dos Santos Barbosa, C.R.; Muniz, D.F.; da Silva, A.C.A.; Rocha, J.E.; de Morais Oliveira-Tintino, C.D.; Ribeiro-Filho, J.; da Silva, L.E.; Confortin, C. GC-MS-FID and potentiation of the antibiotic activity of the essential oil of Baccharis reticulata (ruiz & pav.) pers. and α-pinene. Ind. Crops. Prod. 2020, 145, 112106. [Google Scholar]
- Zhu, Z.; Min, T.; Zhang, X.; Wen, Y. Microencapsulation of Thymol in Poly (lactide-co-glycolide)(PLGA): Physical and Antibacterial Properties. Materials 2019, 12, 1133. [Google Scholar] [CrossRef] [PubMed]
- Khoshbakht, T.; Karami, A.; Tahmasebi, A.; Maggi, F. The variability of thymol and carvacrol contents reveals the level of antibacterial activity of the Essential Oils from different accessions of Oliveria decumbens. Antibiotics 2020, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Almehdar, H.; Abdallah, H.M.; Osman, A.-M.M.; Abdel-Sattar, E.A. In vitro cytotoxic screening of selected Saudi medicinal plants. J. Nat. Med. 2012, 66, 406–412. [Google Scholar] [CrossRef]
- Jo, H.; Cha, B.; Kim, H.; Brito, S.; Kwak, B.M.; Kim, S.T.; Bin, B.-H.; Lee, M.-G. α-pinene enhances the anticancer activity of natural killer cells via ERK/AKT pathway. Int. J. Mol. Sci. 2021, 22, 656. [Google Scholar] [CrossRef]
- Seresht, H.R.; Albadry, B.J.; Al-mosawi, A.K.M.; Gholami, O.; Cheshomi, H. The cytotoxic effects of thymol as the major component of trachyspermum ammi on breast cancer (MCF-7) cells. Pharm. Chem. J. 2019, 53, 101–107. [Google Scholar] [CrossRef]
- Alkhathlan, H.; Khan, M.; Abdullah, M.; AlMayouf, A.; Badjah-Hadj-Ahmed, A.Y.; AlOthman, Z.; Mousa, A. Anticorrosive assay-guided isolation of active phytoconstituents from Anthemis pseudocotula extracts and a detailed study of their effects on the corrosion of mild steel in acidic media. RSC Adv. 2015, 5, 54283–54292. [Google Scholar] [CrossRef]
- Al-Hwaiti, M.S.; Alsbou, E.M.; Abu Sheikha, G.; Bakchiche, B.; Pham, T.H.; Thomas, R.H.; Bardaweel, S.K. Evaluation of the anticancer activity and fatty acids composition of “Handal”(Citrullus colocynthis L.) seed oil, a desert plant from south Jordan. Food Sci. Nutr. 2021, 9, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.; Kaur, J.; Sharma, P.; Kaur, G.; Bhandari, Y.; Kumar, R.; Singh, S. P53-mediated anticancer activity of Citrullus colocynthis extracts. Nat. Prod. J. 2019, 9, 303–311. [Google Scholar] [CrossRef]
- Khan, M.; Al-Saleem, M.S.; Alkhathlan, H.Z. A detailed study on chemical characterization of essential oil components of two Plectranthus species grown in Saudi Arabia. J. Saudi Chem. Soc. 2016, 20, 711–721. [Google Scholar] [CrossRef]
- Acree, T.; Arn, H. Gas Chromatography-Olfactometry (GCO) of Natural Products. Flavornet and Human Odor Space, Sponsored by DATU Inc. 2004. Available online: http://www.flavornet.org (accessed on 25 December 2022).
- NIST Mass Spectrometry Data Center, W.E.W. Director “Retention Indices”. In NIST Chemistry WebBook; NIST Standard Reference Database Number, 69; Linstrom, P.J., Mallard, W.G., Eds.; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2020; p. 20899. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Swapnaja, K.J.M.; Yennam, S.; Chavali, M.; Poornachandra, Y.; Kumar, C.G.; Muthusamy, K.; Jayaraman, V.B.; Arumugam, P.; Balasubramanian, S.; Sriram, K.K. Design, synthesis and biological evaluation of diaziridinyl quinone isoxazole hybrids. Eur. J. Med. Chem. 2016, 117, 85–98. [Google Scholar] [CrossRef]
- Hansen, M.B.; Nielsen, S.E.; Berg, K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef] [PubMed]




| Peaks | Compounds * | M.F | CAS No. | R.T. (min) | LRI | H.E. % | C.E. % | M.E. % |
|---|---|---|---|---|---|---|---|---|
| 1 | cis-2-Pentenol | C5H10O | 1576-95-0 | 5.187 | 772 | - | - | 0.7 |
| 2 | Toluene | C7H8 | 108-88-3 | 5.394 | 778 | 3.6 | - | - |
| 3 | Capronaldehyde | C6H12O | 66-25-1 | 5.996 | 796 | - | - | 0.2 |
| 4 | 1-Octene | C8H16 | 111-66-0 | 6.034 | 797 | - | - | 0.4 |
| 5 | Isopropyl butanoate | C7H14O2 | 638-11-9 | 7.466 | 840 | 10.4 | - | - |
| 6 | p-Xylene | C8H10 | 106-42-3 | 8.422 | 869 | 2.8 | - | - |
| 7 | o-Xylene | C8H10 | 95-47-6 | 9.251 | 894 | 1.6 | - | - |
| 8 | Santolina triene | C10H16 | 2153-66-4 | 9.806 | 909 | 1.6 | - | - |
| 9 | Isobutyl isobutyrate | C8H16O2 | 97-85-8 | 9.958 | 913 | 1.6 | - | - |
| 10 | α-Thujene | C10H16 | 2867-05-2 | 10.43 | 926 | 1.5 | - | - |
| 11 | Benzaldehyde | C7H6O | 100-52-7 | 11.662 | 958 | - | 1.6 | - |
| 12 | α-Pinene | C10H16 | 80-56-8 | 10.754 | 934 | 30.6 | - | - |
| 13 | Sabinene | C10H16 | 3387-41-5 | 12.29 | 974 | 1.6 | - | - |
| 14 | Pseudocumene | C9H12 | 95-63-6 | 13.058 | 994 | 2.7 | - | - |
| 15 | Undecane | C11H24 | 1120-21-4 | 17.098 | 1100 | - | - | 1.1 |
| 16 | δ3-Carene | C10H16 | 13466-78-9 | 13.696 | 1011 | 5.1 | - | - |
| 17 | o-Methylacetophenone | C9H10O | 577-16-2 | 18.527 | 1138 | 10.8 | - | - |
| 18 | Dodecane | C12H26 | 112-40-3 | 20.783 | 1200 | 1.4 | - | - |
| 19 | Ethyl phenyl acetate | C10H12O2 | 101-97-3 | 22.604 | 1253 | - | 3.1 | - |
| 20 | 2E-Decenal | C10H18O | 3913-81-3 | 22.971 | 1263 | - | 3.4 | - |
| 21 | Thymol | C10H14O | 499-75-2 | 23.988 | 1293 | - | 37.2 | 3.0 |
| 22 | Filifolide-A | C10H14O2 | - | 24.83 | 1318 | - | 3.9 | - |
| 23 | Tetradecane | C14H30 | 629-59-4 | 27.483 | 1400 | 2.8 | - | 1.5 |
| 24 | Coumarin | C9H6O2 | 91-64-5 | 28.542 | 1434 | - | - | 0.3 |
| 25 | 2-Methyl butyl benzoate | C12H16O2 | 52513-03-8 | 28.696 | 1439 | - | - | 0.5 |
| 26 | α-Guaiene | C15H24 | 3691-12-1 | 28.795 | 1443 | - | - | 0.2 |
| 27 | β-Ionol | C13H22O | 22029-76-1 | 31.038 | 1517 | - | 4.8 | - |
| 28 | Caryophyllene oxide | C15H24O | 1139-30-6 | 33.252 | 1593 | 1.4 | - | - |
| 29 | Hexadecane | C16H34 | 544-76-3 | 33.459 | 1600 | 3.1 | - | - |
| 30 | 8-Cedren-13-ol | C15H24O | 18319-35-2 | 35.846 | 1686 | - | 2.8 | - |
| 31 | Octadecane | C18H38 | 593-45-3 | 38.831 | 1800 | 1.7 | - | - |
| 32 | 7-Hydroxycoumarin | C9H6O3 | 93-35-6 | 39.837 | 1840 | 3.9 | - | - |
| 33 | Methyl hexadecanoate | C17H34O2 | 112-39-0 | 41.982 | 1927 | - | 5.6 | 18.3 |
| 34 | n-Hexadecanoic acid | C16H32O2 | 57-10-3 | 42.789 | 1960 | - | - | 1.6 |
| 35 | Ethyl hexadecanoate | C18H36O2 | 628-97-7 | 43.603 | 1994 | 3.3 | - | - |
| 36 | 8,11-Octadecadienoic acid, methyl ester | C19H34O2 | 56599-58-7 | 45.91 | 2091 | - | 13.0 | 28.6 |
| 37 | (Z)-9-Octadecenoic acid methyl ester | C19H36O2 | 112-62-9 | 46.029 | 2096 | - | 5.3 | 20.4 |
| 38 | Methyl oleate | C19H36O2 | 112-62-9 | 46.319 | 2108 | 2.1 | - | - |
| 39 | n-Octadecanoic acid, methyl ester | C19H38O2 | 112-61-8 | 46.599 | 2120 | - | - | 7.4 |
| 40 | Ethyl linoleate | C20H36O2 | 544-35-4 | 47.605 | 2162 | 1.9 | - | - |
| 41 | Tetracosane | C24H50 | 646-31-1 | 53.297 | 2400 | - | 2.1 | 1.4 |
| 42 | trans-Ferruginyl acetate | C22H32O2 | 15340-79-1 | 53.562 | 2411 | - | 8.1 | - |
| 43 | 6-Ketoferruginol | 54.625 | 2456 | - | - | 9.6 | ||
| Monoterpenes hydrocarbons | 40.4 | - | - | |||||
| Oxygenated monoterpenes | - | 41.1 | 3.0 | |||||
| Sesquiterpene hydrocarbons | - | - | 0.2 | |||||
| Oxygenated sesquiterpenes | 1.4 | 7.6 | - | |||||
| Aliphatic hydrocarbons | 9 | 2.1 | 4.4 | |||||
| Oxygenated aliphatic hydrocarbons | 19.3 | 27.3 | 77.2 | |||||
| Aromatics | 21.5 | 4.7 | 0.5 | |||||
| Diterpenoids | - | 8.1 | 9.6 | |||||
| Others | 3.9 | - | 0.3 | |||||
| Total identified | 95.5 | 90.9 | 95.2 | |||||
| No. | Country | City | Major Components (%) | Reference |
|---|---|---|---|---|
| 1. | Morocco | Tangier | Nonadienal (15.4), linalool propanoate (14.3), 2,4-decadienal (7.8), pentadecane (7.2), hexanal (4.5), and butylated hydroxy anisol (4.3). | [44] |
| 2. | India | Parangipettai | 2-Methyl,4-heptanone (48.0), 3-Methyl,2-heptanone (12.9), trimethylsilylmethanol (9.1), pentane, 1-propoxy (6.5), and 2-pentanol, 5-methoxy-2-methyl- (5.3). | [45] |
| Southern Haryana | n-Hexadecanoic acid (12.4), morphine (9.1), narceine (10.3), isoquinoline, 1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy- (7.5), codein (6.6), and glycerol (5.5). | [46] | ||
| 3. | Saudi Arabia | Riyadh | Thymol (3.0–37.2), α-pinene (0–30.0), 8,11-octadecadienoic acid, methyl ester (13.0–28.6), (Z)-9-octadecenoic acid methyl ester (0–20.4), methyl hexadecanoate (5.6–18.3), o-methylacetophenone (0–10.8), isopropyl butanoate (0–10.4), 6-ketoferruginol (0–9.6), trans-ferruginyl acetate (0.0–8.1), n-octadecanoic acid, methyl ester (0–7.4), trans-sabinyl acetate (0–6.0). | Present study |
| Tested Extracts of C. colocynthis Seeds | Minimum Inhibitory Concentration (µg/mL) | |||
|---|---|---|---|---|
| Gram-Positive | Gram-Negative | |||
| S. aureus MTCC 96 | M. luteus MTCC 2470 | K. planticola MTCC 530 | E. coli MTCC 739 | |
| M.E. | 62.5 | 62.5 | 7.8 | >250 |
| H.E. | 3.9 | >250 | 0.9 | >250 |
| C.E. | 7.8 | >250 | 1.9 | >250 |
| Ciprofloxacin | 0.9 | 0.9 | 0.9 | 0.9 |
| Tested Extracts of C. colocynthis Seeds | IC50 (µg/mL) | |||
|---|---|---|---|---|
| HepG2 | DU145 | Hela | A549 | |
| M.E. | 126.65 ± 11.48 | 91.94 ± 7.88 | 99.96 ± 9.70 | 70.18 ± 1.17 |
| H.E. | 177.05 ± 4.84 | 48.49 ± 0.50 | 197.28 ± 9.45 | 82.99 ± 6.5 |
| C.E. | NA | 53.32 ± 1.59 | 83.87 ± 4.61 | 154.05 ± 14.25 |
| Doxorubicin | 0.72 ± 0.012 (µM) | 0.36 ± 0.01 (µM) | 0.8 ± 0.71 (µM) | 0.55 ± 0.16 (µM) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.; Khan, M.; Al-hamoud, K.; Adil, S.F.; Shaik, M.R.; Alkhathlan, H.Z. Diversity of Citrullus colocynthis (L.) Schrad Seeds Extracts: Detailed Chemical Profiling and Evaluation of Their Medicinal Properties. Plants 2023, 12, 567. https://doi.org/10.3390/plants12030567
Khan M, Khan M, Al-hamoud K, Adil SF, Shaik MR, Alkhathlan HZ. Diversity of Citrullus colocynthis (L.) Schrad Seeds Extracts: Detailed Chemical Profiling and Evaluation of Their Medicinal Properties. Plants. 2023; 12(3):567. https://doi.org/10.3390/plants12030567
Chicago/Turabian StyleKhan, Merajuddin, Mujeeb Khan, Khaleel Al-hamoud, Syed Farooq Adil, Mohammed Rafi Shaik, and Hamad Z. Alkhathlan. 2023. "Diversity of Citrullus colocynthis (L.) Schrad Seeds Extracts: Detailed Chemical Profiling and Evaluation of Their Medicinal Properties" Plants 12, no. 3: 567. https://doi.org/10.3390/plants12030567