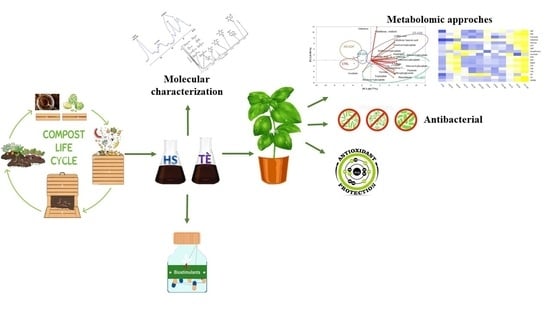
Evaluation of Sustainable Recycled Products to Increase the Production of Nutraceutical and Antibacterial Molecules in Basil Plants by a Combined Metabolomic Approach
Abstract
:1. Introduction
2. Results
2.1. Molecular Characterization of Compost Extracts
2.2. Phenological Parameter of Basil Plants
2.3. Antioxidant Capacity of Basil Extracts
2.4. Antibacterial Activity of Basil Methanolic Extracts
2.5. Untargeted and Targeted Metabolomic Approach
3. Discussion
3.1. Plant Development, Antioxidant and Antimicrobial Activities
3.1.1. Plant Biomass
3.1.2. Antioxidant Activity
3.1.3. Antimicrobial Activity
3.2. Combined Nontarget and Target Metabolomic Approaches
3.2.1. Effect of Organic Derivates on Plants Biochemical Pathways to Produce Metabolites by Nutraceutical Application
3.2.2. Effect of Compost Derivates on Plants Phenylpropanoid Derivatives
4. Materials and Methods
4.1. Humic Substances (HS) and Compost Teas (CTs)
4.2. Experimental Design, Plant Growth, Sampling, and Analyses
- A: CTRL: Control (H2O) + mineral fertilizers;
- B: A + HS artichoke 10 mg L−1; C: A + HS artichoke 50 mg L−1; D: A + HS artichoke 100 mg L−1;
- E: A + CT artichoke 10 mg L−1; F: A + CT artichoke 50 mg L−1; G: A + CT artichoke 100 mg L−1;
- H: HS coffee 10 mg L−1; I: A + HS coffee 50 mg L−1; L: A + HS coffee 100 mg L−1;
- M: CT coffee 10 mg L−1; N: A + CT coffee 50 mg L−1; O: A + CT coffee 100 mg L−1.
4.3. Extraction of Plant Leaf Metabolites
4.4. 13C-CPMAS-NMR Spectroscopy
- the hydrophobic index is the ratio of signal intensities of apolar alkyl and aromatic C components over those of hydrophilic C moleculesHB/HI = Σ[(0–45) + (45–60)/2 + (110–160)]/Σ[(45–60)/2 + (60–110) + (160–190)];
- the aromaticity index compares the number of aromatic compounds to that of the alkyl groupsARM = [(110–160)/Σ(0–45) + (60–110)];
- the alkyl ratio is determined by the relative abundance of apolar aliphatic molecules over that of the polar-alkyl fractionsA/OA = (0–45)/(60–110);
- the lignin ratio, relates the area of methoxyl-C and N-alkyl groups to that of O-aryl-C functionsLigR = (45–60)/(145–160).
4.5. Antioxidant Activity of Methanolic Basil Extracts
4.5.1. Sample Extraction
4.5.2. Total Phenolic Compounds (TPC)
4.5.3. DPPH Radical Scavenging Assay
4.6. Antimicrobial Activity of Leaf Methanolic Extracts
4.7. Metabolomic Approaches
4.7.1. Standard References
4.7.2. Liquid Chromatography High Resolution Mass Spectrometry (LC-HRMS)
4.7.3. Data Processing LC/HRMS Results
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huiart, L.; Bouhnik, A.D.; Rey, D.; Rousseau, F.; Retornaz, F.; Meresse, M.; Giorgi, R. Complementary or Alternative Medicine as Possible Determinant of Decreased Persistence to Aromatase Inhibitor Therapy among Older Women with Non-Metastatic Breast Cancer. PLoS ONE 2013, 8, e81677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thirumurugan, D.; Cholarajan, A.; Raja SS, S.; Vijayakumar, R. An Introductory Chapter: Secondary Metabolites. In Secondary Metabolites-Sources and Applications; Vijayakumar, R., Raja, S.S., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Datta, R.; Sharma, A.K.; Thakur, A.; Datta, R.; Sharma, A.; Thakur, A. Secondary Metabolites from Plants: Role in Plant Diseases and Health Care. In Plant Secondary Metabolites; Sharma, A.K., Sharma, A., Eds.; Springer Nature: Singapore, 2022; pp. 355–369. [Google Scholar] [CrossRef]
- Dorosh, O.; Rodrigues, F.; Delerue-Matos, C.; Moreira, M.M. Increasing the added value of vine-canes as a sustainable source of phenolic compounds: A review. Sci. Total Environ. 2022, 830, 154600. [Google Scholar] [CrossRef]
- De Luca, I.; Di Cristo, F.; Valentino, A.; Peluso, G.; Di Salle, A.; Calarco, A. Food-Derived Bioactive Molecules from Mediterranean Diet: Nanotechnological Approaches and Waste Valorization as Strategies to Improve Human Wellness. Polymers 2022, 14, 1726. [Google Scholar] [CrossRef] [PubMed]
- Fraga-Corral, M.; Otero, P.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Jarboui, A.; Prieto, M.A. By-Products of Agri-Food Industry as Tannin-Rich Sources: A Review of Tannins’ Biological Activities and Their Potential for Valorization. Foods 2021, 10, 137. [Google Scholar] [CrossRef]
- Skrypnik, L.; Novikova, A.; Tokupova, E. Improvement of Phenolic Compounds, Essential Oil Content and Antioxidant Properties of Sweet Basil (Ocimum basilicum L.) Depending on Type and Concentration of Selenium Application. Plants 2019, 8, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, M.A.; De Souza, L.P.; Serag, A.; Fernie, A.R.; Farag, M.A.; Ezzat, S.M.; Alseekh, S. Metabolomics in the Context of Plant Natural Products Research: From Sample Preparation to Metabolite Analysis. Metabolites 2020, 10, 37. [Google Scholar] [CrossRef] [Green Version]
- Twaij, B.M.; Hasan, M.N. Bioactive Secondary Metabolites from Plant Sources: Types, Synthesis, and Their Therapeutic Uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Bagnasco, D.; Paggiaro, P.; Latorre, M.; Folli, C.; Testino, E.; Bassi, A.; Cicero, S.L. Severe asthma: One disease and multiple definitions. World Allergy Organ. J. 2021, 14, 100606. [Google Scholar] [CrossRef] [PubMed]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From Central to Specialized Metabolism: An Overview of Some Secondary Compounds Derived From the Primary Metabolism for Their Role in Conferring Nutritional and Organoleptic Characteristics to Fruit. Front. Plant Sci. 2019, 10, 835. [Google Scholar] [CrossRef] [Green Version]
- Canellas, N.A.; Olivares, F.L.; da Silva, R.M.; Canellas, L.P. Changes in Metabolic Profile of Rice Leaves Induced by Humic Acids. Plants 2022, 11, 3261. [Google Scholar] [CrossRef]
- Pane, C.; Spaccini, R.; Caputo, M.; De Falco, E.; Zaccardelli, M. Multi-Parameter Characterization of Disease-Suppressive Bio-composts from Aromatic Plant Residues Evaluated for Garden Cress (Lepidium sativum L.) Cultivation. Horticulturae 2022, 8, 632. [Google Scholar] [CrossRef]
- Venezia, V.; Pota, G.; Silvestri, B.; Vitiello, G.; Di Donato, P.; Landi, G.; Luciani, G. A study on structural evolution of hybrid humic Acids-SiO2 nanostructures in pure water: Effects on physico-chemical and functional properties. Chemosphere 2022, 287, 131985. [Google Scholar] [CrossRef] [PubMed]
- Verrillo, M.; Parisi, M.; Savy, D.; Caiazzo, G.; Di Caprio, R.; Luciano, M.A.; Fabbrocini, G.; Piccolo, A. Antiflammatory activity and potential dermatological applications of characterized humic acids from a lignite and a green compost. Sci. Rep. 2022, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Verrillo, M.; Salzano, M.; Savy, D.; Di Meo, V.; Valentini, M.; Cozzolino, V.; Piccolo, A. Antibacterial and antioxidant properties of humic substances from composted agricultural biomasses. Chem. Biol. Technol. Agric. 2022, 9, 1–15. [Google Scholar] [CrossRef]
- Luo, F.; Yu, Z.; Zhou, Q.; Huang, A. Multi-Omics-Based Discovery of Plant Signaling Molecules. Metabolites 2022, 12, 76. [Google Scholar] [CrossRef]
- Narad, P.; Gupta, R.; Sengupta, A. Plant metabolomics: A new era in the advancement of agricultural research. In Bioinformatics in Agriculture; Sharma, P., Yadav, D., Gaur, R.K., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2022; pp. 139–160. [Google Scholar] [CrossRef]
- Roca, M.; Pérez-Gálvez, A. Metabolomics of Chlorophylls and Carotenoids: Analytical Methods and Metabolome-Based Studies. Antioxidants 2021, 10, 1622. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Saand, M.A.; Huang, L.; Abdelaal, W.B.; Zhang, J.; Wu, Y.; Wang, F. Applications of Multi-Omics Technologies for Crop Improvement. Front. Plant Sci. 2021, 12, 1846. [Google Scholar] [CrossRef]
- Rodrigues Sá, R.; da Cruz Caldas, J.; de Andrade Santana, D.; de Freitas Santos Júnior, A. Multielementar/centesimal composition and determination of bioactive phenolics in dried fruits and capsules containing Goji berries (Lycium barbarum L.). Food Chem. 2019, 273, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Serra Moura, H.; de Souza Dias, F.; Beatriz Souza e Souza, L.; de Andrade Santana, D.; de Freitas Santos Júnior, A. Evaluation of multielement/proximate composition and bioactive phenolics contents of unconventional edible plants from Brazil using multivariate analysis techniques. Food Chem. 2021, 363, 129995. [Google Scholar] [CrossRef]
- Stavropoulou, M.I.; Termentzi, A.; Kasiotis, K.M.; Cheilari, A.; Stathopoulou, K.; Machera, K.; Aligiannis, N. Untargeted Ultrahigh-Performance Liquid Chromatography-Hybrid Quadrupole-Orbitrap Mass Spectrometry (UHPLC-HRMS) Metabolomics Reveals Propolis Markers of Greek and Chinese Origin. Molecules 2021, 26, 456. [Google Scholar] [CrossRef]
- Canellas, L.P.; Canellas, N.O.A.; da Silva, R.M.; Spaccini, R.; Mota, G.P.; Olivares, F.L. Biostimulants Using Humic Substances and Plant-Growth-Promoting Bacteria: Effects on Cassava (Manihot esculentus) and Okra (Abelmoschus esculentus) Yield. Agronomy 2023, 13, 80. [Google Scholar] [CrossRef]
- de Aquino, A.M.; Canellas, L.P.; da Silva AP, S.; Canellas, N.O.; da SLima, L.; Olivares, F.L.; Spaccini, R. Evaluation of molecular properties of humic acids from vermicompost by 13 C-CPMAS-NMR spectroscopy and thermochemolysis–GC–MS. J. Anal. Appl. Pyrol. 2019, 141, 104634. [Google Scholar] [CrossRef]
- da Silva, S.F.; Spaccini, R.; Mazzei, P.; de Rezende, C.E.; Canellas, L.P. Changes in water-extractable organic matter in tropical forest and agricultural soils as detected by the DRIFT spectroscopy technique. Land Degrad. Dev. 2021, 32, 4755–4767. [Google Scholar] [CrossRef]
- Fregolente, L.G.; Dos Santos, J.V.; Vinci, G.; Piccolo, A.; Moreira, A.B.; Ferreira, O.P.; Bisinoti, M.C.; Spaccini, R. Insights on molecular characteristics of hydrochars by13C-NMR and off-line TMAH-GC/MS and assessment of their potential use as plant growth promoters. Molecules 2021, 26, 1026. [Google Scholar] [CrossRef] [PubMed]
- Verrillo, M.; Cozzolino, V.; Spaccini, R.; Piccolo, A. Humic substances from green compost increase bioactivity and antibacterial properties of essential oils in Basil leaves. Chem. Biol. Technol. Agric. 2021, 8, 1–14. [Google Scholar] [CrossRef]
- Verrillo, M.; Savy, D.; Cangemi, S.; Savarese, C.; Cozzolino, V.; Piccolo, A. Valorization of lignins from energy crops and agro-industrial byproducts as antioxidant and antibacterial materials. J. Sci. Food Agric. 2021, 102, 2885–2892. [Google Scholar] [CrossRef]
- Aminifard, M.H.; Aroiee, H.; Azizi, M.; Nemati, H.; Jaafar HZ, E. Effect of Humic Acid on Antioxidant Activities and Fruit Quality of Hot Pepper (Capsicum annuum L.). J. Herbs Spices Med. Plants. 2012, 18, 360–369. [Google Scholar] [CrossRef]
- de Castro, T.A.V.T.; Berbara, R.L.L.; Tavares, O.C.H.; da Graça Mello, D.F.; Pereira, E.G.; de Souza, C.D.C.B.; Espinosa, L.M.; García, A.C. Humic acids induce a eustress state via photosynthesis and nitrogen metabolism leading to a root growth improvement in rice plants. Plant Physiol. Bioch. 2021, 162, 171–184. [Google Scholar] [CrossRef]
- Lee, H.J.; Kremer, D.M.; Sajjakulnukit, P.; Zhang, L.; Lyssiotis, C.A. A large-scale analysis of targeted metabolomics data from heterogeneous biological samples provides insights into metabolite dynamics. Metabolomics 2019, 15, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamany Djande, C.Y.; Pretorius, C.; Tugizimana, F.; Piater, L.A.; Dubery, I.A. Metabolomics: A Tool for Cultivar Phenotyping and Investigation of Grain Crops. Agronomy 2020, 10, 831. [Google Scholar] [CrossRef]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nature Reviews. Molecular Cell Biology 2016, 17, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lelli, V.; Belardo, A.; Timperio, A.M. From Targeted Quantification to Untargeted Metabolomics. In Metabolomics–Methodology and Applications in Medical Sciences and Life Sciences; Zhan, X., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Vinci, G.; Cozzolino, V.; Mazzei, P.; Monda, H.; Spaccini, R.; Piccolo, A. An alternative to mineral phosphorus fertilizers: The combined effects of Trichoderma harzianum and compost on Zea mays, as revealed by 1H NMR and GC-MS metabolomics. PLoS ONE 2018, 13, e0209664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinson, C.C.; Mota AP, Z.; Porto, B.N.; Oliveira, T.N.; Sampaio, I.; Lacerda, A.L.; Brasileiro, A.C.M. Characterization of raffinose metabolism genes uncovers a wild Arachis galactinol synthase conferring tolerance to abiotic stresses. Sci. Rep. 2020, 10, 1–19. [Google Scholar] [CrossRef]
- Zhang, L.; Becker, D.F. Connecting proline metabolism and signaling pathways in plant senescence. Front. Plant Sci. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [Green Version]
- Eudoxie, G.; Martin, M. Compost Tea Quality and Fertility. In Organic Fertilizers–History, Production and Applications; Larramendy, M.L., Solonesky, S., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical structure and biological activity of humic substances define their role as plant growth promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef]
- Chernysheva, M.G.; Badun, G.; Kulikova, N.; Perminova, I.V. Behavior of humic substances in the liquid-liquid system directly measured using tritium label. Chemosphere 2020, 238, 124646. [Google Scholar] [CrossRef]
- Savarese, C.; di Meo, V.; Cangemi, S.; Verrillo, M.; Savy, D.; Cozzolino, V.; Piccolo, A. Bioactivity of two different humic materials and their combination on plants growth as a function of their molecular properties. Plant Soil 2022, 472, 509–526. [Google Scholar] [CrossRef]
- Aguiar, N.O.; Olivares, F.L.; Novotny, E.H.; Canellas, L.P. Changes in metabolic profiling of sugarcane leaves induced by endophytic diazotrophic bacteria and humic acids. PeerJ 2018, 2018, e5445. [Google Scholar] [CrossRef]
- Olaetxea, M.; De Hita, D.; Garcia, C.A.; Fuentes, M.; Baigorri, R.; Mora, V.; Garcia-Mina, J.M. Hypothetical framework integrating the main mechanisms involved in the promoting action of rhizospheric humic substances on plant root- and shoot- growth. Appl. Soil Ecol. 2018, 123, 521–537. [Google Scholar] [CrossRef]
- Baía, D.C.; Olivares, F.L.; Zandonadi, D.B.; de Paula Soares, C.; Spaccini, R.; Canellas, L.P. Humic acids trigger the weak acids stress response in maize seedlings. Chem. Biol. Technol. Agric. 2020, 7, 31. [Google Scholar] [CrossRef]
- Rahbardar, M.G.; Hosseinzadeh, H. Therapeutic effects of rosemary (Rosmarinus officinalis L.) and its active constituents on nervous system disorders. Iran. J. Basic Med. Sci. 2020, 23, 1100. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [Green Version]
- Deng, G.F.; Lin, X.; Xu, X.R.; Gao, L.L.; Xie, J.F.; Li, H.B. Antioxidant capacities and total phenolic contents of 56 vegetables. J. Funct. Foods 2013, 5, 260–266. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic Acid Pathway in Biosynthesis of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds; Soto-Hernández, M., García-Mateos, R., Palma-Tenango, M., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Kaurinovic, B.; Popovic, M.; Vlaisavljevic, S.; Trivic, S. Antioxidant Capacity of Ocimum basilicum L. and Origanum vulgare L. Extracts. Molecules 2011, 16, 7401. [Google Scholar] [CrossRef]
- Khojasteh, A.; Mirjalili, M.H.; Alcalde, M.A.; Cusido, R.M.; Eibl, R.; Palazon, J. Powerful Plant Antioxidants: A New Biosustainable Approach to the Production of Rosmarinic Acid. Antioxidants 2020, 9, 1273. [Google Scholar] [CrossRef]
- Garciá, A.C.; De Souza, L.G.A.; Pereira, M.G.; Castro, R.N.; Garciá-Mina, J.M.; Zonta, E.; Berbara, R.L.L. Structure-Property-Function Relationship in Humic Substances to Explain the Biological Activity in Plants. Sci. Rep. 2016, 6, 20798. [Google Scholar] [CrossRef] [Green Version]
- Schiavon, M.; Pizzeghello, D.; Muscolo, A.S.; Francioso, O.; Nardi, S. High molecular size humic substances enhance phenylpropanoid metabolism in maize (Zea mays L.). J. Chem. Ecol. 2010, 36, 662–669. [Google Scholar] [CrossRef]
- Trevisan, S.; Francioso, O.; Quaggiotti, S.; Nardi, S. Humic substances biological activity at the plant-soil interface: From environmental aspects to molecular factors. Plant Signal. Behav. 2010, 5, 635. [Google Scholar] [CrossRef] [Green Version]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Papadochristopoulos, A.; Kerry, J.P.; Fegan, N.; Burgess, C.M.; Duffy, G. Natural anti-microbials for enhanced microbial safety and shelf-life of processed packaged meat. Foods 2021, 10, 1598. [Google Scholar] [CrossRef] [PubMed]
- Mishra, L.K.; Sarkar, D.; Mentreddy, R.; Shetty, K. Evaluation of phenolic bioactive-linked anti-hyperglycemic and Helicobacter pylori inhibitory activities of Asian Basil (Ocimum spp.) varieties. J. Herb. Med. 2020, 20, 100310. [Google Scholar] [CrossRef]
- Malaka, M.J.; Araya, N.A.; Soundy, P.; du Plooy, C.P.; Araya, H.T.; Jansen Van Rensburg, W.S.; Watkinson, E.; Levember, E.; Wadiwala, E.; Amoo, S.O. Biomass, Essential Oil Yield, and Composition of Marjoram as Influenced by Interactions of Different Agronomic Practices under Controlled Conditions. Plants 2023, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, H.; Wang, S.; Du, Y.; Wei, B.; Wu, Q.; Wang, H. Inhibitors of Bacterial Extracellular Vesicles. Front. Microbiol. 2022, 13, 319. [Google Scholar] [CrossRef]
- Ribbenstedt, A.; Ziarrusta, H.; Benskin, J.P. Development, characterization and comparisons of targeted and non-targeted metabolomics methods. PLoS ONE 2018, 13, e0207082. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Fokou PV, T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [Green Version]
- Kiokias, S.; Oreopoulou, V. A Review of the Health Protective Effects of Phenolic Acids against a Range of Severe Pathologic Conditions (Including Coronavirus-Based Infections). Molecules 2021, 26, 5405. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [Green Version]
- Conte, P.; Kucerik, J. Water Dynamics and Its Role in Structural Hysteresis of Dissolved Organic Matter. Environ. Sci. Technol. 2016, 50, 2210–2216. [Google Scholar] [CrossRef] [Green Version]
- Robson, T.M.; Aphalo, P.J.; Banaś, A.K.; Barnes, P.W.; Brelsford, C.C.; Jenkins, G.I.; Kotilainen, T.K.; Łabuz, J.; Martínez-Abaigar, J.; Morales, L.O.; et al. A perspective on ecologically relevant plant-UV research and its practical application. Photochem. Photobiol. Sci. 2019, 18, 970–988. [Google Scholar] [CrossRef] [Green Version]
- Chutimanukul, P.; Wanichananan, P.; Janta, S.; Toojinda, T.; Darwell, C.T.; Mosaleeyanon, K. The influence of different light spectra on physiological responses, antioxidant capacity and chemical compositions in two holy basil cultivars. Sci. Rep. 2022, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Cai, Y.; Sun, M.; Wang, G.; Corke, H. Anthocyanins, flavonols, and free radical scavenging activity of Chinese Bayberry (Myrica rubra) extracts and their color properties and stability. J. Agric. Food Chem. 2005, 53, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Ezzat, S.M.; Salama, M.M.; Tadros, M.G. Anti-acetylcholinesterase potential and metabolome classification of 4 Ocimum species as determined via UPLC/qTOF/MS and chemometric tools. J. Pharmaceutic. Biomed. 2016, 125, 292–302. [Google Scholar] [CrossRef]
- Rampler, E.; Abiead YEl Schoeny, H.; Rusz, M.; Hildebrand, F.; Fitz, V.; Koellensperger, G. Recurrent Topics in Mass Spectrometry-Based Metabolomics and Lipidomics—Standardization, Coverage, and Throughput. Anal. Chem. 2021, 93, 519. [Google Scholar] [CrossRef] [PubMed]






| Organic Extracts | Carbonyl-C (190–160) | O-Aryl-C (160–145) | Aromatic-C (145–110) | O-Alkyl-C (110–60) | CH3O/CN (60–45) | Alkyl-C (45–0) | HB/HI | A/OA | ARM | LigR |
|---|---|---|---|---|---|---|---|---|---|---|
| HS-ART | 10.6 | 5.6 | 28.9 | 24.7 | 13.8 | 16.4 | 1.4 | 0.7 | 0.8 | 2.5 |
| HS-COF | 11.8 | 4 | 14.9 | 25.3 | 13.4 | 30.6 | 1.3 | 1.2 | 0.3 | 3.4 |
| CT-ART | 14.8 | 4.1 | 13.3 | 27.6 | 12.8 | 27.4 | 1.0 | 1.0 | 0.3 | 3.1 |
| CT-COF | 13.2 | 5.5 | 16.6 | 25.4 | 10.7 | 28.6 | 1.3 | 1.1 | 0.4 | 1.9 |
| Inhibition Zones (mm) | BSA 2 | AMP 3 | CTRL | HS-ART | HS-COF | CT-ART | CT-COF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 50 | 100 | 10 | 50 | 100 | 10 | 50 | 100 | 10 | 50 | 100 | ||||
| S. aureus | n.i. 1 | 21.13 ** | 9.3 | 10.3 * | 20.1 ** | 11.4 * | 8.5 | 10.3 * | 10.1 | 8.3 | 10.2 ** | 9.7 | 9.1 ** | 18.4 ** | 17.1 ** |
| ±0.1 | ±0.3 | ±0.2 | ±0.3 | ±0.3 | ±0.1 | ±0.4 | ±0.2 | ±0.2 | ±0.3 | ±0.2 | ±0.2 | ±0.5 | ±0.2 | ||
| E. faecalis | n.i. | 14.32 * | 8.15 | 8.1 | 11.3 | 12.4 | 7.4 | 7.3 | 7.5 | 7.2 * | 7.2 | 7.1 | 7.6 | 10.6 * | 9.2 * |
| ±0.5 | ±0.4 | ±0.4 | ±0.3 | ±0.2 | ±0.3 | ±0.1 | ±0.6 | ±0.2 | ±0.4 | ±0.7 | ±0.1 | ±0.2 | ±0.2 | ||
| E. coli | n.i. | 11.4 | 7.5 | 7.3 | 6.4 | 10.2 | 5.3 | 5.2 | 5.3 | 7.4 | 6.9 | 6.3 | 7.2 * | 5.8 | 9.7 * |
| ±0.1 | ±0.6 | ±0.6 | ±0.6 | ±0.4 | ±0.5 | ±0.1 | ±0.3 | ±0.1 | ±0.2 | ±0.1 | ±0.4 | ±0.2 | ±0.6 | ||
| P. aeruginosa | n.i. | 13.6 | 8.4 | 8.2 ** | 9.6 * | 9.8 | 8.4 | 8.2 * | 5.7 | 5.2 * | 5.6 | 6.1 | 7.9 | 9.2 * | 8.7 |
| ±0.6 | ±0.1 | ±0.1 | ±0.4 | ±0.3 | ±0.2 | ±0.6 | ±0.3 | ±0.2 | ±0.2 * | ±0.1 | ±0.2 | ±0.7 | ±0.4 | ||
| S. typhi | n.i. | 18.5 * | 7.3 | 6.4 | 8.3 | 8.6 | 7.4 | 7.9 | 7.3 | 7.9 | 7.4 | 7.1 | 6.70 * | 8.1 | 7.9 * |
| ±0.3 | ±0.7 | ±0.5 | ±0.2 | ±0.2 | ±0.2 | ±0.2 | ±0.2 | ±0.2 | ±0.1 | ±0.7 | ±0.6 | ±0.4 | ±0.3 | ||
| L. monocytogenes | n.i. | 10.3 | 9.1 | 8.2 | 9.4 | 11.2 ** | 10.2 | 8.2 | 7.1 | 6.9 | 7.1 | 5.3 | 7.1 | 9.1 | 10.1 |
| ±0.6 | ±0.4 | ±0.04 | ±0.3 | ±0.4 | ±0.3 | ±0.5 | ±0.6 | ±0.6 | ±0.6 | ±0.3 | ±0.2 | ±0.8 | ±0.02 | ||
| MIC µg mL−1 | BSA | AMP | CTRL | HS-ART | HS-COF | CT-ART | CT-COF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 50 | 100 | 10 | 50 | 100 | 10 | 50 | 100 | 10 | 50 | 100 | ||||
| S. aureus | n.i. | 1.8 | 2.3 | 1.8 | 1.2 | 1.4 | 2.4 | 2.1 | 1.9 | 2.8 | 2.4 | 3.7 | 2 | 1.9 | 2.1 |
| E. faecalis | n.i. | 2.1 | 2.1 | 1.4 | 1.4 | 1.7 | 2.5 | 2.6 | 2.8 | 2.3 | 2.6 | 3.7 | 1.6 | 1.6 | 1.4 |
| E. coli | n.i. | 2.2 | 2.5 | 1.3 | 1.5 | 1.9 | 2.7 | 2.3 | 3.1 | 3.2 | 2.8 | 3.2 | 1.7 | 1.7 | 1.9 |
| P. aeruginosa | n.i. | 3.2 | 2.9 | 1.8 | 2.1 | 2.5 | 2.3 | 2.7 | 2.1 | 3.6 | 2.6 | 2.5 | 1.9 | 1.9 | 2.1 |
| S. typhi | n.i. | 1.6 | 2.1 | 1.9 | 2.1 | 2.7 | 2.7 | 2.5 | 3.6 | 3.9 | 2.9 | 2.7 | 2.1 | 1.4 | 1.5 |
| L. monocytogenes | n.i. | 1.7 | 1.9 | 1.6 | 1.3 | 2.3 | 2.6 | 2.6 | 2.3 | 3.5 | 3.1 | 3.8 | 1.3 | 1.3 | 1.7 |
| HS-ART | HS-COF | CT-ART | CT-COF | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabolites | Ctrl | 10 | 50 | 100 | 10 | 50 | 100 | 10 | 50 | 100 | 10 | 50 | 100 |
| 3-Methyl-2-oxovaleric acid | 0.79 ±2.1 | 0.93 ±4.2 | 1.07 ±1.5 | 0.76 ±2.5 | 0.82 ±0.1 | 0.73 ±2.1 | 0.72 ±3.9 | 1.20 ±1.9 | 0.89 ±2.1 | 1.04 ±0.4 | 1.17 ±1.1 | 1.07 ±1.2 | 0.87 ±3.1 |
| 3-Phosphoglycerate | 1.16 ±1.3 | 1.23 ±3.6 | 1.43 * ±1.8 | 1.63 ±2.5 | 0.87 ** ±1.3 | 1.13 ±2.5 | 1.07 * ±2.1 | 1.11 ±8.1 | 1.19 ±2.1 | 1.29 ±0.1 | 1.24 * ±0.8 | 1.34 * ±1.3 | 1.44 ** ±1.9 |
| 3-AMP | 1.26 ±0.2 | 1.4 * ±0.5 | 1.55 ** ±2.1 | 1.68 * ±4.2 | 1.22 ±2.1 | 1.3 6±1.9 | 1.47 ±2.1 | 1.27 ±3.1 | 1.36 ±0.1 | 1.4 ±0.6 | 1.33 ** ±0.2 | 1.46 * ±1.5 | 1.58 ** ±1.3 |
| 5′-Deoxy-5′-Methylthioadenosine | 1.63 ±2.1 | 2.46 ±0.1 | 2.33 ±2.1 | 2.0 ±2.6 | 2.15 ±2.1 | 1.85 ±3.1 | 1.67 ±0.1 | 2.43 ±0.5 | 2.17 ±0.7 | 2.45 ±0.1 | 2.46 ±1.6 | 1.78 ±3.1 | 2.16 ±4.8 |
| Adenine | 2.62 ±2.1 | 2.61 ±0.6 | 2.49 ±0.8 | 2.50 ±0.9 | 2.22 ±0.1 | 2.02 ±0.8 | 1.82 ±0.1 | 2.63 ±0.9 | 2.37 ±0.1 | 2.37 ±.1.4 | 2.64 ±1.7 | 1.98 ±1.4 | 2.10 ±1.4 |
| Arginine | 0.98 ±3.1 | 2.36 ±1.2 | 1.16 ±1.6 | 0.88 ±2.1 | 6.44 ±2.1 | 1.37 ±2.7 | 2.28 ±3.1 | 3.88 ±4.1 | 2.28 ±1.2 | 6.8 ±6.1 | 1.6 ±0.1 | 1.17 ±0.4 | 4.43 ±0.5 |
| Asparagine | 1.8 * ±0.1 | 2.79 ±0.5 | 3.91 ±0.4 | 1.69 * ±0.4 | 2.28 ±0.6 | 1.99 ±0.7 | 1.58 * ±0.8 | 1.74 ±0.3 | 1.75 ±0.5 | 2.53 ±0.7 | 2.24 ** ±0.6 | 3.33 * ±2.1 | 4.02 * ±0.6 |
| Aspartate | 5.65 ±0.4 | 10.31 ±0.6 | 14.93 ±0.6 | 10.84 ±0.7 | 6.99 ±0.4 | 8.91 ±0.8 | 14.96 ±0.2 | 0.8 ±0.6 | 0.9 ±0.2 | 12.78 ±0.5 | 18.68 ±0.6 | 16.25 ±0.6 | 8.13 ±0.1 |
| Cis aconitate | 2.1 ±0.5 | 2.48 ±0.7 | 2.09 ±0.1 | 1.7 ±0.5 | 1.89 ±0.6 | 1.68 ±0.6 | 1.38 ±0.3 | 2.13 ±0.1 | 1.89 ±0.6 | 1.93 ±0.5 | 2.10 ±0.2 | 1.60 ±0.6 | 1.53 ±0.2 |
| Citrate | 20.68 ±0.1 | 24.96 ** ±0.5 | 26.23 * ±0.1 | 28.16 ±0.6 * | 21.26 ±0.6 | 17.53 * ±0.6 | 22.1 ±0.9 | 18.94 * ±0.9 | 22.04 ±2.5 | 26.8 ±3.1 | 25.42 ** ±2.1 | 27.74 ** ±5.1 | 29.87 * ±0.6 |
| Dihydroxyisovalerate | 0.85 ±0.5 | 2.03 ±0.8 | 2.11 ± 0.7 | 2.37 ± 0.7 | 1.71 ± 0.3 | 1.81 ± 1.4 | 1.63 ± 1.9 | 1.76 ± 3.1 | 1.87 ± 2.1 | 2.30 ± 2.1 | 1.32 ± 0.5 | 1.46 ± 2.1 | 1.34 ± 0.2 |
| Erythrose-4-phosphate | 0.45 ± 0.2 | 0.66 * ± 0.7 | 0.72 * ± 2.1 | 0.86 ** ± 1.5 | 0.55 ± 5.1 | 0.51 ± 0.5 | 0.58 ± 2.1 | 0.73 ± 0.6 | 0.68 ± 2.1 | 0.73 ± 3.1 | 0.63 * ± 1.2 | 0.76 ± 1.2 | 0.88 * ± 0.4 |
| Flavinadenin dinucleotide | 0.33 ±2.1 | 0.43 ±1.4 | 0.34 ±2.1 | 0.33 ±0.3 | 0.33 ±0.6 | 0.33 ±0.1 | 0.30 ±1.2 | 0.41 ±2.1 | 0.42 ±0.6 | 0.34 ±0.7 | 0.43 ±2.1 | 0.29 ±2.1 | 0.36 ±0.4 |
| Fructose-6-phosphate | 1.63 ±0.1 | 1.72 * ±0.5 | 1.99 ±0.5 | 2.10 ±0.6 | 1.16 ±0.7 | 1.33 ±1.5 | 1.16 ±3.1 | 2.01 ±1.8 | 1.60 ±0.4 | 1.78 ±0.5 | 2.30 ±0.6 | 3.53 ±1.8 | 3.81 ±2.1 |
| Fumarate | 27.48 ±0.6 | 32.45 ** ±0.8 | 38.84 ±2.1 | 40.88 ±3.8 | 26.72 ±4.2 | 22.90 ±2.1 | 25.29 ±2.8 | 36.1 8±1.8 | 22.05 ±1.4 | 25.66 ±1.8 | 37.19 ±2.1 | 39.22 ±0.17 | 40.85 ±0.5 |
| Galactose | 1.06 ±1.4 | 1.84 ±1.7* | 4.45 ±1.5 | 7.67 ±1.8 | 8.77 ±1.9 | 8.55 ±2.1 | 9.21 ±2.7 | 9.74 ±3.1 | 10.72 ±2.8 | 9.04 ±2.1 | 9.04 ±2.6 | 10.97 ±2.5 | 12.63 ±1.7 |
| Glucose | 6.97 ±0.5 | 9.80 ±0.3 | 15.93 * ±0.1 | 17.81 ±3.1 | 8.11 ±0.6* | 8.11 ±2.1 | 8.43 ±2.1 | 10.44 ±1.7 | 6.96 ±2.1 | 7.16 ±5.1 | 14.68 * ±2.1 | 19.20 * ±2.6 | 19.49 * ±2.1 |
| Glucose-1-phosphate | 1.53 ±2.1 | 1.99 * ±0.4 | 2.89 ±1.2 | 1.29 * ±0.4 | 0.92 ±1.2 | 1.24 ±0.3 | 1.09 ±1.3 | 1.85 * ±1.9 | 1.50 ±1.2 | 1.63 * ±1.7 | 1.21 ±0.2 | 2.43 ±0.9 | 3.04 ±1.9 |
| Glucose-6-phosphate | 1.55 ±2.1 | 2.6 ** ±0.3 | 3.90 * ±0.2 | 1.31 ±1.3 | 0.90 ±2.1 | 1.24 * ±1.4 | 1.08 ±1.2 | 1.88 ±1.8 | 1.50 ±2.1 | 1.61 ±0.1 | 1.19 ±0.4 | 2.44 ±1.2 | 3.05 ±1.2 |
| Glutathione, oxidized | 2.61 ±0.2 | 2.46 * ±0.5 | 3.14 ±0.6 | 3.24 ±0.1 | 4.92 ±0.9 | 4.90 ±2.1 | 4.09 ±1.2 | 1.37 ±0.5 | 3.91 ±1.2 | 2.18 ±0.4 | 2.40 ±0.6 | 2.43 ±1.2 | 6.42 ±0.3 |
| Glutathione, reduced | 0.27 ±0.1 | 0.23 ±0.5 | 0.24 ±0.2 | 0.23 ±0.1 | 0.23 ±0.6 | 0.24 ±0.1 | 0.22 ±0.1 | 0.23 ±0.4 | 0.23 ±0.3 | 0.23 ±0.2 | 0.23 ±0.5 | 0.23 ±0.1 | 0.23 ±0.4 |
| GMP | 0.21 ±0.1 | 0.22 ±0.6 | 0.22 ±0.1 | 0.22 ±0.5 | 0.25 ±0.5 | 0.25 ±0.6 | 0.24 ±0.9 | 0.21 ±1.2 | 0.23 ±2.1 | 0.23 ±0.1 | 0.26 ±0.7 | 0.21 ±2.1 | 0.22 ±2.1 |
| GTP | 0.32 ±0.1 | 0.26 ±1.6 | 0.21 ±0.8 | 0.27 ±2.1 | 0.21 ±1.2 | 0.21 ±0.6 | 0.19 ±0.8 | 0.20 ±0.1 | 0.21 ±0.8 | 0.21 ±1.6 | 0.32 ±0.6 | 0.21 ±0.9 | 0.21 ±1.5 |
| Histidine | 3.36 ±0.1 | 2.72 ±2.1 | 4.21 ±0.1 | 2.43 ±0.6 | 3.81 ±0.7 | 2.96 ±1.4 | 2.55 ±0.5 | 2.45 ±0.1 | 2.28 ±0.7 | 4.30 ±2.1 | 2.77 ±0.7 | 1.29 ±2.1 | 3.07 ±0.6 |
| Inosine | 0.26 ±0.1 | 0.34 ±0.5 | 0.22 ±0.6 | 0.30 ±0.8 | 0.34 ±1.2 | 0.33 ±0.4 | 0.27 ±0.9 | 0.31 ±0.9 | 0.26 ±0.4 | 0.25 ±0.1 | 0.29 ±0.2 | 0.26 ±0.8 | 0.29 ±0.1 |
| Isocitrate | 18.99 ±0.1 | 22.71 * ±0.7 | 18.79 ± 0.8 ** | 19.96 ±0.1 | 27.09 * ±1.2 | 19.88 ± 2.1 | 16.46 ±0.6 | 21.12 ±0.1 | 17.71 * ±0.6 | 21.11 ±0.4 | 25.56 * ±1.2 | 14.33 ** ±2.1 | 18.75 ±0.6 |
| Ketoisovalerate | 1.07 ±1.3 | 1.26 ±0.5 | 1.39 ±1.2 | 1.00 ±0.6 | 0.95 ±1.2 | 0.91 ±0.6 | 0.88 ±1.2 | 1.32 ±0.6 | 1.07 ±0.1 | 1.15 ±0.5 | 1.33 ±0.2 | 1.22 ±0.7 | 0.99 ±0.1 |
| Kynurenine | 0.15 ± 0.1 | 0.15 ± 0.4 | 0.15 ± 0.7 | 0.15 ±0.4 | 0.16 ±1.3 | 0.16 ±0.7 | 0.14 ±0.1 | 0.15 ±0.4 | 0.15 ±0.1 | 0.15 ±0.4 | 0.14 ±0.1 | 0.14 ±0.1 | 0.15 ±0.9 |
| Leucine | 11.46 ± 1.2 | 16.35 ± 1.8 | 11.25 ±1.3 | 10.53 ±2.1 | 18.86 ±1.6 | 0.15 ±1.3 | 13.60 ±1.4 | 14.11 ±2.1 | 17.36 ±0.3 | 0.11 ±0.1 | 18.09 ±0.7 | 10.16 ±0.7 | 3.57 ±0.7 |
| Methionine | 0.86 ± 2.1 | 1.06 ± 1.3 | 0.85 ± 1.7 | 1.02 ±2.1 | 1.15 ±4.1 | 1.15 ±1.3 | 0.70 ±3.1 | 1.49 ±0.1 | 0.82 ±0.5 | 1.17 ±0.3 | 0.81 ±0.1 | 0.73 ±0.5 | 1.04 ±0.1 |
| Mevalonic acid | 4.62 ± 0.4 | 6.09 ± 0.1 | 5.52 ±0.2 | 4.99 ±0.4 | 4.30 ±0.1 | 4.03 ±0.1 | 3.65 ±0.6 | 5.93 ±0.1 | 4.46 ±0.6 | 4.57 ±0.1 | 5.19 ±0.1 | 4.52 ±0.2 | 4.03 ±0.2 |
| N-Acetyl-L-aspartic acid | 0.21 ± 0.1 | 0.21 ± 0.5 | 0.20 ±0.1 | 0.22 ±0.2 | 0.24 ±0.5 | 0.23 ±0.2 | 0.21 ±0.01 | 0.20 ±0.6 | 0.19 ±0.3 | 0.20 ±0.9 | 0.23 ±0.4 | 0.16 ±0.2 | 0.19 ±0.8 |
| N4-Acetylcytidine | 0.17 ± 0.3 | 0.17 ±0.02 | 0.17 ±0.3 | 0.17 ±0.16 | 0.17 ±0.4 | 0.17 ±0.5 | 0.16 ±0.3 | 0.17 ±0.2 | 0.17 ±0.1 | 0.17 ±0.2 | 0.17 ±0.2 | 0.17 ±0.4 | 0.17 ±0.2 |
| NAD+ | 0.58 ± 0.3 | 0.72 ±1.5 | 0.65 ± 2.3 | 0.65 ± 0.5 | 0.66 ± 0.3 | 0.68 ± 0.3 | 0.56 ± 0.6 | 0.64 ± 0.6 | 0.67 ± 0.7 | 0.61 ± 0.3 | 0.63 ± 0.3 | 0.52 ± 0.2 | 0.54 ± 0.5 |
| NADH | 0.21 ± 0.2 | 0.21 ± 0.2 | 0.21 ± 0.7 | 0.21 ± 0.6 | 0.22 ± 0.7 | 0.21 ± 0.6 | 0.19 ± 0.1 | 0.20 ± 0.7 | 0.20 ± 0.2 | 0.21 ± 0.3 | 0.21 ± 0.7 | 0.20 ± 0.3 | 0.21 ± 0.2 |
| NADP+ | 0.26 ± 0.3 | 0.26 ± 0.2 | 0.26 ± 1.5 | 0.26 ± 2.1 | 0.26 ± 0.4 | 0.26 ± 0.3 | 0.26 ± 0.6 | 0.26 ± 0.7 | 0.26 ± 0.3 | 0.26 ± 0.2 | 0.27 ± 0.2 | 0.27 ± 1.2 | 0.27 ± 0.6 |
| Ornithine | 1.60 ± 2.1 | 1.65 ± 2.4 | 3.73 ± 1.2 | 2.09 ± 0.4 | 2.77 ± 1.2 | 2.63 ± 1.6 | 2.61 ± 1.8 | 2.52 ± 1.8 | 2.48 ± 0.6 | 3.79 ± 2.1 | 3.30 ± 2.1 | 1.98 ± 1.5 | 1.98 ± 0.4 |
| Phenylalanine | 0.15 ± 0.1 | 2.39 * ± 2.1 | 4.07 ± 3.1 | 6.14 ± 1.2 ** | 0.14 ± 0.2 | 0.13 ± 1.6 | 0.13 ± 1.7 | 0.81 ± 1.2 | 0.14 ± 1.1 | 0.13 ± 0.2 | 1.54 ± 2.1 | 2.13 ** ± 1.5 | 3.13 * ± 0.3 |
| Ribose-5-phosphate | 0.22 ± 1.3 | 0.49 * ± 1.3 | 0.50 * ± 1.6 | 0.76 ± 2.1 | 0.23 ± 0.4 | 0.22 ± 0.6 | 0.27 ± 1.7 | 0.32 ± 2.1 | 0.30 ± 0.5 | 0.27 ± 0.4 | 0.48 ± 2.1 | 0.76 ± 0.5 | 0.86 ± 0.5 |
| Ribulose-5-phosphate | 0.23 ± 1.4 | 0.59 ± 2.5 | 0.70 ** ± 1.4 | 0.96 * ±1.5 | 0.23 ±2.1 | 0.22 ** ± 1.7 | 0.27 ± 0.6 | 0.31 ± 0.7 | 0.30 ± 1.5 | 0.27 * ± 0.7 | 0.38 * ± 0.7 | 0.46 * ± 0.4 | 0.63 ± 0.5 |
| Sedoheptulose-7-phosphate | 0.24 ± 1.5 | 0.75 ± 1.2 | 1.20 ± 2.6 | 1.72 ± 3.2 | 0.23 ** ± 3.6 | 0.25 ± 1.4 | 0.20 ± 0.2 | 0.26 ± 0.6 | 0.27 ± 0.8 | 0.23 ± 0.3 | 1.26 ± 0.3 | 1.45 ± 0.3 | 1.76 ± 0.1 |
| Seleno-methionine | 0.12 ± 2.5 | 0.11 ± 1.6 | 0.11 ± 1.7 | 0.12 ± 1.8 | 0.12 ± 2.1 | 0.12 ± 2.1 | 0.12 ± 1.4 | 0.12 ± 1.2 | 0.12 ± 0.6 | 0.12 ± 1.5 | 0.12 ± 1.8 | 0.12 ± 2.6 | 0.12 ± 2.6 |
| Serine | 4.37 ± 0.4 | 3.59 ± 0.6 | 3.98 ± 0.7 | 4.45 ± 0.7 | 3.80 ± 0.7 | 5.70 ± 0.8 | 3.48 ± 0.8 | 3.75 ± 0.9 | 3.82 ± 0.9 | 4.6 1 ± 0.5 | 4.83 ± 0.6 | 2.3 5 ± 0.6 | 3.35 ± 0.8 |
| Threonine | 0.82 ± 0.8 | 0.87 ± 0.7 | 0.90 ± 0.3 | 0.83 ± 1.5 | 0.93 ± 0.7 | 0.77 ± 0.3 | 0.68 ± 0.6 | 0.95 ± 0.7 | 0.78 ± 0.4 | 0.94 ± 0.3 | 1.04 ± 2.1 | 0.73 ± 1.7 | 0.82 ± 1.8 |
| Thymidine | 0.16 ± 1.4 | 0.18 ± 1.6 | 0.17 ± 1.8 | 0.17 ± 1.5 | 0.18 ± 2.1 | 0.17 ± 1.8 | 0.15 ± 1.2 | 0.17 ± 1.8 | 0.17 ± 0.5 | 0.17 ± 0.6 | 0.18 ± 0.7 | 0.16 ± 0.4 | 0.17 ± 0.2 |
| Thymine | 0.22 ± 1.7 | 0.25 ± 1.5 * | 0.23 ± 1.6 | 0.23 ± 2.1 | 0.24 ± 2.7 | 0.23 ± 1.8 | 0.22 ± 1.6 | 0.24 * ± 1.5 | 0.24 ± 1.2 | 0.24 ± 1.4 | 0.26 ± 1.7 | 0.26 ± 1.6 | 0.26 ± 1.2 |
| Tryptophan | 18.65 ± 2.1 | 33.77 ** ± 1.5 | 46.50 ** ± 1.7 | 53.99 ± 1.6 * | 27.44 ± 0.4 * | 15.97 ± 0.5 | 19.16 ± 1.4 | 24.68 ± 1.4 | 17.90 ± 0.5 | 27.47 ± 0.7 | 30.04 * ± 0.2 | 34.35 * ± 0.6 | 44.25 ± 0.2 |
| Tyrosine | 8.66 ± 0.3 | 9.49 ± 0.6 | 15.27 ± 0.3 | 17.32 ± 0.3 | 9.85 ± 0.2 | 4.69 ± 0.3 | 6.29 ± 0.6 | 7.03 ± 0.5 | 5.61 ± 2.1 | 7.68 ± 1.3 | 11.32 ± 1.7 | 15.22 ± 1.8 | 19.96 ± 2.7 |
| Valine | 0.06 ± 0.2 | 5.82 ± 0.7 | 4.40 ± 0.8 | 4.71 ± 0.5 | 5.10 ± 0.7 | 5.10 ± 0.7 | 7.32 ± 0.4 | 8.47 ± 0.6 | 4.94 ± 0.7 | 0.14 ± 0.3 | 8.16 ± 0.8 | 8.19 ± 0.5 | 4.83 ± 0.7 |
| Xanthine | 0.14 ± 2.1 | 0.15 ± 4.1 | 0.17 ± 0.7 | 0.19 ± 1.6 | 0.23 ± 2.5 | 0.18 ± 2.8 | 0.14 ± 1.6 | 0.16 ± 1.8 | 0.12 ± 1.6 | 0.16 ± 2.1 | 0.16 ± 4.1 | 0.16 ± 2.6 | 0.13 ± 2.5 |
| Rosmarinic Acid | 9.57 * ± 1.6 | 12.85 * ± 1.8 | 14.45 ± 1.7 | 19.31 ** ± 1.7 | 16.11 ± 2.5 | 18.88 * ± 2.9 | 20.96 ± 2.5 | 18.84 ± 2.4 | 12.39 ± 2.1 | 11.85 ± 4.6 | 14.59 ± 2.4 | 12.81 * ± 1.5 | 15.15 ** ± 1.5 |
| Caffeic acid | 21.33 ± 2.1 | 27.33 ± 4.1 | 32.94 ± 1.7* | 43.96 ± 1.8° | 29.11 ± 2.7 | 27.75 ± 1.7 | 28.20 ± 2.1 | 27.00 ± 2.6 | 33.06 ** ± 1.7 | 27.74 ± 1.4 | 34.77 ± 1.6 | 47.83 ± 0.5 | 60.37 * ± 0.5 |
| 4-Hydroxy benzoic acid | 2.18 ± 4.1 | 2.96 ± 0.1 | 3.94 ± 0.2 | 3.5 2 ± 1.5 | 2.9 8 ± 1.3 | 2.20 ± 1.7 | 2.30 ± 1.1 | 2.03 ± 0.9 | 2.49 ± 2.1 | 1.85 ± 1.5 | 3.04 ± 1.4 | 6.43 ± 1.8 | 8.24 ± 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verrillo, M.; Koellensperger, G.; Puehringer, M.; Cozzolino, V.; Spaccini, R.; Rampler, E. Evaluation of Sustainable Recycled Products to Increase the Production of Nutraceutical and Antibacterial Molecules in Basil Plants by a Combined Metabolomic Approach. Plants 2023, 12, 513. https://doi.org/10.3390/plants12030513
Verrillo M, Koellensperger G, Puehringer M, Cozzolino V, Spaccini R, Rampler E. Evaluation of Sustainable Recycled Products to Increase the Production of Nutraceutical and Antibacterial Molecules in Basil Plants by a Combined Metabolomic Approach. Plants. 2023; 12(3):513. https://doi.org/10.3390/plants12030513
Chicago/Turabian StyleVerrillo, Mariavittoria, Gunda Koellensperger, Marlene Puehringer, Vincenza Cozzolino, Riccardo Spaccini, and Evelyn Rampler. 2023. "Evaluation of Sustainable Recycled Products to Increase the Production of Nutraceutical and Antibacterial Molecules in Basil Plants by a Combined Metabolomic Approach" Plants 12, no. 3: 513. https://doi.org/10.3390/plants12030513