Chemical Composition and Skin-Whitening Activities of Siegesbeckia glabrescens Makino Flower Absolute in Melanocytes
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of SGMFAb
2.2. Effects of SGMFAb on the Viability and Proliferation of B16BL6 Melanoma Cells
2.3. Effects of SGMFAb on Melanin Synthesis and Tyrosinase Activity in B16BL6 Melanoma Cells
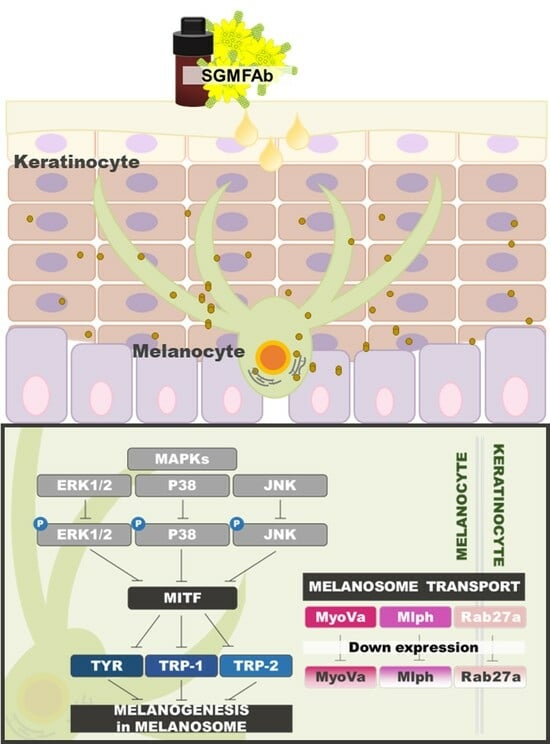
2.4. Changes in Expression of Melanogenesis Regulatory Molecules Induced by SGMFAb in B16BL6 Melanoma Cells
2.5. Changes in the Phosphorylations of MAPKs in B16BL6 Melanoma Cells Exposed to SGMFAb
2.6. Changes in the Expressions of Melanosome-Transport-Related Proteins by SGMFAb in B16BL6 Melanoma Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction of Siegesbeckia glabrescens Makino Flower Absolute
4.3. Analysis of Chemical Compounds of SGMFAb
4.4. Cell Culture
4.5. Cell Viability Assay
4.6. Proliferation Assay
4.7. Melanin Content Assay
4.8. Tyrosinase Activity Assays
4.9. Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Huang, J.; Lu, J.; Ding, Y.; Jiang, L.; Hu, S.; Chen, J.; Zeng, Q. The role and mechanism of Asian medicinal plants in treating skin pigmentary disorders. J. Ethnopharmacol. 2019, 245, 112173. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Liu, W.; Zhu, D.; Cao, Y.; Tang, A.; Gong, G.; Su, H. Natural skin-whitening compounds for the treatment of melanogenesis (Review). Exp. Ther. Med. 2020, 20, 173–185. [Google Scholar] [CrossRef]
- Ortonne, J.P.; Passeron, T. Melanin pigmentary disorders: Treatment update. Dermatol. Clin. 2005, 23, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Giménez García, R.M.; Carrasco Molina, S. Drug-induced hyperpigmentation: Review and case series. J. Am. Board. Fam. Med. 2019, 32, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Ohbayashi, N.; Fukuda, M. Role of Rab family GTPases and their effectors in melanosomal logistics. J. Biochem. 2012, 151, 343–351. [Google Scholar] [CrossRef]
- Boissy, R.E. Melanosome transfer to and translocation in the keratinocyte. Exp. Dermatol. 2003, 12 (Suppl. S2), 5–12. [Google Scholar] [CrossRef]
- Kim, K.; Huh, Y.; Lim, K.M. Anti-pigmentary natural compounds and their mode of action. Int. J. Mol. Sci. 2021, 22, 6206. [Google Scholar] [CrossRef]
- Ye, Y.; Chu, J.H.; Wang, H.; Xu, H.; Chou, G.X.; Leung, A.K.; Fong, W.F.; Yu, Z.L. Involvement of p38 MAPK signaling pathway in the anti-melanogenic effect of San-bai-tang, a Chinese herbal formula, in B16 cells. J. Ethnopharmacol. 2010, 132, 533–535. [Google Scholar] [CrossRef]
- Choi, H.; Yoon, J.H.; Youn, K.; Jun, M. Decursin prevents melanogenesis by suppressing MITF expression through the regulation of PKA/CREB, MAPKs, and PI3K/Akt/GSK-3β cascades. Biomed. Pharmacother. 2022, 147, 112651. [Google Scholar] [CrossRef]
- Seabra, M.C.; Coudrier, E. Rab GTPases and myosin motors in organelle motility. Traffic 2004, 5, 393–399. [Google Scholar] [CrossRef]
- Kuroda, T.S.; Itoh, T.; Fukuda, M. Functional analysis of slac2-a/melanophilin as a linker protein between Rab27A and myosin Va in melanosome transport. Methods Enzymol. 2005, 403, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.; Onderwater, J.; Vander Haeghen, Y.; Vancoillie, G.; Koerten, H.K.; Mommaas, A.M.; Naeyaert, J.M. Myosin V colocalizes with melanosomes and subcortical actin bundles not associated with stress fibers in human epidermal melanocytes. J. Investig. Dermatol. 1998, 111, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Bellere, A.D.; Yu, D.; Oh, S.; Kim, M.; Jung, J.; Fang, M.; Zheng, S.; Yi, T.H. Antiperiodontitis effects of Siegesbeckia glabrescens in vitro. Antioxidants 2023, 12, 471. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Ko, H.Y.; Choi, S.W.; Seo, D.W. Anti-angiogenic effects of Siegesbeckia glabrescens are mediated by suppression of the Akt and p70S6K-dependent signaling pathways. Oncol. Rep. 2015, 33, 699–704. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, J.H.; Won, J.H.; Park, E.J.; Chae, H.J.; Kim, H.R.; Kim, C.H.; Baek, S.H. Inhibitory effect on immunoglobulin E production in vivo and in vitro by Siegesbeckia glabrescens. Phytother. Res. 2001, 15, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, M.B.; Yun, J.G.; Hwang, J.K. Protective effects of standardized Siegesbeckia glabrescens extract and its active compound Kirenol against UVB-induced photoaging through inhibition of MAPK/NF-κB pathways. J. Microbiol. Biotechnol. 2017, 27, 242–250. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, H.; Jung, E.; Kim, J.H.; Hwang, W.; Kang, E.J.; Lee, S.; Ha, B.J.; Lee, J.; Park, D. A novel antibacterial compound from Siegesbeckia glabrescens. Molecules 2012, 17, 12469–12477. [Google Scholar] [CrossRef]
- Lee, H.N.; Joo, J.H.; Oh, J.S.; Choi, S.W.; Seo, D.W. Regulatory effects of Siegesbeckia glabrescens on non-small cell lung cancer cell proliferation and invasion. Am. J. Chin. Med. 2014, 42, 453–463. [Google Scholar] [CrossRef]
- Shim, S.Y.; Lee, Y.E.; Lee, M. Antioxidant compounds, kirenol and methyl ent-16α, 17-dihydroxy-kauran-19-oate bioactivity-guided isolated from Siegesbeckia glabrescens attenuates MITF-mediated melanogenesis via inhibition of intracellular ROS production. Molecules 2021, 26, 1940. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Brenner, M.; Hearing, V.J. The regulation of skin pigmentation. J. Biol. Chem. 2007, 282, 27557–27561. [Google Scholar] [CrossRef]
- Kim, J.H.; Baek, S.H.; Kim, D.H.; Choi, T.Y.; Yoon, T.J.; Hwang, J.S.; Kim, M.R.; Kwon, H.J.; Lee, C.H. Downregulation of melanin synthesis by haginin A and its application to in vivo lightening model. J. Investig. Dermatol. 2008, 128, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Downregulation of melanogenesis: Drug discovery and therapeutic options. Drug Discov. Today 2017, 22, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Insaf, A.; Parveen, R.; Gautam, G.; Samal, M.; Zahiruddin, S.; Ahmad, S. A comprehensive study to explore tyrosinase inhibitory medicinal plants and respective phytochemicals for hyperpigmentation; molecular approach and future perspectives. Curr. Pharm. Biotechnol. 2023, 24, 780–813. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.R.; Won, K.J.; Kim, D.Y.; Kim, M.J.; Hong, B.S.; Lee, H.M. Chemical composition of Impatiens textori Miq. flower absolute and its potential wound repair and anti-melanogenesis-promoting activities in skin cells. Pharmaceuticals 2022, 15, 1397. [Google Scholar] [CrossRef]
- Yardman-Frank, J.M.; Fisher, D.E. Skin pigmentation and its control: From ultraviolet radiation to stem cells. Exp. Dermatol. 2021, 30, 560–571. [Google Scholar] [CrossRef]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Wu, Y.; Yang, T.; Yang, K.; Wang, R.; Yang, J.; Guo, H. Wnt5a inhibits the proliferation and melanogenesis of melanocytes. Int. J. Med. Sci. 2013, 10, 699–706. [Google Scholar] [CrossRef]
- Makino, E.T.; Mehta, R.C.; Banga, A.; Jain, P.; Sigler, M.L.; Sonti, S. Evaluation of a hydroquinone-free skin brightening product using in vitro inhibition of melanogenesis and clinical reduction of ultraviolet-induced hyperpigmentation. J. Drugs Dermatol. 2013, 12, s16–s20. [Google Scholar]
- Kameyama, K.; Sakai, C.; Kuge, S.; Nishiyama, S.; Tomita, Y.; Ito, S.; Wakamatsu, K.; Hearing, V.J. The expression of tyrosinase, tyrosinase-related proteins 1 and 2 (TRP1 and TRP2), the silver protein, and a melanogenic inhibitor in human melanoma cells of differing melanogenic activities. Pigment Cell. Res. 1995, 8, 97–104. [Google Scholar] [CrossRef]
- Maeda, K.; Yokokawa, Y.; Hatao, M.; Naganuma, M.; Tomita, Y. Comparison of the melanogenesis in human black and light brown melanocytes. J. Dermatol. Sci. 1997, 14, 199–206. [Google Scholar] [CrossRef]
- Lee, R.; Ko, H.J.; Kim, K.; Sohn, Y.; Min, S.Y.; Kim, J.A.; Na, D.; Yeon, J.H. Anti-melanogenic effects of extracellular vesicles derived from plant leaves and stems in mouse melanoma cells and human healthy skin. J. Extracell. Vesicles 2019, 9, 1703480. [Google Scholar] [CrossRef]
- Salah Maamoun, H.; Rabie, G.H.; Shaker, I.A.; Alaidaroos, B.; El-Sayed, A.S.A. Biochemical properties of tyrosinase from Aspergillus terreus and Penicillium copticola; undecanoic acid from Aspergillus flavus, an endophyte of Moringa oleifera, is a novel potent tyrosinase inhibitor. Molecules 2021, 26, 1309. [Google Scholar] [CrossRef] [PubMed]
- Joo, I.H.; Choi, J.H.; Kim, D.H.; Chung, M.J.; Lim, M.H. Ligularia fischeri ethanol extract: An inhibitor of alpha-melanocyte-stimulating hormone-stimulated melanogenesis in B16F10 melanoma cells. J. Cosmet. Dermatol. 2023, 22, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Kim, M.; Song, N.; Sun, S.; Choi, J.; Park, K. Antioxidant and anti-melanogenesis effects of colloidal gold Camellia sinensis L. extracts. Molecules 2022, 27, 5593. [Google Scholar] [CrossRef]
- Karunarathne, W.A.H.M.; Molagoda, I.M.N.; Kim, M.S.; Choi, Y.H.; Oren, M.; Park, E.K.; Kim, G.Y. Flumequine-mediated upregulation of p38 MAPK and JNK results in melanogenesis in B16F10 cells and zebrafish larvae. Biomolecules 2019, 9, 596. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Hyun, C. Acenocoumarol, an anticoagulant drug, prevents melanogenesis in B16F10 melanoma cells. Pharmaceuticals 2023, 16, 604. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Hemesath, T.J.; Takemoto, C.M.; Horstmann, M.A.; Wells, A.G.; Price, E.R.; Fisher, D.Z.; Fisher, D.E. c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev. 2000, 14, 301–312. [Google Scholar] [CrossRef]
- Kim, D.S.; Hwang, E.S.; Lee, J.E.; Kim, S.Y.; Kwon, S.B.; Park, K.C. Sphingosine-1-phosphate decreases melanin synthesis via sustained ERK activation and subsequent MITF degradation. J. Cell. Sci. 2003, 116 Pt 9, 1699–1706. [Google Scholar] [CrossRef]
- Ng, L.T.; Lin, L.T.; Chen, C.L.; Chen, H.W.; Wu, S.J.; Lin, C.C. Anti-melanogenic effects of δ-tocotrienol are associated with tyrosinase-related proteins and MAPK signaling pathway in B16 melanoma cells. Phytomedicine 2014, 21, 978–983. [Google Scholar] [CrossRef]
- Van Den Bossche, K.; Naeyaert, J.M.; Lambert, J. The quest for the mechanism of melanin transfer. Traffic 2006, 7, 769–778. [Google Scholar] [CrossRef]
- Kudo, M.; Kobayashi-Nakamura, K.; Tsuji-Naito, K. Bifunctional effects of O-methylated flavones from Scutellaria baicalensis Georgi on melanocytes: Inhibition of melanin production and intracellular melanosome transport. PLoS ONE 2017, 12, e0171513. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.E.; Choi, N.; Sung, J.H. Inhibition of Rab27a and Rab27b has opposite effects on the regulation of hair cycle and hair growth. Int. J. Mol. Sci. 2020, 21, 5672. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; An, X.; Jiang, S.; Yang, Y.; Song, G.; Gao, R. Protoporphyrin IX stimulates melanogenesis, melanocyte dendricity, and melanosome transport through the cGMP/PKG pathway. Front. Pharmacol. 2020, 11, 569368. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M. Rab GTPases: Key players in melanosome biogenesis, transport, and transfer. Pigment Cell. Melanoma Res. 2021, 34, 222–235. [Google Scholar] [CrossRef]
- Van Gele, M.; Dynoodt, P.; Lambert, J. Griscelli syndrome: A model system to study vesicular trafficking. Pigment Cell. Melanoma Res. 2009, 22, 268–282. [Google Scholar] [CrossRef]
- Wei, X.; Huang, M.; Yang, Y.; Liu, Y.; Chi, S.; Li, C. Silencing of Rab23 by siRNA inhibits ultraviolet B-induced melanogenesis via downregulation of PKA/CREB/MITF. Exp. Dermatol. 2022, 31, 1253–1263. [Google Scholar] [CrossRef]
- Van Gele, M.; Geusens, B.; Schmitt, A.M.; Aguilar, L.; Lambert, J. Knockdown of myosin Va isoforms by RNAi as a tool to block melanosome transport in primary human melanocytes. J. Investig. Dermatol. 2008, 128, 2474–2484. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, R.; Zhou, J.; Xu, X.; Sun, Z.; Li, J.; Chen, X.; Li, Z.; Yan, X.; Zhao, D.; et al. Salicylic acid in ginseng root alleviates skin hyperpigmentation disorders by inhibiting melanogenesis and melanosome transport. Eur. J. Pharmacol. 2021, 910, 174458. [Google Scholar] [CrossRef]
- Park, J.I.; Lee, H.Y.; Lee, J.E.; Myung, C.H.; Hwang, J.S. Inhibitory effect of 2-methyl-naphtho[1,2,3-de]quinolin-8-one on melanosome transport and skin pigmentation. Sci. Rep. 2016, 6, 29189. [Google Scholar] [CrossRef]
- Kim, N.Y.; Won, K.J.; Kim, H.B.; Kim, D.Y.; Kim, M.J.; Won, Y.R.; Lee, H.M. Chemical composition of Salix koreensis Anderss flower absolute and its skin wound healing activities in vitro. Plants 2022, 11, 246. [Google Scholar] [CrossRef]
- Kang, H.M.; Won, K.J.; Kim, D.Y.; Lee, S.Y.; Kim, M.J.; Won, Y.R.; Kim, B.; Lee, H.M. Chemical composition of Miscanthus sinensis var. purpurascens flower absolute and its beneficial effects on skin wound healing and melanogenesis-related cell activities. Chem. Biodivers. 2021, 18, e2100383. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, J.; Abe, E.; Suda, T.; Kuroki, T. Regulation of melanin synthesis of B16 mouse melanoma cells by 1 alpha, 25-dihydroxyvitamin D3 and retinoic acid. Cancer Res. 1985, 45, 1474–1478. [Google Scholar] [PubMed]






| No | Component Name | RT 1 | RI 2 | Area (%) | CAS No. | |
|---|---|---|---|---|---|---|
| Observed | Literature | |||||
| 1 | Geijerene | 46.48 | 1144 | 1143 | 0.73 | 6902-73-4 |
| 2 | 2-Isopropyl-5-methylanisole | 52.17 | 1227 | 1227 | 2.85 | 1076-56-8 |
| 3 | 2-Methoxy-3-(tert-butyl)-5-methylphenol | 64.72 | 1417 | - | 0.60 | NA |
| 4 | Methyl undecanoate | 65.12 | 1426 | 1427 | 25.65 | 1731-86-8 |
| 5 | Undecanoic acid | 66.76 | 1467 | 1466 | 7.21 | 112-37-4 |
| 6 | TBMP | 67.75 | 1491 | 1490 | 0.78 | 88-32-4 |
| 7 | γ-Elemene | 68.24 | 1503 | 1482 | 6.16 | 29873-99-2 |
| 8 | Capric acid | 68.96 | 1526 | 1404 | 0.87 | 334-48-5 |
| 9 | trans-α-Bisabolene | 69.62 | 1547 | 1547 | 1.42 | 25532-79-0 |
| 10 | Lauric acid | 70.25 | 1566 | 1566 | 34.41 | 143-07-7 |
| 11 | Spathulenol | 71.00 | 1590 | 1590 | 0.71 | 6750-60-3 |
| 12 | Tridecanoic acid | 72.97 | 1661 | 1662 | 2.65 | 638-53-9 |
| 13 | Oxacyclotetradeca-4,11-diyne | 82.01 | 1755 | 1639 | 3.72 | 6568-32-7 |
| 14 | α-springene | 93.29 | 2561 | 1781 | 12.25 | 77898-97-6 |
| Total Identified (%) | 100.00 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.K.; Won, K.J.; Kim, D.Y.; Kim, Y.Y.; Lee, H.M. Chemical Composition and Skin-Whitening Activities of Siegesbeckia glabrescens Makino Flower Absolute in Melanocytes. Plants 2023, 12, 3930. https://doi.org/10.3390/plants12233930
Lee DK, Won KJ, Kim DY, Kim YY, Lee HM. Chemical Composition and Skin-Whitening Activities of Siegesbeckia glabrescens Makino Flower Absolute in Melanocytes. Plants. 2023; 12(23):3930. https://doi.org/10.3390/plants12233930
Chicago/Turabian StyleLee, Da Kyoung, Kyung Jong Won, Do Yoon Kim, Yoon Yi Kim, and Hwan Myung Lee. 2023. "Chemical Composition and Skin-Whitening Activities of Siegesbeckia glabrescens Makino Flower Absolute in Melanocytes" Plants 12, no. 23: 3930. https://doi.org/10.3390/plants12233930