Rowanberry—A Source of Bioactive Compounds and Their Biopharmaceutical Properties
Abstract
:1. Introduction
2. History and Origins
3. Botanical Description
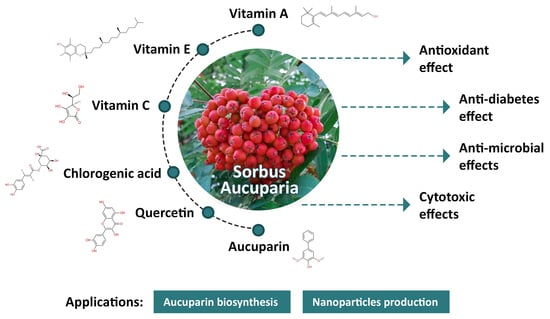
4. The Chemical Composition of Rowanberries
5. Biopharmaceutical Properties of Rowanberries
5.1. Antioxidant Effect
5.2. Antidiabetic Effect
5.3. Antimicrobial Effects
5.4. Cytotoxic Effects
6. Applications
6.1. Aucuparin Biosynthesis
6.2. Nanoparticles Production
6.3. Nutritional and Pharmaceutical Applications of Rowanberry
6.4. Toxicology
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anunciato, T.P.; da Rocha Filho, P.A. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J. Cosmet. Dermatol. 2012, 11, 51–54. [Google Scholar] [CrossRef]
- Sarv, V.; Venskutonis, P.R.; Bhat, R. The Sorbus spp.—Underutilised Plants for Foods and Nutraceuticals: Review on Polyphenolic Phytochemicals and Antioxidant Potential. Antioxidants 2020, 9, 813. [Google Scholar] [CrossRef]
- Sarv, V.; Venskutonis, P.R.; Rätsep, R.; Aluvee, A.; Kazernavičiūtė, R.; Bhat, R. Antioxidants Characterization of the Fruit, Juice, and Pomace of Sweet Rowanberry (Sorbus aucuparia L.) Cultivated in Estonia. Antioxidants 2021, 10, 1779. [Google Scholar] [CrossRef]
- Zymone, K.; Raudone, L.; Žvikas, V.; Jakštas, V.; Janulis, V. Phytoprofiling of Sorbus L. Inflorescences: A Valuable and Promising Resource for Phenolics. Plants 2022, 11, 3421. [Google Scholar] [CrossRef] [PubMed]
- Arvinte, O.; Amariei, S. Chemical composition of peatland small cranberry (Vaccinium oxycoccus) for potential use as functional ingredient. Ukr. Food J. 2022, 11, 416–428. [Google Scholar] [CrossRef]
- Rushforth, K.D. Collins Wildlife Trust Guide: Trees: A Photographic Guide to the Trees of Britain and Europe; HarperCollins: New York, NY, USA, 1999; ISBN 0002200139. [Google Scholar]
- Šedivá, J.; Velebil, J.; Zahradník, D. Micropropagation as a Tool for the Conservation of Autochthonous Sorbus Species of Czechia. Plants 2023, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, M.; Kolodziejczyk-Czepas, J.; Owczarek, A.; Zakrzewska, A.; Magiera, A.; Olszewska, M.A. Novel insight into biological activity and phytochemical composition of Sorbus aucuparia L. fruits: Fractionated extracts as inhibitors of protein glycation and oxidative/nitrative damage of human plasma components. Food Res. Int. 2021, 147, 110526. [Google Scholar] [CrossRef] [PubMed]
- Bobinaitė, R.; Grootaert, C.; Van Camp, J.; Šarkinas, A.; Liaudanskas, M.; Žvikas, V.; Viškelis, P.; Venskutonis, P.R. Chemical composition, antioxidant, antimicrobial and antiproliferative activities of the extracts isolated from the pomace of rowanberry (Sorbus aucuparia L.). Food Res. Int. 2020, 136, 109310. [Google Scholar] [CrossRef]
- Räty, M.; Caudullo, G.; Rigo, D. Sorbus Aucuparia in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; Publications Office of the EU: Luxembourg, 2016; pp. 176–177. [Google Scholar]
- Mikulic-Petkovsek, M.; Krska, B.; Kiprovski, B.; Veberic, R. Bioactive Components and Antioxidant Capacity of Fruits from Nine Sorbus Genotypes. J. Food Sci. 2017, 82, 647–658. [Google Scholar] [CrossRef]
- Hukkanen, A.T.; Pölönen, S.S.; Kärenlampi, S.O.; Kokko, H.I. Antioxidant Capacity and Phenolic Content of Sweet Rowanberries. J. Agric. Food Chem. 2006, 54, 112–119. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Mellenthin, A. Identification and quantitation of flavonols in rowanberry (Sorbus aucuparia L.) juice. Eur. Food Res. Technol. 2001, 213, 12–17. [Google Scholar] [CrossRef]
- Vitasović-Kosić, I.; Hodak, A.; Łuczaj, Ł.; Marić, M.; Juračak, J. Traditional Ethnobotanical Knowledge of the Central Lika Region (Continental Croatia)—First Record of Edible Use of Fungus Taphrina pruni. Plants 2022, 11, 3133. [Google Scholar] [CrossRef] [PubMed]
- Bexultanova, G.; Prakofjewa, J.; Sartori, M.; Kalle, R.; Pieroni, A.; Sõukand, R. Promotion of Wild Food Plant Use Diversity in the Soviet Union, 1922–1991. Plants 2022, 11, 2670. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Mijakovic, I. Rowan Berries: A Potential Source for Green Synthesis of Extremely Monodisperse Gold and Silver Nanoparticles and Their Antimicrobial Property. Pharmaceutics 2022, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Wagner, H.; Verpoorte, R.; Heinrich, M. Medicinal Plants of the Russian Pharmacopoeia; their history and applications. J. Ethnopharmacol. 2014, 154, 481–536. [Google Scholar] [CrossRef]
- Sołtys, A.; Galanty, A.; Podolak, I. Ethnopharmacologically Important but Underestimated Genus Sorbus: A Comprehensive Review; Springer: Dordrecht, The Netherlands, 2020; Volume 19, ISBN 0123456789. [Google Scholar]
- Sak, K.; Jürisoo, K.; Raal, A. Estonian folk traditional experiences on natural anticancer remedies: From past to the future. Pharm. Biol. 2014, 52, 855–866. [Google Scholar] [CrossRef]
- Săvulescu, T. Flora Republicii Populare Române, Vol. IV; Academiei Republicii Populare Române: Bucharest, Romania, 1952. [Google Scholar]
- Poljak, I.; Vahčić, N.; Liber, Z.; Tumpa, K.; Pintar, V.; Zegnal, I.; Vidaković, A.; Valković, B.; Kajba, D.; Idžojtić, M. Morphological and Chemical Diversity and Antioxidant Capacity of the Service Tree (Sorbus domestica L.) Fruits from Two Eco-Geographical Regions. Plants 2021, 10, 1691. [Google Scholar] [CrossRef]
- Raspé, O.; Findlay, C.; Jacquemart, A.-L. Sorbus aucuparia L. J. Ecol. 2000, 88, 910–930. [Google Scholar] [CrossRef]
- Botany, S.S.; Jun, N.A.; Aldasoro, J. The Genus Sorbus (Maloideae, Rosaceae) in Europe and in North Africa: Morphological Analysis and Systematics. Syst. Bot. 2011, 23, 189–212. [Google Scholar]
- Yousefzadeh, H.; Raeisi, S.; Esmailzadeh, O.; Jalali, G.; Nasiri, M.; Walas, Ł.; Kozlowski, G. Genetic Diversity and Structure of Rear Edge Populations of Sorbus aucuparia (Rosaceae) in the Hyrcanian Forest. Plants 2021, 10, 1471. [Google Scholar] [CrossRef]
- Caudullo, G.; Welk, E.; San-Miguel-Ayanz, J. Chorological maps for the main European woody species. Data Brief 2017, 12, 662–666. [Google Scholar] [CrossRef]
- Termentzi, A.; Kefalas, P.; Kokkalou, E. LC–DAD–MS (ESI+) analysis of the phenolic content of Sorbus domestica fruits in relation to their maturity stage. Food Chem. 2008, 106, 1234–1245. [Google Scholar] [CrossRef]
- Sergunova, E.V.; Bokov, D.O. Some Pharmacognostic Studies of the Bird Cherry (Padus avium Mill.) and Mountain Ash (Sorbus aucuparia L.) Fruits Collected from Moscow (Russia). Pharmacogn. J. 2019, 11, 996–1002. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of Sugars, Organic Acids, and Total Phenolics in 25 Wild or Cultivated Berry Species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef] [PubMed]
- Bujor, A.; Miron, A.; Luca, S.V.; Skalicka-Wozniak, K.; Silion, M.; Ancuceanu, R.; Dinu, M.; Girard, C.; Demougeot, C.; Totoson, P. Metabolite profiling, arginase inhibition and vasorelaxant activity of Cornus mas, Sorbus aucuparia and Viburnum opulus fruit extracts. Food Chem. Toxicol. 2019, 133, 110764. [Google Scholar] [CrossRef]
- Cristea, E.; Ghendov-Mosanu, A.; Patras, A.; Socaciu, C.; Pintea, A.; Tudor, C.; Sturza, R. The Influence of Temperature, Storage Conditions, PH, and Ionic Strength on the Antioxidant Activity and Color Parameters of Rowan Berry Extracts Elena. Molecules 2021, 26, 3786. [Google Scholar] [CrossRef]
- Krivoruchko, E.V.; Andrushchenko, O.A.; Kononenko, A.V. Carboxylic Acids from Sorbus aucuparia and S. aria. Chem. Nat. Compd. 2013, 49, 742–743. [Google Scholar] [CrossRef]
- Pasko, P. South Siberian fruits: Their selected chemical constituents, biological activity, and traditional use in folk medicine and daily nutrition. J. Med. Plants Res. 2012, 6, 4698–4706. [Google Scholar] [CrossRef]
- Mrkonjić, Z.O.; Nadjpal, J.; Beara, I.N.; Aleksić-Sabo, V.S.; Četojević-Simin, D.D.; Mimica-Dukić, N.M.; Lesjak, M.M. Phenolic profiling and bioactivities of fresh fruits and jam of Sorbus species. J. Serbian Chem. Soc. 2017, 82, 651–664. [Google Scholar] [CrossRef]
- Šavikin, K.P.; Zdunić, G.M.; Krstić-Milošević, D.B.; Šircelj, H.J.; Stešević, D.D.; Pljevljakušić, D.S. Sorbus aucuparia and Sorbus aria as a Source of Antioxidant Phenolics, Tocopherols, and Pigments. Chem. Biodivers. 2017, 14, e1700329. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ahotupa, M.; Määttä, P.; Kallio, H. Composition and antioxidative activities of supercritical CO2-extracted oils from seeds and soft parts of northern berries. Food Res. Int. 2011, 44, 2009–2017. [Google Scholar] [CrossRef]
- Klavins, L.; Kviesis, J.; Steinberga, I.; Klavina, L.; Klavins, M. Gas Chromatography-Mass Spectrometry Study of Lipids in Northern Berries. Agron. Res. 2016, 14, 1328–1346. [Google Scholar]
- Berņa, E.; Kampuse, S. The Marmalades of Sweet Rowanberries as an Example of a Functional Food. In Proceedings of the 7th International Congress of Food Technologists, Biotechnologists and Nutritionists, Opatija, Croatia, 20–23 September 2011; pp. 139–251. [Google Scholar]
- Bozhuyuk, M.R. Morphological and Biochemical Diversity in Fruits of Rowanberry (Sorbus aucuparia L.) Genotypes. Erwerbs-Obstbau 2021, 63, 431–435. [Google Scholar] [CrossRef]
- Kampuss, K.; Kampuse, S.; Berna, E.; Kruma, Z.; Krasnova, I. Biochemical Composition and Antiradical Activity of Rowanberry (Sorbus L.) Cultivars and Hybrids with Different Rosaceae L. Cultivars Pīlādžu (Sorbus L.) Šėirĥu Un to Hibrīdu Ar Citiem Rosaceae L. Augěaugiem Antioksidatīvā Aktivitāte un Bioėīmiskais. Latv. J. Agron. 2009, 12, 59–65. [Google Scholar]
- Azimova, S.S.; Glushenkova, A.I. Sorbus aucuparia L. (S. Boissieri Schneid.). In Lipids, Lipophilic Components and Essential Oils from Plant Sources; Azimova, S.S., Glushenkova, A.I., Eds.; Springer Science & Business Media: London, UK, 2012; pp. 778–779. ISBN 9780857293237. [Google Scholar]
- Zymone, K.; Raudone, L.; Raudonis, R.; Marksa, M.; Ivanauskas, L.; Janulis, V. Phytochemical Profiling of Fruit Powders of Twenty Sorbus L. Cultivars. Molecules 2018, 23, 2593. [Google Scholar] [CrossRef]
- Valadon, L.R.G.; Mummery, R.S. Carotenoids of Rowan Berries. Ann. Bot. 1972, 36, 471–474. [Google Scholar] [CrossRef]
- Mlcek, J.; Rop, O.; Jurikova, T.; Sochor, J.; Fisera, M.; Balla, S.; Baron, M.; Hrabe, J. Bioactive compounds in sweet rowanberry fruits of interspecific Rowan crosses. Open Life Sci. 2014, 9, 1078–1086. [Google Scholar] [CrossRef]
- Perezjimenez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; González-Paramás, A.M.; Oludemi, T.; Ayuda-Durán, B.; González-Manzano, S. Plant Phenolics as Functional Food Ingredients, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 90, ISBN 9780128165676. [Google Scholar]
- Aladedunye, F.; Matthäus, B. Phenolic extracts from Sorbus aucuparia (L.) and Malus baccata (L.) berries: Antioxidant activity and performance in rapeseed oil during frying and storage. Food Chem. 2014, 159, 273–281. [Google Scholar] [CrossRef]
- Denev, P.; Kratchanova, M.; Ciz, M.; Lojek, A.; Vasicek, O.; Nedelcheva, P.; Blazheva, D.; Toshkova, R.; Gardeva, E.; Yossifova, L.; et al. Biological activities of selected polyphenol-rich fruits related to immunity and gastrointestinal health. Food Chem. 2014, 157, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, M.A.; Michel, P. Antioxidant activity of inflorescences, leaves and fruits of three Sorbuss pecies in relation to their polyphenolic composition. Nat. Prod. Res. 2009, 23, 1507–1521. [Google Scholar] [CrossRef] [PubMed]
- Raudonis, R.; Raudonė, L.; Gaivelytė, K.; Viškelis, P.; Janulis, V. Phenolic and antioxidant profiles of rowan (Sorbus L.) fruits. Nat. Prod. Res. 2014, 28, 1231–1240. [Google Scholar] [CrossRef]
- Kylli, P.; Nohynek, L.; Puupponen-Pimiä, R.; Westerlund-Wikström, B.; McDougall, G.; Stewart, D.; Heinonen, M. Rowanberry Phenolics: Compositional Analysis and Bioactivities. J. Agric. Food Chem. 2010, 58, 11985–11992. [Google Scholar] [CrossRef]
- Aladedunye, F.; Niehaus, K.; Bednarz, H.; Thiyam-Hollander, U.; Fehling, E.; Matthäus, B. Enzymatic lipophilization of phenolic extract from rowanberry (Sorbus aucuparia) and evaluation of antioxidative activity in edible oil. LWT 2015, 60, 56–62. [Google Scholar] [CrossRef]
- Tian, Y.; Liimatainen, J.; Alanne, A.-L.; Lindstedt, A.; Liu, P.; Sinkkonen, J.; Kallio, H.; Yang, B. Phenolic compounds extracted by acidic aqueous ethanol from berries and leaves of different berry plants. Food Chem. 2017, 220, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Deren’Ko, S.A.; Suprunov, N.I. Ursolic acid from the fruit ofSorbus aucuparia. Chem. Nat. Compd. 1979, 15, 363. [Google Scholar] [CrossRef]
- Khalil, M.N.; Beuerle, T.; Müller, A.; Ernst, L.; Bhavanam, V.B.; Liu, B.; Beerhues, L. Biosynthesis of the biphenyl phytoalexin aucuparin in Sorbus aucuparia cell cultures treated with Venturia inaequalis. Phytochemistry 2013, 96, 101–109. [Google Scholar] [CrossRef]
- Forino, M.; Tenore, G.C.; Tartaglione, L.; Carmela, D.; Novellino, E.; Ciminiello, P. (1S,3R,4S,5R)5-O-Caffeoylquinic acid: Isolation, stereo-structure characterization and biological activity. Food Chem. 2015, 178, 306–310. [Google Scholar] [CrossRef]
- Clifford, M.N.; Kerimi, A.; Williamson, G. Bioavailability and metabolism of chlorogenic acids (acyl-quinic acids) in humans. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1299–1352. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef]
- Miftode, A.M. Antioxidants: Mechanisms of Action and Therapeutic Applications; Medichub Media: București, Romania, 2022; Volume 6, p. 8. [Google Scholar] [CrossRef]
- Bensid, A.; El Abed, N.; Houicher, A.; Regenstein, J.M.; Özogul, F. Antioxidant and antimicrobial preservatives: Properties, mechanism of action and applications in food—A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2985–3001. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380–3410. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Presler, A.; Michel, P. Profiling of Phenolic Compounds and Antioxidant Activity of Dry Extracts from the Selected Sorbus Species. Molecules 2012, 17, 3093–3113. [Google Scholar] [CrossRef]
- Smeriglio, A.; Davide, B.; Laganà, G.; Bellocco, E.; Trombetta, D. Bilberry (Vaccinium myrtyllus L.) . In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 159–163. [Google Scholar] [CrossRef]
- Tunde, J.; Vicas, L.G.; Toth, I.; Braun, M. Mineral elements profile, bioactive compounds and antioxidant capacity of wild blueberry and of pharmaceutical preparations from blueberry (Vaccinium myrtillus). Farmacia 2016, 64, 581–587. [Google Scholar]
- Neamtu, A.-A.; Szoke-Kovacs, R.; Mihok, E.; Georgescu, C.; Turcus, V.; Olah, N.K.; Frum, A.; Tita, O.; Neamtu, C.; Szoke-Kovacs, Z.; et al. Bilberry (Vaccinium myrtillus L.) Extracts Comparative Analysis Regarding Their Phytonutrient Profiles, Antioxidant Capacity along with the In Vivo Rescue Effects Tested on a Drosophila melanogaster High-Sugar Diet Model. Antioxidants 2020, 9, 1067. [Google Scholar] [CrossRef] [PubMed]
- Urbonaviciene, D.; Bobinaite, R.; Viskelis, P.; Bobinas, C.; Petruskevicius, A.; Klavins, L.; Viskelis, J. Geographic Variability of Biologically Active Compounds, Antioxidant Activity and Physico-Chemical Properties in Wild Bilberries (Vaccinium myrtillus L.). Antioxidants 2022, 11, 588. [Google Scholar] [CrossRef]
- Ciulca, S.; Roma, G.; Alexa, E.; Radulov, I.; Cocan, I.; Madosa, E.; Ciulca, A. Variation of Polyphenol Content and Antioxidant Activity in Some Bilberry (Vaccinium myrtillus L.) Populations from Romania. Agronomy 2021, 11, 2557. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Taie, H.A.; Wahab, W.A.A. Antioxidant capacity and antitumor activity of the bioactive protein prepared from orange peel residues as a by-product using fungal protease. Int. J. Biol. Macromol. 2023, 234, 123578. [Google Scholar] [CrossRef]
- Stolarczyk, E.U.; Strzempek, W.; Łaszcz, M.; Leś, A.; Menaszek, E.; Stolarczyk, K. Thiogenistein—Antioxidant Chemistry, Antitumor Activity, and Structure Elucidation of New Oxidation Products. Int. J. Mol. Sci. 2022, 23, 7816. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Chang, Q.; Zhou, Z.; Han, R.; Liang, Z. Antioxidant and Antidiabetic Activity of Proanthocyanidins from Fagopyrum dibotrys. Molecules 2021, 26, 2417. [Google Scholar] [CrossRef]
- Nisar, J.; Shah, S.M.A.; Ayaz, S.; Akram, M.; Rashid, A.; Mustafa, I.; Nisar, Z. In vitro comparative evaluation of Tamarix gallica extracts for antioxidant and antidiabetic activity. Exp. Biol. Med. 2022, 248, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Ahangarpour, A.; Sayahi, M.; Sayahi, M. The antidiabetic and antioxidant properties of some phenolic phytochemicals: A review study. Diabetes Metab. Syndr. 2019, 13, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.-M.; Reyes-Maldonado, O.K.; Puebla-Pérez, A.M.; Arreola, M.P.G.; Velasco-Ramírez, S.F.; Zúñiga-Mayo, V.; Sánchez-Fernández, R.E.; Delgado-Saucedo, J.-I.; Velázquez-Juárez, G. GC/MS Analysis, Antioxidant Activity, and Antimicrobial Effect of Pelargonium peltatum (Geraniaceae). Molecules 2022, 27, 3436. [Google Scholar] [CrossRef] [PubMed]
- Toledo, A.G.; Souza, J.G.d.L.d.; Santana, C.B.; Mallmann, A.P.; dos Santos, C.V.; Corrêa, J.M.; Pinto, F.G.d.S. Antimicrobial, antioxidant activity and phytochemical prospection of Eugenia involucrata DC. leaf extracts. Braz. J. Biol. 2021, 83, e245753. [Google Scholar] [CrossRef]
- Varga, E.; Becsek, E.; Bartha, S.G.; Stranczinger, S.; Mihalovits, F.; Papp, N. Determination of polyphenols and in vitro antimicrobial and antioxidant activity of Calluna vulgaris (L.) Hull. Biol. Futur. 2021, 72, 251–256. [Google Scholar] [CrossRef]
- Bailie, A.; Renaut, S.; Ubalijoro, E.; Guerrero-Analco, J.A.; Saleem, A.; Haddad, P.; Arnason, J.T.; Johns, T.; Cuerrier, A. Phytogeographic and genetic variation in Sorbus, a traditional antidiabetic medicine—Adaptation in action in both a plant and a discipline. PeerJ 2016, 4, e2645. [Google Scholar] [CrossRef] [PubMed]
- Nohynek, L.J.; Alakomi, H.-L.; Kähkönen, M.P.; Heinonen, M.; Helander, I.M.; Oksman-Caldentey, K.-M.; Puupponen-Pimiä, R.H. Berry Phenolics: Antimicrobial Properties and Mechanisms of Action Against Severe Human Pathogens. Nutr. Cancer 2006, 54, 18–32. [Google Scholar] [CrossRef]
- Augusti, P.R.; Conterato, G.M.; Denardin, C.C.; Prazeres, I.D.; Serra, A.T.; Bronze, M.R.; Emanuelli, T. Bioactivity, bioavailability, and gut microbiota transformations of dietary phenolic compounds: Implications for COVID-19. J. Nutr. Biochem. 2021, 97, 108787. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, S.; Almatroudi, A.; Khan, A.A.; Alsahli, M.A.; Almatroodi, S.A.; Rahmani, A.H. A review on mechanism of inhibition of advanced glycation end products formation by plant derived polyphenolic compounds. Mol. Biol. Rep. 2021, 48, 787–805. [Google Scholar] [CrossRef]
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; E Smith, A.; E Dalton, B.; Duprey, J.; A Cruz, J.; Hagins, H.; et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Boath, A.S.; Stewart, D.; McDougall, G.J. Berry components inhibit α-glucosidase in vitro: Synergies between acarbose and polyphenols from black currant and rowanberry. Food Chem. 2012, 135, 929–936. [Google Scholar] [CrossRef]
- Rutkowska, M.; Kolodziejczyk-Czepas, J.; Olszewska, M.A. The Effects of Sorbus aucuparia L. Fruit Extracts on Oxidative/Nitrative Modifications of Human Fibrinogen, Impact on Enzymatic Properties of Thrombin, and Hyaluronidase Activity In Vitro. Antioxidants 2021, 10, 2009. [Google Scholar] [CrossRef] [PubMed]
- Turumtay, H.; Midilli, A.; Turumtay, E.A.; Demir, A.; Selvi, E.K.; Budak, E.E.; Er, H.; Kocaimamoglu, F.; Baykal, H.; Belduz, A.O.; et al. Gram (−) microorganisms DNA polymerase inhibition, antibacterial and chemical properties of fruit and leaf extracts of Sorbus acuparia and Sorbus caucasica var. yaltirikii. Biomed. Chromatogr. 2017, 31, e3901. [Google Scholar] [CrossRef] [PubMed]
- Liepiņa, I.; Nikolajeva, V.; Jākobsone, I. Antimicrobial Activity of Extracts from Fruits of Aronia Melanocarpa and Sorbus Aucuparia. Environ. Exp. Biol. 2013, 11, 195–199. [Google Scholar]
- Otsuka, Y. Potent Antibiotics Active against Multidrug-Resistant Gram-Negative Bacteria. Chem. Pharm. Bull. 2020, 68, 182–190. [Google Scholar] [CrossRef]
- Ruhal, R.; Kataria, R. Biofilm patterns in gram-positive and gram-negative bacteria. Microbiol. Res. 2021, 251, 126829. [Google Scholar] [CrossRef]
- Qi, W.; Qi, W.; Xiong, D.; Long, M. Quercetin: Its Antioxidant Mechanism, Antibacterial Properties and Potential Application in Prevention and Control of Toxipathy. Molecules 2022, 27, 6545. [Google Scholar] [CrossRef]
- Boncler, M.; Golanski, J.; Lukasiak, M.; Redzynia, M.; Dastych, J.; Watala, C. A new approach for the assessment of the toxicity of polyphenol-rich compounds with the use of high content screening analysis. PLoS ONE 2017, 12, e0180022. [Google Scholar] [CrossRef]
- Krisch, J.; Galgóczy, L.; Tölgyesì, M.; Papp, T.; Vágvölgyi, C. Effect of Fruit Juices and Pomace Extracts on the Growth of Gram-Positive and Gram-Negative Bacteria. Acta Biol. Szeged. 2008, 52, 267–270. [Google Scholar]
- McDougall, G.J.; Ross, H.A.; Ikeji, M.; Stewart, D. Berry Extracts Exert Different Antiproliferative Effects against Cervical and Colon Cancer Cells Grown in Vitro. J. Agric. Food Chem. 2008, 56, 3016–3023. [Google Scholar] [CrossRef]
- Harborne, J.B. Encyclopedia of Applied Plant Sciences; Academic Press: Cambridge, MA, USA, 2003. [Google Scholar]
- George, N. Agrios. In Plant Pathology, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Egbuonu, A.C.C.; Eneogwe, J.C. Phytoalexins: Current and possible future applications in human health and diseases control. Int. J. Mol. Biol. 2018, 3, 61. [Google Scholar] [CrossRef]
- Sarkate, A.; Saini, S.S.; Teotia, D.; Gaid, M.; Mir, J.I.; Roy, P.; Agrawal, P.K.; Sircar, D. Comparative metabolomics of scab-resistant and susceptible apple cell cultures in response to scab fungus elicitor treatment. Sci. Rep. 2018, 8, 17844. [Google Scholar] [CrossRef] [PubMed]
- Chizzali, C.; Khalil, M.N.; Beuerle, T.; Schuehly, W.; Richter, K.; Flachowsky, H.; Peil, A.; Hanke, M.-V.; Liu, B.; Beerhues, L. Formation of biphenyl and dibenzofuran phytoalexins in the transition zones of fire blight-infected stems of Malus domestica cv. ‘Holsteiner Cox’ and Pyrus communis cv. ‘Conference’. Phytochemistry 2012, 77, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wang, X.; Yang, J.; Kuang, Y.; Wang, Y.; Yang, S.; Qin, J.; Guo, L. Antifungal Biphenyl Derivatives from Sorbus pohuashanensis Leaves Infected by Alternaria tenuissi and Their Effect against Crop Pathogens. Chem. Biodivers. 2021, 18, e2100079. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fu, H.-Y.; He, W.; Xiang, Y.-T.; Yang, Z.-C.; Kuang, Y.; Yang, S.-X. Synthesis and Antibacterial Activity Evaluation of Biphenyl and Dibenzofuran Derivatives as Potential Antimicrobial Agents against Antibiotic-Resistant Bacteria. Curr. Issues Mol. Biol. 2022, 44, 4087–4099. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, J.; Yang, X.-L.; Zhang, L.; Wang, J.; Li, Q.; Lin, D.-M.; Zhang, M.; Xia, S.-N.; Xu, L.-L.; et al. Novel dibenzofuran and biphenyl phytoalexins from Sorbus pohuashanensis suspension cell and their antimicrobial activities. Fitoterapia 2021, 152, 104914. [Google Scholar] [CrossRef]
- Azam, S.; Hadi, N.; Khan, N.; Hadi, S. Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: Implications for anticancer properties. Toxicol. Vitr. 2004, 18, 555–561. [Google Scholar] [CrossRef]
- Farhan, M.; Khan, H.Y.; Oves, M.; Al-Harrasi, A.; Rehmani, N.; Arif, H.; Hadi, S.M.; Ahmad, A. Cancer Therapy by Catechins Involves Redox Cycling of Copper Ions and Generation of Reactive Oxygen Species. Toxins 2016, 8, 37. [Google Scholar] [CrossRef]
- Hazafa, A.; Rehman, K.-U.; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2019, 72, 386–397. [Google Scholar] [CrossRef]
- Woźniak, M.; Krajewski, R.; Makuch, S.; Agrawal, S. Phytochemicals in Gynecological Cancer Prevention. Int. J. Mol. Sci. 2021, 22, 1219. [Google Scholar] [CrossRef] [PubMed]
- Isacescu, E.; Chiroi, P.; Zanoaga, O.; Nutu, A.; Budisan, L.; Pirlog, R.; Atanasov, A.G.; Berindan-Neagoe, I. Melanoma Cellular Signaling Transduction Pathways Targeted by Polyphenols Action Mechanisms. Antioxidants 2023, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kaur, M.; Katnoria, J.K.; Nagpal, A.K.; Sharma, M.K.A. Polyphenols in Food: Cancer Prevention and Apoptosis Induction. Curr. Med. Chem. 2018, 25, 4740–4757. [Google Scholar] [CrossRef]
- Suganuma, M.; Rawangkan, A.; Wongsirisin, P.; Kobayashi, N.; Matsuzaki, T.; Yoshikawa, H.Y.; Watanabe, T. Stiffening of Cancer Cell Membranes Is a Key Biophysical Mechanism of Primary and Tertiary Cancer Prevention with Green Tea Polyphenols. Chem. Pharm. Bull. 2020, 68, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Augimeri, G.; Montalto, F.I.; Giordano, C.; Barone, I.; Lanzino, M.; Catalano, S.; Andò, S.; De Amicis, F.; Bonofiglio, D. Nutraceuticals in the Mediterranean Diet: Potential Avenues for Breast Cancer Treatment. Nutrients 2021, 13, 2557. [Google Scholar] [CrossRef]
- Rabelo, A.C.S.; Borghesi, J.; Noratto, G.D. The role of dietary polyphenols in osteosarcoma: A possible clue about the molecular mechanisms involved in a process that is just in its infancy. J. Food Biochem. 2022, 46, e14026. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Lin, Y.; Ni, Z.; Chen, T.; Wang, X. Therapeutic effects of natural polyphenols on colorectal adenomas: Focus on preclinical studies (Review). Oncol. Rep. 2023, 49, 112. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, B.; Wang, N.; Gu, J. Tumor immunomodulatory effects of polyphenols. Front. Immunol. 2022, 13, 1041138. [Google Scholar] [CrossRef]
- Rajendran, P.; Abdelsalam, S.A.; Renu, K.; Veeraraghavan, V.; Ben Ammar, R.; Ahmed, E.A. Polyphenols as Potent Epigenetics Agents for Cancer. Int. J. Mol. Sci. 2022, 23, 11712. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary polyphenols in chemoprevention and synergistic effect in cancer: Clinical evidences and molecular mechanisms of action. Phytomedicine 2021, 90, 153554. [Google Scholar] [CrossRef]
- Oruganti, L.; Meriga, B. Plant Polyphenolic Compounds Potentiates Therapeutic Efficiency of Anticancer Chemotherapeutic Drugs: A Review. Endocr. Metab. Immune Disord.—Drug Targets 2021, 21, 246–252. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Y.; Zhao, J.; Liu, M.; Shu, X.; Li, Q.; Wang, Y.; Zhou, Y. Polyphenol Mechanisms against Gastric Cancer and Their Interactions with Gut Microbiota: A Review. Curr. Oncol. 2022, 29, 5247–5261. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Wang, X.-L.; He, D.-H.; Cheng, Y.-X. Protection against chemotherapy- and radiotherapy-induced side effects: A review based on the mechanisms and therapeutic opportunities of phytochemicals. Phytomedicine 2021, 80, 153402. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Bahramsoltani, R.; Rahimi, R. Phytochemicals as Adjunctive with Conventional Anticancer Therapies. Curr. Pharm. Des. 2016, 22, 4201–4218. [Google Scholar] [CrossRef] [PubMed]
- Sharma, E.; Attri, D.C.; Sati, P.; Dhyani, P.; Szopa, A.; Sharifi-Rad, J.; Hano, C.; Calina, D.; Cho, W.C. Recent updates on anticancer mechanisms of polyphenols. Front. Cell Dev. Biol. 2022, 10, 1005910. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt-Guzel, C.; Kultur, S. Cytotoxic Activities of Some Turkish Medicinal Plants against HeLa Cells in Vitro. Indian J. Tradit. Knowl. 2018, 17, 43–49. [Google Scholar]
- Goun, E.A.; Petrichenko, V.; Solodnikov, S.; Suhinina, T.; Kline, M.A.; Cunningham, G.; Nguyen, C.; Miles, H. Anticancer and antithrombin activity of Russian plants. J. Ethnopharmacol. 2002, 81, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Isaikina, N.V.; Kalinkina, G.I.; Razina, T.G.; Zueva, E.P.; Rybalkina, O.Y.; Ulirich, A.V.; Fedorova, E.P.; Shilova, A.B. Sorbus aucuparia L. Fruit Is a Source of the Drug for Increasing the Efficiency of Tumor Chemotherapy. Russ. J. Bioorgan. Chem. 2018, 44, 899–905. [Google Scholar] [CrossRef]
- Razina, T.G.; Zueva, E.P.; Ulrich, A.V.; Rybalkina, O.Y.; Chaikovskii, A.V.; Isaikina, N.V.; Kalinkina, G.I.; Zhdanov, V.V.; Zyuz’kov, G.N. Antitumor Effects of Sorbus aucuparia L. Extract Highly Saturated with Anthocyans and Their Mechanisms. Bull. Exp. Biol. Med. 2016, 162, 93–97. [Google Scholar] [CrossRef]
- Rybalkina, O.Y.; Fedorova, E.P.; Chaikovsky, A.V.; Razina, T.G.; Kalinkina, G.I.; Isaikina, N.V.; Kiseleva, E.A.; Zueva, E.P.; Zhdanov, V.V. Erythropoiesis-Stimulating Properties of Anthocyanin-Containing Complex from Sorbus aucuparia L. in Cytostatic Anemic Syndrome in Mice with Lewis Lung Carcinoma. Bull. Exp. Biol. Med. 2022, 173, 199–204. [Google Scholar] [CrossRef]
- Rybalkina, O.Y.; Neupokoeva, O.V.; Voronova, O.L.; Razina, T.G.; Kalinkina, G.I.; Isaikina, N.V.; Kiseleva, E.A.; Churin, A.A.; Zueva, E.P.; Zhdanov, V.V. Prevention of the Genotoxic Effects of Doxorubicin with Anthocyanin-Containing Complex from Sorbus aucuparia L. Fruit. Bull. Exp. Biol. Med. 2023, 175, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.; Kroon, P.A.; Quideau, S.; Treutter, D. Plant Phenolics—Secondary Metabolites with Diverse Functions. In Recent Advances in Polyphenol Research; Wiley: New York, NY, USA, 2008; Volume 1, ISBN 9781444302400. [Google Scholar]
- Hüttner, C.; Beuerle, T.; Scharnhop, H.; Ernst, L.; Beerhues, L. Differential Effect of Elicitors on Biphenyl and Dibenzofuran Formation in Sorbus aucuparia Cell Cultures. J. Agric. Food Chem. 2010, 58, 11977–11984. [Google Scholar] [CrossRef] [PubMed]
- Chizzali, C.; Beerhues, L. Phytoalexins of the Pyrinae: Biphenyls and dibenzofurans. Beilstein J. Org. Chem. 2012, 8, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Erdtman, H.; Eriksson, G.; Norin, T.; Forsén, S.; Meisingseth, E. Aucuparin and Methoxyaucuparin, Two Phenolic Biphenyl Derivatives from the Heartwood of Sorbus aucuparia (L.). Acta Chem. Scand. 1963, 17, 1151–1156. [Google Scholar] [CrossRef]
- Liu, B.; Beuerle, T.; Klundt, T.; Beerhues, L. Biphenyl synthase from yeast-extract-treated cell cultures of Sorbus aucuparia. Planta 2004, 218, 492–496. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, S.; Liu, Y.-H.; Li, J.-X.; Zhou, L.-Y.; Li, T.; Zhou, L.; Zhang, W.-J.; Guo, L.-P.; Huang, L.-Q. Preliminary study on memory function of Sorbus aucuparia suspension cell to biotic stress. China J. Chin. Mater. Med. 2021, 46, 2467–2473. [Google Scholar] [CrossRef]
- Dubey, S.P.; Lahtinen, M.; Särkkä, H.; Sillanpää, M. Bioprospective of Sorbus aucuparia leaf extract in development of silver and gold nanocolloids. Colloids Surf. B Biointerfaces 2010, 80, 26–33. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. Vitamin C: A Review on its Role in the Management of Metabolic Syndrome. Int. J. Med. Sci. 2020, 17, 1625–1638. [Google Scholar] [CrossRef]
- Brzezińska, O.; Styrzyński, F.; Makowska, J.; Walczak, K. Role of Vitamin C in Prophylaxis and Treatment of Gout—A Literature Review. Nutrients 2021, 13, 701. [Google Scholar] [CrossRef]
- Tada, A.; Miura, H. The Relationship between Vitamin C and Periodontal Diseases: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 2472. [Google Scholar] [CrossRef]
- Xu, K.; Peng, R.; Zou, Y.; Jiang, X.; Sun, Q.; Song, C. Vitamin C intake and multiple health outcomes: An umbrella review of systematic reviews and meta-analyses. Int. J. Food Sci. Nutr. 2022, 73, 588–599. [Google Scholar] [CrossRef]
- Milani, G.P.; Macchi, M.; Guz-Mark, A. Vitamin C in the Treatment of COVID-19. Nutrients 2021, 13, 1172. [Google Scholar] [CrossRef]
- van Gorkom, G.N.; Lookermans, E.L.; Van Elssen, C.H.; Bos, G.M. The Effect of Vitamin C (Ascorbic Acid) in the Treatment of Patients with Cancer: A Systematic Review. Nutrients 2019, 11, 977. [Google Scholar] [CrossRef]
- Ngo, B.; Van Riper, J.M.; Cantley, L.C.; Yun, J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat. Rev. Cancer 2019, 19, 271–282. [Google Scholar] [CrossRef]
- Böttger, F.; Vallés-Martí, A.; Cahn, L.; Jimenez, C.R. High-dose intravenous vitamin C, a promising multi-targeting agent in the treatment of cancer. J. Exp. Clin. Cancer Res. 2021, 40, 343. [Google Scholar] [CrossRef]
- Aschoff, J.K.; Kaufmann, S.; Kalkan, O.; Neidhart, S.; Carle, R.; Schweiggert, R.M. In Vitro Bioaccessibility of Carotenoids, Flavonoids, and Vitamin C from Differently Processed Oranges and Orange Juices [Citrus sinensis (L.) Osbeck]. J. Agric. Food Chem. 2015, 63, 578–587. [Google Scholar] [CrossRef]
- Hof, K.H.V.H.; De Boer, B.C.; Tijburg, L.B.; Lucius, B.R.; Zijp, I.; West, C.E.; Hautvast, J.G.; Weststrate, J.A. Carotenoid Bioavailability in Humans from Tomatoes Processed in Different Ways Determined from the Carotenoid Response in the Triglyceride-Rich Lipoprotein Fraction of Plasma after a Single Consumption and in Plasma after Four Days of Consumption. J. Nutr. 2000, 130, 1189–1196. [Google Scholar] [CrossRef]
- Jayedi, A.; Rashidy-Pour, A.; Parohan, M.; Zargar, M.S.; Shab-Bidar, S. Dietary and circulating vitamin C, vitamin E, β-caroten and risk of total cardiovascular mortality; a systematic review and dose-response meta-analysis of prospective observational studies. Public Health Nutr. 2019, 22, 1872–1887. [Google Scholar] [CrossRef]
- Miao, M.; Xiang, L. Pharmacological action and potential targets of chlorogenic acid. Adv. Pharmacol. 2020, 87, 71–88. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. BioMed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Pimpley, V.; Patil, S.; Srinivasan, K.; Desai, N.; Murthy, P.S. The chemistry of chlorogenic acid from green coffee and its role in attenuation of obesity and diabetes. Prep. Biochem. Biotechnol. 2020, 50, 969–978. [Google Scholar] [CrossRef]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef]
- Gupta, A.; Atanasov, A.G.; Li, Y.; Kumar, N.; Bishayee, A. Chlorogenic acid for cancer prevention and therapy: Current status on efficacy and mechanisms of action. Pharmacol. Res. 2022, 186, 106505. [Google Scholar] [CrossRef]
- Pandey, A.K.; Singh, A.K.; Singla, R.K. Chlorogenic acid: A dietary phenolic acid with promising pharmacotherapeutic potential. Curr. Med. Chem. 2023, 30, 3905–3926. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Q.; Shen, L.; Guo, K.; Zhou, X. Chlorogenic acid improves glucose tolerance, lipid metabolism, inflammation and microbiota composition in diabetic db/db mice. Front. Endocrinol. 2022, 13, 1042044. [Google Scholar] [CrossRef]
- Chen, X.-D.; Tang, J.-J.; Feng, S.; Huang, H.; Lu, F.-N.; Lu, X.-M.; Wang, Y.-T. Chlorogenic Acid Improves PTSD-like Symptoms and Associated Mechanisms. Curr. Neuropharmacol. 2021, 19, 2180–2187. [Google Scholar] [CrossRef]
- Rashidi, R.; Rezaee, R.; Shakeri, A.; Hayes, A.W.; Karimi, G. A review of the protective effects of chlorogenic acid against different chemicals. J. Food Biochem. 2022, 46, e14254. [Google Scholar] [CrossRef]
- Suganthy, N.; Devi, K.P.; Braidy, N.; Nabavi, S.M. Bioactive effects of quercetin in the central nervous system: Focusing on the mechanisms of actions. BioMed. Pharmacother. 2016, 84, 892–908. [Google Scholar] [CrossRef]
- Pandey, A.K.; Patnaik, R.; Muresanu, D.F.; Sharma, A.; Sharma, H.S. Quercetin in Hypoxia-Induced Oxidative Stress: Novel Target for Neuroprotection. Int. Rev. Neurobiol. 2012, 102, 107–146. [Google Scholar] [CrossRef]
- Palle, S.; Neerati, P. Quercetin nanoparticles attenuates scopolamine induced spatial memory deficits and pathological damages in rats. Bull. Fac. Pharmacy Cairo Univ. 2017, 55, 101–106. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and Its Anti-Allergic Immune Response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef]
- Formica, J.; Regelson, W. Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Lin, C.-H.; Zhao, T.; Zuo, D.; Ye, Z.; Liu, L.; Lin, M.-T. Quercetin protects against heat stroke-induced myocardial injury in male rats: Antioxidative and antiinflammatory mechanisms. Chem.-Biolog. Interact. 2017, 265, 47–54. [Google Scholar] [CrossRef]
- Yang, M.-S.; Choi, H.-G.; Sung, N.-Y.; Song, D.-S.; Sin, S.-J.; Byun, E.-H. Quercetin negatively regulates TLR4 signaling induced by lipopolysaccharide through Tollip expression. Biochem. Biophys. Res. Commun. 2013, 431, 698–705. [Google Scholar] [CrossRef]
- Roslan, J.; Giribabu, N.; Karim, K.; Salleh, N. Quercetin ameliorates oxidative stress, inflammation and apoptosis in the heart of streptozotocin-nicotinamide-induced adult male diabetic rats. BioMed. Pharmacother. 2017, 86, 570–582. [Google Scholar] [CrossRef]
- Lu, X.-L.; Zhao, C.-H.; Yao, X.-L.; Zhang, H. Quercetin attenuates high fructose feeding-induced atherosclerosis by suppressing inflammation and apoptosis via ROS-regulated PI3K/AKT signaling pathway. BioMed. Pharmacother. 2017, 85, 658–671. [Google Scholar] [CrossRef]
- Elbarbry, F.; Ung, A.; Abdelkawy, K. Studying the Inhibitory Effect of Quercetin and Thymoquinone on Human Cytochrome P450 Enzyme Activities. Pharmacogn. Mag. 2018, 13 (Suppl. S4), S895–S899. [Google Scholar]
- Wu, W.; Li, R.; Li, X.; He, J.; Jiang, S.; Liu, S.; Yang, J. Quercetin as an Antiviral Agent Inhibits Influenza A Virus (IAV) Entry. Viruses 2015, 8, 6. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, J.-E.; Song, Y.-J. Antiviral Activities of Quercetin and Isoquercitrin Against Human Herpesviruses. Molecules 2020, 25, 2379. [Google Scholar] [CrossRef]
- Noor, N.; Gani, A.; Gani, A.; Shah, A.; Ashraf, Z.U. Exploitation of polyphenols and proteins using nanoencapsulation for anti-viral and brain boosting properties—Evoking a synergistic strategy to combat COVID-19 pandemic. Int. J. Biol. Macromol. 2021, 180, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Bachevski, D.; Damevska, K.; Simeonovski, V.; Dimova, M. Back to the basics: Propolis and COVID -19. Dermatol. Ther. 2020, 33, e13780. [Google Scholar] [CrossRef] [PubMed]
- Mrityunjaya, M.; Pavithra, V.; Neelam, R.; Janhavi, P.; Halami, P.M.; Ravindra, P.V. Immune-Boosting, Antioxidant and Anti-inflammatory Food Supplements Targeting Pathogenesis of COVID-19. Front. Immunol. 2020, 11, 570122. [Google Scholar] [CrossRef]
- Demopoulos, C.; Antonopoulou, S.; Theoharides, T.C. COVID-19, microthromboses, inflammation, and platelet activating factor. BioFactors 2020, 46, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Glinsky, G.V. Tripartite Combination of Candidate Pandemic Mitigation Agents: Vitamin D, Quercetin, and Estradiol Manifest Properties of Medicinal Agents for Targeted Mitigation of the COVID-19 Pandemic Defined by Genomics-Guided Tracing of SARS-CoV-2 Targets in Human Cells. Biomedicines 2020, 8, 129. [Google Scholar] [CrossRef]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/prunasin (accessed on 15 July 2023).





| Phytotherapheutic Phytoconstituents | Quantity % | Source |
|---|---|---|
| Total sugars | 8.73–21.69 | [11,27,28] |
| Glucose | 3.76–5.29 | [11,27,28] |
| Fructose | 2.48–4.16 | [11,27,28] |
| Sucrose | 0.17–0.81 | [11,27,28] |
| Sorbitol | 2.68–13.41 | [11,28,29] |
| Organic acids | 0.2566 | [11] |
| Acetic acid | 0.008 | |
| Citric acid | 0.12–10.9 | [11,27,28,30,31] |
| Fumaric acid | 0–0.0016 | [11,27,28] |
| Malic acid | 2.20–3.34 | [11,27,28,30,31] |
| Oxalic acid | 0.38 | [27] |
| Shikimic acid | 0.0028–0.003 | [11,20] |
| Sorbic acid | 0.75 | [27] |
| Tartric acid | 0.01–0.03 | [11,27,28] |
| Total minerals | 8–9 | [27] |
| Quantity mg/100 g | ||
| Al | 0.0041 | [27] |
| Ca | 0.249–29.9 | [2,27] |
| Cl | <0.03 | [27] |
| Cu | 0.0004–0.294 | [2,27,32] |
| Fe | 0.0079–2.42 | [2,27] |
| K | 1.288–154 | [2,27] |
| Mg | 0.095–27.84 | [2,27] |
| Mn | 0.0062–0.503 | [2,27] |
| Na | 0.0056 | [27] |
| P | 0.139–123 | [2,27] |
| S | 0.053 | [27] |
| Si | 0.0233 | [27] |
| Zn | 0.0026–0.861 | [2,27,32] |
| Vitamin C | 10–42 | [27,30,33] |
| Vitamin E | 0.423–0.718 | [34,35,36] |
| α-Tocopherol | 0.34–0.489 | [34,35,36] |
| γ-Tocopherol | 0.025–0.171 | [34,35,36] |
| δ-Tocopherol | 0.058 | [35,36] |
| Carotenoids | 2.5–21.65 | [30,37] |
| Carotenes | 0.098–0.1 | [30,34] |
| Cis-carotenes | 0.15 | [30] |
| All-trans-carotenes | 1.78 | [30] |
| Cryptoxanthin | 1.37 | [30] |
| Lutein | 0.013 | [30] |
| Lycopene | 0.007 | [34] |
| Zeaxanthin | 0.003–1.11 | [30,34] |
| Compounds | Content in Fruits mg/g dw | Content in Fruits mg/g fw |
|---|---|---|
| Totalpolyphenols content mg eqGAE/100 g dw | 190–54,000 [8,28,30,32,43,46,47] | |
| Phenolic acids | 0.0002–269.43 [8,37,47,48] | 5.25–15.91 [34] |
| 1-caffeoylquinicacid | 6.81 [8] | |
| 3-caffeoylquinicacid (neochlorogenic acid) | 0.000705–24.65 [4,8,33,47,49,50] | 0.0064–0.369 [11,48] |
| 4-caffeoylquinicacid (cryptochlorogenic acid) | 8.33 [8] | 4.9 [11] |
| 5-caffeoylquinicacid (chlorogenic acid) | 0.0004–155.12 [4,8,30,34,47,49,50,51] | 0.0029–0.399 [11,48] |
| Gallic acid | 0.039 [30] | |
| Caffeic acid | 0.021–3.46 [8,30] | |
| Ferulic acid | 0.0078–9.59 [30,50] | |
| p-coumaric acid | 0.004 [30] | |
| Hydroxybenzoic acid | 0.107–0.11 [30,50] | |
| Protocatechuic acid | 0.018–2.80 [8,30] | |
| Flavonoids | 0.681–1.84 [37,50] | |
| Flavonols | 1.54 [50] | 0.1488 [11] |
| Quercetin | 0.0028–1.06 [4,8,30,34] | 0.00051 [48] |
| Kaempferol | 0.006 [48] | |
| Proanthocyanidins | 5 × 10−6–0.92 [34,37,50] | 0.0107 [48] |
| Flavanols | 0.97 [50] | 0.2494 [11] |
| Catechin | 0.017–1.3 [4,30] | |
| Epycatechin | 0.033–0.074 [4,30] | 0.0191 [11] |
| Procyanidin B1 | 0.022–0.085 [4,30] | |
| Procyanidin B2 | 0.092 [4] | |
| Procyanidin C1 | 0.103 [4] | |
| Anthocyanins | 0.12 [50] | 0.1012 [11] |
| Cyanidin-3-glucoside | 0.001 [4] | 0.00209 [11] |
| Cyanidin-3-galactoside | 0.183 [4] | 0.09912 [11] |
| Quercetin-3-galactoside (Hyperoside) | 0.024–1.36 [8,33,34,49] | |
| Quercetin-3-rutinoside (Rutin) | 4.01 × 10−5–0.0006 [33,34,49] | |
| Quercitin-3-glucoside (Isoquercitin) | 0.0061–2.29 [4,8,33,34,49] | 0.02612 [11] |
| Kaempferol—3-O-glucoside | 0.0085–0.009 [34] | 0.0024 [11] |
| Quercetin-3-O β-sophoroside | 3.57 [8] | |
| Hydrolyzable tannins | 0.0305 [11] |
| Antioxidant Activity Rowanberries | Ranges of Values | Antioxidant Activity Bilberries |
|---|---|---|
| Antioxidant activity IC 50 g/mL | 8.93–4260 [33,34,46,48,62] | 3.99 [63] |
| TP FRAP mmol Fe2+/kg dry fruits) | 315–54,670 [32,33,48,49,62] | 73.16 [64] |
| TP ABTS mmolTE/100 g | 0.76–5.84 [30,49] | 6–35.34 [65,66] |
| AA% | 65.2–92.2 [46] | 81.94–82.38 [63,67] |
| DPPH Trolox mmol Trolox/kg dry fruits | 10.84–7380 [30,32,33,48,62] | 5366 [63] |
| ORAC mmol TE/L | 23.689 [47] | 50.87 [65] |
| Extracts | Gram-Positive Bacteria | Gram-Negative Bacteria | Fungi | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B. c. | S. a. | C. p. | B. s. | E. f. | L. m. | E. c. | P. a. | C. j. | S. m. | P. v. | S. e. | K. p. | C. f. | P. f. | C. a. | |
| Aqueos extract | 1 | 1 | 1 | 4 | ||||||||||||
| Water–ethanol 50% | 3 | 3 | 0 | 3 | ||||||||||||
| Water–methanol | 2 | 2 | ||||||||||||||
| Methanol | 4 | 0 | 0–2 | 0 | 1–3 | 3 | 1 | |||||||||
| Acetone | 1 | 2 | 1 | 3 | 2 | 3 | 1 | |||||||||
| Phenolic 1 mg·mL | 4 | 1 | 0 | 1 | 0 | |||||||||||
| Rich in polyphenols | 0 | 2 | 3 | 3 | 3 | 3 | ||||||||||
| Pomace | 3 | 3 | 3 | 1 | 1 | 3 | 3 | 1 | ||||||||
| jus | 2 | 1 | 1 | 4 | ||||||||||||
| Solvents | HeLa | HepG2 | Caco-2 | A549 | HMEC-1 | 3T3 | Lewis Lung Carcinoma | Prostate Cancer |
|---|---|---|---|---|---|---|---|---|
| Acetone + water | 3 | 3 | 3 | 3 | 3 | 3 | ||
| Methanol + water | 0–1 | 4 | ||||||
| Water, ethanol | 3 | |||||||
| Methylene chloride | 2–3 |
| Phytotherapeutic Phytoconstituents | Diet Dosage | Effects |
|---|---|---|
| Vitamin C | 60 mg/day 5–7 mg/day prevents scurvy | Powerful antioxidant Sustains immunity Gene regulation Iron absorption Hormone synthesis Metabolic syndrome—prevention and treatment Gout—prevention and treatment Periodontal disease—prevention Respiratory infections—prevention and treatment Cancer treatment—assists chemotherapy |
| Vitamin E | 15 mg/day | Cancer prevention Dementia prevention Cardiovascular-diseases prevention |
| Vitamin A | 2–7 mg/day | Lower risk of cardiovascular mortality Eyesight enhancer Strengthen the immune system Sustains the bound healt |
| Chlorogenic acid | Antioxidant Anti-inflammatory Antibacterial Antiviral Hypoglycaemic and lipid lowering Antihypertension Liver, gastrointestinal, renal protection Stimulates nervous system Antimutagenic, anticarcinogenic Protects against chemicals toxicity | |
| Quercetin | Antioxidant Anti-inflammatory Antidiabetic Antiviral Improves mental and physical performance Degenerative diseases—prevention and treatment Prevent atherosclerotic plaque formation Regulating arterial tension, antiarrhythmic Cytotoxic | |
| Aucuparin | Pulmonary fibrosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arvinte, O.M.; Senila, L.; Becze, A.; Amariei, S. Rowanberry—A Source of Bioactive Compounds and Their Biopharmaceutical Properties. Plants 2023, 12, 3225. https://doi.org/10.3390/plants12183225
Arvinte OM, Senila L, Becze A, Amariei S. Rowanberry—A Source of Bioactive Compounds and Their Biopharmaceutical Properties. Plants. 2023; 12(18):3225. https://doi.org/10.3390/plants12183225
Chicago/Turabian StyleArvinte, Ofelia Marioara, Lăcrimioara Senila, Anca Becze, and Sonia Amariei. 2023. "Rowanberry—A Source of Bioactive Compounds and Their Biopharmaceutical Properties" Plants 12, no. 18: 3225. https://doi.org/10.3390/plants12183225