Callus Derived from Petals of the Rosa hybrida Breeding Line 15R-12-2 as New Material Useful for Fragrance Production
Abstract
:1. Introduction
2. Results and Discussion
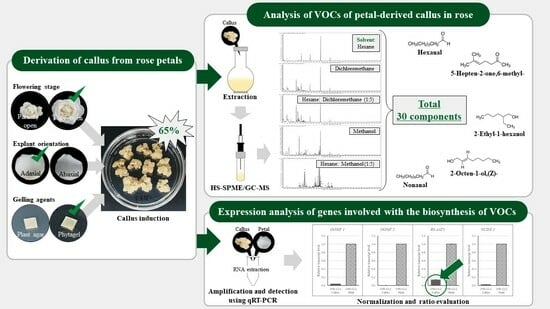
2.1. Callus Induction
2.2. Analysis of the VOCs of Petal-Derived Callus in Rose
2.3. Expression Analysis of Genes Involved in the Biosynthesis of VOCs of Rose-Petal-Derived Callus
3. Materials and Method
3.1. Derivation of Callus from Petals
3.1.1. Explant Material and Surface Sterilization
3.1.2. Condition of the Medium and Culture Used to Induce Callus from the Petals
3.2. Extract and Identification of VOC Analysis of Petal-Derived Callus Using HS-SPME/GC-MS
3.3. Analysis of Gene Expression Involved in the Biosynthesis of VOCs Using Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
3.4. Statistical Analysis
4. Conclusions
5. Patent
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naeem, A.; Abbas, T.; Ali, T.M.; Hasnain, A. Essential oils: Brief background and uses. Ann. Short Rep. 2018, 1, 1–6. [Google Scholar]
- Flament, I.; Debonneville, C.; Furrer, A. Volatile constituents of roses: Characterization of cultivars based on the headspace analysis of living flower emissions. In Bioactive Volatile Compounds from Plants; American Chemical Society: Washington, DC, USA, 1993; pp. 269–281. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E. Biochemical and molecular genetic aspects of floral scents. Plant Physiol. 2000, 122, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Shalit, M.; Guterman, I.; Volpin, H.; Bar, E.; Tamari, T.; Menda, N.; Adam, Z.; Zamir, D.; Vainstein, A.; Weiss, D.; et al. Volatile ester formation in roses. Identification of an acetyl-coenzyme A. Geraniol/citronellol acetyltransferase in developing rose petals. Plant Physiol. 2003, 131, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Bergougnoux, V.; Caissard, J.C.; Jullien, F.; Magnard, J.L.; Scalliet, G.; Cock, J.M.; Hugueney, P.; Baudino, S. Both the adaxial and abaxial epidermal layers of the rose petal emit volatile scent compounds. Planta 2007, 226, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Essential Oils Market Size, Share & Growth Report (2021–2028). Available online: https://www.Fortunebusinessinsights.com/enquiry/request-sample-pdf/essential-oils-market-101063 (accessed on 17 June 2023).
- Baser, K.H.C. Turkish rose oil. Perfum. Flavor. 1992, 17, 45. [Google Scholar]
- Lee, K.Y.; Shin, J.Y.; Lee, Y.A.; Ahn, C.H.; Kim, Y.J.; Park, P.M.; An, H.R.; Lee, K.Y.; Jung, H.H. The induction of somatic embryogenic callus from petals-derived callus in Rosa hybrida. Korean J. Plant Res. 2022, 35, 652–658. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Srivastava, A.K.; Bhojwani, S.S.; Bisaria, V.S. Production of podophyllotoxin by plant cell cultures of Podophyllum hexandrum in bioreactor. J. Biosci. Bioeng. 2002, 93, 215–220. [Google Scholar] [CrossRef]
- Hirner, A.A.; Veit, S.; Seitz, H.U. Regulation of anthocyanin biosynthesis in UV-A-irradiated cell cultures of carrot and in organs of intact carrot plants. Plant Sci. 2001, 161, 315–322. [Google Scholar] [CrossRef]
- Saw, N.M.M.T.; Riedel, H.; Cai, Z.; Kütük, O.; Smetanska, I. Stimulation of anthocyanin synthesis in grape (Vitis vinifera) cell cultures by pulsed electric fields and ethephon. Plant Cell Tissue Organ Cult. 2012, 108, 47–54. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Akita, M.; Sakamoto, K.; Liu, H.; Shigeoka, T.; Koyano, T.; Kawamura, M.; Furuya, T. Large-scale production of anthocyanin by Aralia cordata cell suspension cultures. Appl. Microbiol. Biotechnol. 1993, 40, 215–218. [Google Scholar] [CrossRef]
- Yu, K.W.; Gao, W.Y.; Hahn, E.J.; Paek, K.Y. Jasmonic acid improves ginsenoside accumulation in adventitious root culture of Panax ginseng C.A. Meyer. Biochem. Eng. J. 2002, 11, 211–215. [Google Scholar] [CrossRef]
- Huang, C.; Zhong, J.J. Elicitation of ginsenoside biosynthesis in cell cultures of Panax ginseng by vanadate. Process Biochem. 2013, 48, 1227–1234. [Google Scholar] [CrossRef]
- Wang, J.W.; Zheng, L.P.; Wu, J.Y.; Tan, R.X. Involvement of nitric oxide in oxidative burst, phenylalanine ammonia-lyase activation and Taxol production induced by low-energy ultrasound in Taxus yunnanensis cell suspension cultures. Nitric. Oxide 2006, 15, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Park, D.E.; Adhikari, D.; Pangeni, R.; Panthi, V.K.; Kim, H.J.; Park, J.W. Preparation and characterization of callus extract from Pyrus pyrifolia and investigation of its effects on skin regeneration. Cosmetics 2018, 5, 71. [Google Scholar] [CrossRef]
- Duan, Y.; Su, Y.; Chao, E.; Zhang, G.; Zhao, F.; Xue, T.; Sheng, W.; Teng, J.; Xue, J. Callus-mediated plant regeneration in Isodon amethystoides using young seedling leaves as starting materials. Plant Cell Tissue Organ Cult. 2019, 136, 247–253. [Google Scholar] [CrossRef]
- Zhou, C.; Sun, C.; Chen, K.; Li, X. Flavonoids, phenolics, and antioxidant capacity in the flower of Eriobotrya japonica Lindl. Int. J. Mol. Sci. 2011, 12, 2935–2945. [Google Scholar] [CrossRef]
- Fu, M.; He, Z.; Zhao, Y.; Yang, J.; Mao, L. Antioxidant properties and involved compounds of daylily flowers in relation to maturity. Food Chem. 2009, 114, 1192–1197. [Google Scholar] [CrossRef]
- Schmitzer, V.; Veberic, R.; Osterc, G.; Stampar, F. Color and phenolic content changes during flower development in groundcover rose. J. Am. Soc. Hortic. Sci. 2010, 135, 195–202. [Google Scholar] [CrossRef]
- Sulborska, A.; Weryszko-Chmielewska, E.; Chwil, M. Micromorphology of Rosa rugosa Thunb. petal epidermis secreting fragrant substances. Acta Agrobot. 2012, 65, 4. [Google Scholar] [CrossRef]
- Stubbs, J.M.; Francis, M.J.O. Electron microscopical studies of rose petal cells during flower maturation. Planta Med. 1971, 20, 211–218. [Google Scholar] [CrossRef]
- Liu, Y.; Chaturvedi, P.; Fu, J.; Cai, Q.; Weckwerth, W.; Yang, P. Induction and quantitative proteomic analysis of cell dedifferentiation during callus formation of lotus (Nelumbo nucifera Gaertn. spp. baijianlian). J. Proteom. 2016, 131, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Beruto, M.; Beruto, D.; Debergh, P. Influence of agar on in vitro cultures: I. Physicochemical properties of agar and agar gelled media. Vitro Cell. Dev. Biol. Plant. 1999, 35, 86–93. [Google Scholar] [CrossRef]
- Das, N.; Tripathi, N.; Basu, S.; Bose, C.; Maitra, S.; Khurana, S. Progress in the development of gelling agents for improved culturability of microorganisms. Front. Microbiol. 2015, 6, 698. [Google Scholar] [CrossRef] [PubMed]
- Ram, M.; Prasad, K.V.; Singh, S.K.; Hada, B.S.; Kumar, S. Influence of salicylic acid and methyl jasmonate elicitation on anthocyanin production in callus cultures of Rosa hybrida L. Plant Cell Tissue Organ Cult. 2013, 113, 459–467. [Google Scholar] [CrossRef]
- Darwish, H.Y.; Ahmed, S.M. Elicitors Enhancing Phenolics Content and Related Gene Expression Variation in Petal-Derived Calli of Rosa damascena Mill. Egypt. J. Bot. 2020, 60, 71–79. [Google Scholar] [CrossRef]
- Almenar, E.; Auras, R.; Rubino, M.; Harte, B. A new technique to prevent the main post harvest diseases in berries during storage: Inclusion complexes β-cyclodextrin-hexanal. Int. J. Food Microbiol. 2007, 118, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, M.; Kekäläinen, S.; Nikkilä, I.; Kilpeläinen, P.; Tenkanen, M.; Mikkonen, K.S. Active food packaging through controlled in situ production and release of hexanal. Food Chem. X 2020, 5, 100074. [Google Scholar] [CrossRef]
- Ragaini, V.; Bianchi, C.L.; Pirola, C. Kinetic of esterification of diluted acetic acid with pure 2-ethyl-1-hexanol. Chem. Eng. J. 2007, 131, 257–262. [Google Scholar] [CrossRef]
- McGinty, D.; Scognamiglio, J.; Letizia, C.S.; Api, A.M. Fragrance material review on 2-ethyl-1-hexanol. Food Chem. Toxicol. 2010, 48, S115–S129. [Google Scholar] [CrossRef]
- Harada, M.; Ueda, Y.; Iwata, T. Purification and some properties of alcohol acetyltransferase from banana fruit. Plant Cell Physiol. 1985, 26, 1067–1074. [Google Scholar] [CrossRef]
- Lilly, M.; Bauer, F.F.; Lambrechts, M.G.; Swiegers, J.H.; Cozzolino, D.; Pretorius, I.S. The effect of increased yeast alcohol acetyltransferase and esterase activity on the flavour profiles of wine and distillates. Yeast 2006, 23, 641–659. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.T.; Tollsten, L.; Bergström, L.G. Floral scents—A checklist of volatile compounds isolated by head-space techniques. Phytochemistry 1993, 33, 253–280. [Google Scholar] [CrossRef]
- Dennis, E.G.; Keyzers, R.A.; Kalua, C.M.; Maffei, S.M.; Nicholson, E.L.; Boss, P.K. Grape contribution to wine aroma: Production of hexyl acetate, octyl acetate, and benzyl acetate during yeast fermentation is dependent upon precursors in the must. J. Agric. Food Chem. 2012, 60, 2638–2646. [Google Scholar] [CrossRef] [PubMed]
- Gomez, E.; Ledbetter, C.A. Comparative study of the aromatic profiles of two different plum species: Prunus salicina Lindl and Prunus simonii L. J. Sci. Food Agric. 1994, 65, 111–115. [Google Scholar] [CrossRef]
- Zhu, Y.; Rudell, D.R.; Mattheis, J.P. Characterization of cultivar differences in alcohol acyltransferase and 1-aminocyclopropane-1-carboxylate synthase gene expression and volatile ester emission during apple fruit maturation and ripening. Postharvest Biol. Technol. 2008, 49, 330–339. [Google Scholar] [CrossRef]
- Iijima, Y.; Gang, D.R.; Fridman, E.; Lewinsohn, E.; Pichersky, E. Characterization of geraniol synthase from the peltate glands of sweet basil. Plant Physiol. 2004, 134, 370–379. [Google Scholar] [CrossRef]
- Yang, T.; Li, J.; Wang, H.X.; Zeng, Y. A geraniol-synthase gene from Cinnamomum tenuipilum. Phytochemistry 2005, 66, 285–293. [Google Scholar] [CrossRef]
- Ito, M.; Honda, G. Geraniol synthases from perilla and their taxonomical significance. Phytochemistry 2007, 68, 446–453. [Google Scholar] [CrossRef]
- Masumoto, N.; Korin, M.; Ito, M. Geraniol and linalool synthases from wild species of perilla. Phytochemistry 2010, 71, 1068–1075. [Google Scholar] [CrossRef]
- Magnard, J.L.; Roccia, A.; Caissard, J.C.; Vergne, P.; Sun, P.; Hecquet, R.; Dubois, A.; Oyant, L.H.; Jullien, F.; Nicole, F.; et al. Biosynthesis of monoterpene scent compounds in roses. Science 2015, 349, 81–83. [Google Scholar] [CrossRef]
- Lavid, N.; Wang, J.; Shalit, M.; Guterman, I.; Bar, E.; Beuerle, T.; Menda, N.; Shafir, S.; Zamir, D.; Adam, Z.; et al. O-methyltransferases involved in the biosynthesis of volatile phenolic derivatives in rose petals. Plant Physiol. 2002, 129, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Scalliet, G.; Journot, N.; Jullien, F.; Baudino, S.; Magnard, J.L.; Channelière, S.; Vergne, P.; Dumas, C.; Bendahmane, M.; Cock, J.M.; et al. Biosynthesis of the major scent components 3,5-dimethoxytoluene and 1,3,5-trimethoxybenzene by novel rose O-methyltransferases. FEBS Lett. 2002, 523, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Scalliet, G.; Lionnet, C.; Le Bechec, M.; Dutron, L.; Magnard, J.L.; Baudino, S.; Bergougnoux, V.; Jullien, F.; Chambrier, P.; Vergne, P.; et al. Role of petal-specific orcinol O-methyltransferases in the evolution of rose scent. Plant Physiol. 2006, 140, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Schenk, R.U.; Hildebrandt, A.C. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Int. Plant Prop. Soc. Proc. 1980, 30, 421. [Google Scholar]




| Flower Stage | Orientation z | Media | Induction Rate (%) y | Color | Texture | |
|---|---|---|---|---|---|---|
| Fully open | Abaxial | SH11D | 30 | ab | Light yellowish | Soft and Friable |
| SH11DP | 65 | a | Light yellowish | Soft and Friable | ||
| WPM11D | 0 | b | - | - | ||
| Adaxial | SH11D | 20 | b | Light yellowish | Soft and Friable | |
| SH11DP | 30 | a | Light yellowish | Soft and Friable | ||
| WPM11D | 0 | b | - | - | ||
| Partially open | Abaxial | SH11D | 0 | b | - | - |
| SH11DP | 40 | ab | Light yellowish | Soft and Friable | ||
| WPM11D | 0 | b | - | - | ||
| Adaxial | SH11D | 0 | b | - | - | |
| SH11DP | 40 | ab | Light yellowish | Soft and Friable | ||
| WPM11D | 0 | b | - | - | ||
| RI | CAS | Name | Formula | Molecular Weight (g/mol) | Relative Content (%) | Odor Description | Identification Method | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hexane | Dichloromethane | Hexane: Dichloro-methane (1:5) | Methanol | Hexane: Methanol (1:5) | |||||||
| 758 | 2919-23-5 | Cyclobutanol | C4H8O | 72.11 | - | - | - | 0.08 | - | MS | |
| 801 | 66-25-1 | Hexanal | C6H12O | 100.16 | 13.07 | 2.37 | 13.45 | 7.75 | 0.83 | Fresh, green, fatty, aldehydic, grass, leafy, fruity, sweaty | MS, RI |
| 833 | 553-90-2 | Dimethyl oxalate | C4H6O4 | 118.09 | - | - | - | 22.83 | - | MS | |
| 873 | 13952-84-6 | Butan-2-amine | C4H11N | 73.14 | - | - | 0.02 | - | - | Fishy, ammonia | MS |
| 953 | 7383-19-9 | Hept-1-yn-3-ol | C7H12O | 112.17 | - | 24.01 | 19.01 | 1.94 | - | MS | |
| 986 | 110-93-0 | 6-methylhept-5-en-2-one | C8H14O | 126.2 | - | 3.76 | 3.09 | - | - | Citrus, green, musty, lemongrass, apple | MS, RI |
| 991 | 3173-53-3 | Isocyanatocyclohexane | C7H11NO | 125.17 | - | - | - | 1.98 | 0.19 | MS | |
| 998 | 124-18-5 | Decane | C10H22 | 142.28 | - | - | - | 17.89 | 0.47 | MS, RI | |
| 1011 | 17302-01-1 | 3-Ethyl-3-methylheptane | C10H22 | 142.28 | - | - | - | - | 1.83 | MS | |
| 1027 | 104-76-7 | 2-ethylhexan-1-ol | C8H18O | 130.229 | 59.01 | 9.74 | 5.28 | 1.80 | 0.62 | Citrus, fresh, floral, oily, sweet | MS, RI |
| 1042 | 821-97-6 | (Z)-undec-3-ene | C11H22 | 154.29 | - | - | - | - | 0.05 | MS | |
| 1058 | 1000309-20-1 | 2-ethylhexyl octadecyl sulfite | C26H54O3S | 446.8 | - | - | - | - | 0.31 | MS | |
| 1065 | 26001-58-1 | (Z)-oct-2-en-1-ol | C8H16O | 128.21 | - | - | 1.19 | - | - | Sweet, floral | MS |
| 1080 | 55170-80-4 | 2,4-dimethyldec-1-ene | C12H24 | 168.32 | - | - | - | - | 6.82 | MS | |
| 1096 | 1000309-24-3 | 1-O-pentadecyl 2-O-prop-2-enyl oxalate | C20H36O4 | 340.5 | - | - | - | 0.27 | - | MS | |
| 1099 | 3913-02-08 | 2-butyloctan-1-ol | C12H26O | 186.33 | - | - | - | 0.33 | - | MS | |
| 1101 | 124-19-6 | Nonanal | C9H18O | 142.24 | - | - | - | 0.30 | - | waxy, aldehydic, rose, fresh, orris, orange, peel, fatty, peely | MS |
| 1240 | 61141-72-8 | 4,6-dimethyldodecane | C14H30 | 198.39 | - | - | - | 0.62 | - | MS | |
| 1276 | 31295-56-4 | 2,6,11-trimethyldodecane | C15H32 | 212.41 | - | - | - | - | 3.55 | MS, RI | |
| 1301 | 91337-07-4 | 5-methyl-2-propan-2-ylheptan-1-ol | C11H24O | 172.31 | - | - | - | - | 5.37 | MS | |
| 1304 | 1000309-34-3 | 2-O-(6-ethyloctan-3-yl) 1-O-(4-methylpentyl) oxalate | C18H34O4 | 314.5 | - | - | - | 0.27 | - | MS | |
| 1310 | 6750-34-1 | 3,7,11-trimethyldodecan-1-ol | C15H32O | 228.41 | - | - | - | 2.53 | - | MS | |
| 1315 | 13187-99-0 | 2-Bromo dodecane | C12H25Br | 249.23 | - | - | - | - | 0.57 | MS | |
| 1318 | 18675-24-6 | 2-methyldecan-1-ol | C11H24O | 172.31 | - | - | - | 1.36 | - | MS | |
| 1322 | 3891-98-3 | 2,6,10-trimethyldodecane | C15H32 | 212.41 | - | - | - | 0.73 | - | MS | |
| 1366 | 1000406-39-2 | 1-heptoxydodecane | C19H40O | 284.5 | - | - | - | - | 0.18 | MS | |
| 1425 | 1009-61-6 | 1-(4-acetylphenyl)ethanone | C10H10O2 | 162.18 | - | - | - | - | 0.30 | MS | |
| 1475 | 195194-80-0 | 1-(4-bromobutyl)piperidin-2-one | C9H16BrNO | 234.13 | - | - | - | - | 0.08 | MS | |
| 1492 | 504-44-9 | Crocetane | C20H42 | 282.5 | - | - | - | - | 0.64 | MS | |
| 1535 | 1000406-09-9 | Tetradecyl €-hept-2-enoate | C21H40O2 | 324.5 | - | - | - | - | 0.08 | MS | |
| Stage | Temperature (°C) | Time | Cycles | |
|---|---|---|---|---|
| Initial Denaturation | 95 | 3 min | 1 | |
| Amplification | Denaturation | 95 | 5 s | 44 |
| Annealing | 58 | 30 s | ||
| Extension | 72 | 10 s | ||
| Melt Curve | 65–95 | 0.5 °C increment 5 s/step | 1 | |
| Gene Name | Primer Sequence (5′-3′) | |
|---|---|---|
| Forward | Reverse | |
| OOMT1Z | CTGACCTGCAAGGAAGTAAGAA | TCGCTCCAGTCATGCAATATC |
| OOMT2 | CTACCAATCCATCCAACCAAATC | GGGAAGCATCAGTAAGGGTATAA |
| RhAAT1 | AGTTCACTCCCACAACGTATTT | AAAGTGAGAGCCTGGGAAAC |
| RrNUDX1 | GGATGGTATGAGTGGGACAATC | GCTAGCAGCTGTCTCATGTT |
| RhACTIN | GTTCCCAGGAATCGCTGATA | ATCCTCCGATCCAAACACTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.Y.; Shin, J.Y.; Ahn, M.S.; Kim, S.J.; An, H.R.; Kim, Y.J.; Kwon, O.H.; Lee, S.Y. Callus Derived from Petals of the Rosa hybrida Breeding Line 15R-12-2 as New Material Useful for Fragrance Production. Plants 2023, 12, 2986. https://doi.org/10.3390/plants12162986
Lee KY, Shin JY, Ahn MS, Kim SJ, An HR, Kim YJ, Kwon OH, Lee SY. Callus Derived from Petals of the Rosa hybrida Breeding Line 15R-12-2 as New Material Useful for Fragrance Production. Plants. 2023; 12(16):2986. https://doi.org/10.3390/plants12162986
Chicago/Turabian StyleLee, Ka Youn, Ju Young Shin, Myung Suk Ahn, Se Jin Kim, Hye Ryun An, Yae Jin Kim, O Hyeon Kwon, and Su Young Lee. 2023. "Callus Derived from Petals of the Rosa hybrida Breeding Line 15R-12-2 as New Material Useful for Fragrance Production" Plants 12, no. 16: 2986. https://doi.org/10.3390/plants12162986