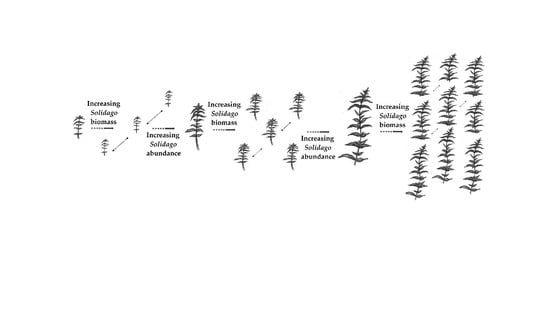
Survey of the Solidago canadensis L. Morphological Traits and Essential Oil Production: Aboveground Biomass Growth and Abundance of the Invasive Goldenrod Appears to Be Reciprocally Enhanced within the Invaded Stands
Abstract
:1. Introduction
- -
- the production of a high number of small, light-winged seeds, spreading mostly by the air, germinating rapidly in the high percentage and, with a wide tolerance for the different values of soil reaction, salinity, and moisture;
- -
- robust asexual reproductive ability of the underground parts (rhizomes, nodes, stem bases), hereby just a naturalized population has a great capacity for a clonal growth, since the clonality is in general concurrently found to offer advantages that facilitate invasion [5];
- -
- -
2. Results
2.1. Morphological Traits
2.1.1. Morphological Traits—Different Phenological Phases
2.1.2. Morphological Traits—Different Degree of Invasion
2.1.3. Relative Daily Growth Rate
2.2. Essential Oil Productivity Rate
2.3. Correlations
3. Discussion
Management Proposal
- invaded stands within the protected, vulnerable, and natural value areas, or those needing to be restored, should receive intensive management, including mowing two times per year, plus shallow ploughing/rotary tilling [21], applied, at the latest, when the inflorescences are already yellow, but before the full opening of the petals [22]; and
4. Materials and Methods
4.1. Sampling Stands
4.2. Material Collection and Processing
- 1st sampling term—between 24 May and 9 June;
- 2nd sampling term—between 27 June and 13 July—the first two sampling terms were characterized by green plants, without inflorescences;
- 3rd sampling term—between 3 and 18 August—the third sampling term was characterized with fully developed inflorescences;
- 4th sampling term—between 3 and 23 September—goldenrod blossoming was coming to the end; however, inflorescences were still considerably yellow; and
- 5th sampling term—between 7 and 28 October—plants passed active blooming period.
4.3. Essential Oil Productivity Rate Assessment
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cvachová, A.; Gojdičová, E. Regulation for Invasive Plant Species Removal; SOPSR, COPK Banská: Bystrica, Slovakia, 2003; pp. 1–37. (In Slovak) [Google Scholar]
- Jakobs, G.; Weber, E.; Edwards, P.J. Introduced Plants of the Invasive Solidago gigantea (Asteraceae) are Larger and Grow Denser than Conspecifics in the Native Range. Divers. Distrib. 2004, 10, 11–19. [Google Scholar] [CrossRef]
- Weber, E.; Jakobs, G. Biological Flora of Central Europe: Solidago gigantea Aiton. Flora 2005, 200, 109–118. [Google Scholar] [CrossRef]
- Koutika, L.S.; Rainey, H.J.; Dassonville, N. Impacts of Solidago gigantea, Prunus serotina, Heracleum mantegazzianum and Fallopia japonica Invasions on Ecosystems. Appl. Ecol. Environ. Res. 2011, 91, 73–83. [Google Scholar] [CrossRef]
- Martin, F.M.; Dommanget, F.; Lavallée, F.; Evette, A. Clonal Growth Strategies of Reynoutria japonica in Response to Light, Shade, and Mowing, and Perspectives for Management. NeoBiota 2020, 56, 89–110. [Google Scholar] [CrossRef]
- Guo, S.L.; Fang, F. Physiological Adaptation of the Invasive Plant to Environments. Chin. J. Plant Ecol. 2003, 27, 47–52. [Google Scholar] [CrossRef]
- Huang, H.; Chen, G.; Guo, S. Reproductive Biology in an Invasive Plant Solidago canadensis. Front. Biol. 2007, 2, 196–204. [Google Scholar] [CrossRef]
- Sun, B.Y.; Tan, J.Z.; Wan, Z.G.; Gu, F.G.; Zhu, M.D. Allelopathic Effects of Extracts from Solidago canadensis L. against Seed Germination and Seedling Growth of Some Plants. J. Environ. Sci. 2006, 18, 304–309. [Google Scholar]
- Abhilasha, D.; Quintana, N.; Vivanco, J.; Joshi, J. Do Allelopathic Compounds in Invasive Solidago canadensis S.L. Restrain the Native European Flora? J. Ecol. 2008, 96, 993–1001. [Google Scholar] [CrossRef]
- Gruľová, D.; Baranová, B.; Ivanova, V.; De Martino, L.; Mancini, E.; De Feo, V. Composition and Bio Activity of Essential Oils of Solidago spp. and their Impact on Radish and Garden Cress. Allelopath. J. 2016, 39, 129–141. [Google Scholar]
- Wang, C.; Xiao, H.; Zhao, L.; Liu, J.; Wang, L.; Zhang, F.; Shi, Y.; Du, D. The Allelopathic Effects of Invasive Plant Solidago Canadensis on Seed Germination and Growth of Lactuca Sativa Enhanced by Different Types of Acid Deposition. Ecotoxicology 2016, 25, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Możdżeń, K.; Barabasz-Krasny, B.; Zandi, P.; Kliszcz, A.; Puła, J. Effect of Aqueous Extracts from Solidago canadensis L. Leaves on Germination and Early Growth Stages of Three Cultivars of Raphanus sativus L. var. radicula Pers. Plants 2020, 9, 1549. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, H.; Wei, M.; Wang, S.; Wu, B.; Du, D. Plant Height and Leaf Size: Which One is More Important in Affecting the Successful Invasion of Solidago canadensis and Conyza canadensis in Urban Ecosystems? Urban For. Urban Green. 2021, 59, 127033. [Google Scholar] [CrossRef]
- Song, D.L.; Zhao, Y.F.; Tang, F.P.; Zhang, Y.H.; Zhou, S.Q.; Don, L.J. Effects of Arbuscular Mycorrhizal Fungi on Solidago Canadensis Growth are Independent of Nitrogen Form. J. Plant Ecol. 2021, 14, 648–661. [Google Scholar] [CrossRef]
- Yu, H.W.; He, W.M. Arbuscular Mycorrhizal Fungi Compete Asymmetrically for Amino Acids with Native and Invasive Solidago. Microb. Ecol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Cheng, J.; Xu, X.; Zhang, Y.; Zhao, Y.; Li, H.; Qiang, S. Polyploidy in Invasive Solidago Canadensis Increased Plant Nitrogen Uptake, And Abundance and Activity of Microbes and Nematodes in Soil. Soil Biol. Biochem. 2019, 138, 107594. [Google Scholar] [CrossRef]
- Szymura, M.; Szymura, T.H. Soil Preferences and Morphological Diversity of Goldenrods (Solidago L.) From South-Western Poland. Acta Soc. Bot. Pol. 2013, 82, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, M.A.; Jafari, A.A. Variation and Relationships of Morphological Traits, Shoot Yields and Essential Oil Contents of Four Species. Folia Hortic. 2016, 28, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, M.A.; Jafari, A.A.; Sayedian, S.E. Evaluation of Aerial Biomass Yield and Essential Oil Content of Seven Species of Tanacetum. J. Hortic. Res. 2017, 25, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Končeková, L.; Zahradníková, E.; Pintér, E.; Halmová, D. Assessment of an Impact of Mechanical Regulation on Selected Morphometric and Productive Parameters of Invasive Species Solidago canadensis Population in Agricultural Land. Agriculture 2016, 61, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Gala-Czekaj, D.; Synowiec, A.; Dąbkowska, T. Self-Renewal of Invasive Goldenrods (Solidago spp.) as a Result of Different Mechanical Management of Fallow. Agronomy 2021, 11, 1065. [Google Scholar] [CrossRef]
- Sá, P.G.; Almeida, D.B.; Alves, C.M.L.; Barbosa, J.G.; Grossi, J.A.S.; Zandonadi, A.S.; Muniz, M.A. Different Harvest Stages and Longevity of Floral Stems of Canadian Goldenrod (Solidago canadensis L.). Acta Hortic. 2015, 1060, 225–228. [Google Scholar] [CrossRef]
- Shelepova, O.; Vinogradova, Y.; Vergun, O.; Grygorieva, O.; Brindza, J. Invasive Solidago canadensis L. as a Resource of Valuable Biological Compounds. Potravin. Slovak J. Food Sci. 2019, 13, 280–286. [Google Scholar] [CrossRef] [Green Version]
- Kalemba, D.; Thiem, B. Constituents of the Essential Oils of Four Micropropagated Solidago Species. Flavour Frag. J. 2004, 19, 40–43. [Google Scholar] [CrossRef]
- Shelepova, O.; Vinogradova, Y.; Zaitchik, B.; Ruzhitsky, A.; Grygorieva, O.; Brindza, J. Constituents of the Essential Oil in Solidago canadensis L. from Eurasia. Potravin. Slovak J. Food Sci. 2018, 12, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Benelli, G.; Pavela, R.; Cianfaglione, K.; Nagy, D.U.; Canale, A.; Maggi, F. Evaluation of two Invasive Plant Invaders in Europe (Solidago canadensis And Solidago gigantea) as Possible Sources of Botanical Insecticides. J. Pest Sci. 2019, 92, 805–821. [Google Scholar] [CrossRef]
- Huang, Y.; Bai, Y.; Wang, Y.; Kong, H. Allelopathic Effects of the Extracts from an Invasive Species Solidago Canadensis L. on Microcystis aeruginosa. Lett. Appl. Microbiol. 2013, 57, 451–458. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Gruľová, D.; Baranová, B.; Caputo, L.; De Martino, L.; Sedlák, V.; Camele, I.; De Feo, V. Antimicrobial Activity and Chemical Composition of Essential Oil Extracted from Solidago canadensis L. Growing Wild in Slovakia. Molecules 2018, 24, 1206. [Google Scholar] [CrossRef] [Green Version]
- González-Coloma, A.; Reina, M.; Díaz, C.E.; Fraga, B.; Santana-Méridas, O. Natural Product—Based Biopesticides for Insect Control. Comprehensive Natural Products II. Chem. Mol. Sci. Chem. Eng. 2010, 3, 237–268. [Google Scholar] [CrossRef]
- Synowiec, A.; Kalemba, D.; Drozdek, E.; Bocianowski, J. Phytotoxic Potential of Essential Oils from Temperate Climate Plants Against the Germination of Selected Weeds and Crops. J. Pest Sci. 2017, 90, 407–419. [Google Scholar] [CrossRef]
- Mariychuk, R.; Gruľová, D.; Grishchenko, L.M.; Linni, R.P.; Lisnyak, V.V. Green Synthesis of Non-spherical Gold Nanoparticles Using Solidago canadensis L. extract. Appl. Nanosci. 2020, 10, 4817–4826. [Google Scholar] [CrossRef]
- Baranová, B.; Fazekašová, D.; Manko, P. Variations of Selected Soil Properties in the Grass Fields Invaded and Uninvaded by Invasive Goldenrod (Solidago canadensis L.). Ekológia 2017, 36, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Koco, Š.; Dubravská, A.; Vilček, J.; Grul’ová, D. Geospatial Approaches to Monitoring the Spread of Invasive Species of Solidago spp. Remote Sens. 2021, 13, 4787. [Google Scholar] [CrossRef]
- Reinhart, K.; Gurnee, J.; Tirado, R.; Callaway, R. Invasion through Quantitative Effects: Intense Shade Drives Native Decline and Invasive Success. Ecol. Appl. 2006, 16, 1821–1831. [Google Scholar] [CrossRef]
- Ye, X.Q.; Yan, Y.N.; Wu, M.; Yu, F.H. High Capacity of Nutrient Accumulation by Invasive Solidago Canadensis in a Coastal Grassland. Front. Plant Sci. 2019, 10, 575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.W.; Yang, J.X.; Gao, Y.; He, W.M. Soil Organic Nitrogen Endows Invasive Solidago canadensis with Greater Advantages in Low-Phosphorus Conditions. Ecosphere 2016, 7, e01254. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wang, J.; Qian, B.Y.; Li, W.H. Effects of the Invader Solidago canadensis on Soil Properties. Appl. Soil Ecol. 2009, 43, 163–169. [Google Scholar] [CrossRef]
- Jin, L.; Gu, Y.; Xiao, M.; Chen, J.; Li, B. The History of Solidago canadensis Invasion and the Development of its Mycorrhizal Associations in Newly-Reclaimed Land. Funct. Plant Biol. 2004, 31, 979–986. [Google Scholar] [CrossRef]
- Bongard, C. A Review of the Influence of Root-Associating Fungi and Root Exudates on the Success of Invasive Plants. NeoBiota 2012, 14, 21–45. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Yang, R.; Tang, J.; Yang, H.; Hu, S.; Chen, X. Positive Feedback Between Mycorrhizal Fungi and Plants Influences Plant Invasion Success and Resistance to Invasion. PLoS ONE 2010, 24, e12380. [Google Scholar] [CrossRef] [Green Version]
- Zandi, P.; Barabasz-Krasny, B.; Stachurska-Swakoń, A.; Puła, J.; Możdżeń, K. Allelopathic Effect of Invasive Canadian Goldenrod (Solidago canadensis L.) on Early Growth of Red Clover (Trifolium pratense L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 2060–2071. [Google Scholar] [CrossRef]
- Yuan, L.; Li, J.M.; Yu, F.H.; Oduor, A.M.O.; van Kleunen, M. Allelopathic and Competitive Interactions Between Native and Alien Plants. Biol. Invasions 2021, 23, 3077–3090. [Google Scholar] [CrossRef]
- Dong, L.J.; Sun, Z.K.; Gao, Y.; He, W.M. Two-Year Interactions Between Invasive Solidago canadensis and Soil Decrease its Subsequent Growth and Competitive Ability. J. Plant Ecol. 2015, 8, 617–622. [Google Scholar] [CrossRef] [Green Version]
- Kajzer-Bonk, J.; Szpiłyk, D.; Woyciechowski, M. Invasive Goldenrods Affect Abundance and Diversity of Grassland Ant Communities (Hymenoptera: Formicidae). J. Insect Conserv. 2016, 20, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Grul’ová, D.; Pl’uchtová, M.; Fejér, J.; De Martino, L.; Caputo, L.; Sedlák, V.; De Feo, V. Influence of Six Essential Oils on Invasive Solidago canadensis L. Seed Germination. Nat. Prod. Res. 2020, 34, 3231–3233. [Google Scholar] [CrossRef] [PubMed]
- Tryjanowski, P.; Hartel, T.; Báldi, A.; Szymański, P.; Tobolka, M.; Herzon, I. Conservation of Farmland Birds Faces Different Challenges in Western and Central-Eastern Europe. Acta Ornithol. 2011, 46, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Baranová, B.; Manko, P.; Jászay, T. Differences in Surface-Dwelling Beetles of Grasslands Invaded and Non-Invaded by Goldenrods (Solidago canadensis, S. gigantea) with Special Reference to Carabidae. J. Insect Conserv. 2014, 18, 623–635. [Google Scholar] [CrossRef]
- Stefanic, E.; Puskadija, Z.; Stefanic, I.; Bubalo, D. Goldenrod: A Valuable Plant for Beekeeping in North-Eastern Croatia. Bee World 2003, 84, 88–92. [Google Scholar] [CrossRef]
- Bjerknes, A.; Totland, Ø.; Hegland, S.J.; Neilsen, A. Do Alien Plant Invasion Really Affect Pollination Success in Native Plant Species? Biol. Conserv. 2007, 138, 1–12. [Google Scholar] [CrossRef]
- Skórka, P.; Lenda, M.; Tryjanowski, P. Invasive Alien Goldenrods Negatively Affect Grassland Bird Communities in Eastern Europe. Biol. Conserv. 2010, 143, 856–861. [Google Scholar] [CrossRef]
- Kalarus, K.; Skórka, P.; Nowicki, P. Resources in Two Contrasting Habitat Types Raises Different Challenges for the Conservation of the Dryad Butterfly Minois Dryas. J. Insect Conserv. 2013, 17, 777–786. [Google Scholar] [CrossRef] [Green Version]
- Fiala, K. Partial Monitoring System-Soil: Mandatory Statues, 1st ed.; SSCRI: Bratislava, Slovakia, 1999; pp. 1–139. (In Slovak) [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Palaeontological Statistics Software Package for Education and Data Analysis, PAST Version 2.17c. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]






| Morphological Traits | A Category 75–100% (Heavy Degree of Invasion) | |||||
|---|---|---|---|---|---|---|
| May/June | June/July | August | September | October | ||
| Plant height (cm) | *** | 100.5 ± 14.5 | 139.8 ± 14.9 * | 167.8 ± 20.2 * | 167.5 ± 22.3 | 159.4 ± 30.9 |
| Entire plant weight (g) | * | 35.2 ± 10.1 * | 47.8 ± 16.3 | 55.1 ± 17.1 | 67.3 ± 34.9 | 62.5 ± 22.2 |
| Relative water content of entire plant (%) | 75.6 ± 6.6 | 61.7 ± 3.6 | 58.6 ± 1.3 | 55.9 ± 1.2 | 57.9 ± 2.4 º | |
| Stem length (cm) | *** | 100.5 ± 14.5 | 139.8 ± 14.9 * | 132.7 ± 12.9 * | 131.4 ± 11.6 * | 129.4 ± 24.9 |
| Stem weight (g) | *** | 21.9 ± 6.3 * | 29.3 ± 11.0 * | 19.9 ± 8.6 * | 9.9 ± 1.8 | 12.7 ± 4.8 * |
| Stem relative water content (%) | 72.8 ± 8.3 | 59.2 ± 3.6 | 53.8 ± 1.8 º | 47.9 ± 3.5 | 51.7 ± 1.9 | |
| Number of all leaves | * | 52.4 ± 8.3 | 93.8 ± 7.1 | 76.4 ± 10.4 º | 67.0 ± 8.2 | 62.0 ± 17.7 |
| Weight of all leaves (g) | ** | 12.9 ± 3.9 | 18.2 ± 5.6 | 12.8 ± 3.9 | 11.4 ± 3.6 | 14.3 ± 8.6 |
| Weight of a single leaf (g) | ** | 0.2 ± 0.05 | 0.2 ± 0.06 | 0.2 ± 0.04 | 0.2 ± 0.08 | 0.2 ± 0.12 |
| Relative water content of all leaves (%) | * | 35.2 ± 10.1 * | 47.8 ± 16.3 | 55.1 ± 17.1 | 67.3 ± 34.9 | 62.5 ± 22.2 |
| Number of assimilating, green, leaves | 43.0 ± 8.9 | 70.3 ± 7.3 | 60.9 ± 2.4 | 60.1 ± 5.9 º | 44.9 ± 16.1 | |
| Weight of assimilating, green, leaves (g) | ** | 11.9 ± 3.7 | 16.9 ± 5.6 | 11.9 ± 3.4 | 10.8 ± 3.4 | 12.4 ± 8.6 |
| Weight of a single assimilating, green, leaf (g) | ** | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 |
| Relative water content of assimilating, green, leaves (%) | ** | 75.1 ± 4.0 | 67.9 ± 3.1 | 64.9 ± 3.1 | 65.6 ± 0.8 *** | 66.2 ± 4.5 |
| Number of non-assimilating, brown, leaves | * | 9.3 ± 0.8 | 23.5 ± 5.0 * | 15.6 ± 8.0 | 6.9 ± 3.7 | 17.1 ± 10.6 |
| Weight of non-assimilating, brown, leaves (g) | ** | 0.9 ± 0.4 * | 1.3 ± 0.4 * | 0.8 ± 0.5 | 0.6 ± 0.4 | 1.9 ± 0.9 |
| Weight of single non-assimilating, brown, leaf (g) | 0.1 ± 0.42 | 0.06 ± 0.01 | 0.05 ± 0.02 | 0.09 ± 0.03 | 0.1 ± 0.05 | |
| Relative water content of non-assimilating, brown, leaves (%) | 59.8 ± 16.9 | 36.9 ± 8.8 | 37.3 ± 7.3 | 55.4 ± 12.9 | 50.2 ± 14.3 | |
| Inflorescence length (cm) | - | - | 35.2 ± 7.9 | 36.1 ± 10.9 | 29.9 ± 7.2 | |
| Inflorescence weight (g) | - | - | 11.1 + 4.4 | 22.6 ± 14.9 | 16.9 ± 7.9 | |
| Inflorescence relative water content (%) | * | - | - | 65.5 + 3.1 | 68.8 ± 4.2 * | 64.8 ± 3.3 |
| Stem EO yield (mg/kg) | ** | 0.7 ± 0.8 | 6.2 ± 5.7 | 4.7 ± 4.0 | 12.3 ± 13.8 * | 7.8 ± 4.6 ** |
| Leaf EO yield (mg/kg) | * | 1.9 ± 2.5 | 5.9 ± 6.2 | 3.5 ± 2.9 * | 17.9 ± 10.7 ** | 248.3 ± 29.7 ** |
| Inflorescence EO yield (mg/kg) | - | - | 6.7 ± 9.3 | 20.9 ± 10.4 | 18.5 ± 6.7 *,º | |
| Morphological Traits | B Category 50–75% (Middle Degree of Invasion) | |||||
|---|---|---|---|---|---|---|
| May/June | June/July | August | September | October | ||
| Plant height (cm) | °° | 95.2 ± 8.9 | 121.8 ± 11.6 | 162.9 ± 6.8 ° | 146.9 ± 13.3 | 140.9 ± 22.4 |
| Entire plant weight (g) | 32.3 ± 5.9 ° | 42.0 ± 8.5 | 60.5 ± 13.8 | 59.1 ± 25.5 | 45.3 ± 9.3 | |
| Relative water content of entire plant (%) | 73.2 ± 3.4 | 61.2 ± 2.6 | 56.9 ± 1.8 | 55.9 ± 6.5 | 49.6 ± 6.2 ° | |
| Stem length (cm) | °°° | 95.2 ± 8.9 | 121.8 ± 11.6 | 127.7 ± 3.2 ° | 115.9 ± 10.1 | 110.4 ± 17.3 |
| Stem weight (g) | °°° | 19.5 ± 3.0 ° | 24.3 ± 5.5 | 18.9 ± 2.7 °° | 10.1 + 2.3 | 11.9 ± 6.0 |
| Stem relative water content (%) | 72.8 ± 8.3 | 59.2 ± 3.6 | 53.8 ± 1.8 ° | 47.9 ± 3.5 | 51.7 ± 1.9 | |
| Number of all leaves | °°° | 50.3 ± 8.4 | 89.5 ± 12.4 | 89.2 ± 7.3 °° | 79.8 ± 6.6 ° | 68.4 ± 11.1 |
| Weight of all leaves (g) | °° | 13.1 ± 5.6 ° | 17.2 ± 2.9 | 15.5 ± 1.7 °° | 11.5 ± 3.1 | 8.9 ± 4.1 |
| Weight of a single leaf (g) | 0.3 ± 0.01 °° | 0.2 ± 0.02 ° | 0.2 ± 0.01 | 0.2 ± 0.04 | 0.1 ± 0.04 | |
| Relative water content of all leaves (%) | 32.3 ± 5.9 ° | 42.0 ± 8.5 | 60.5 ± 13.8 | 59.1 ± 25.5 | 45.3 ± 9.3 | |
| Number of assimilating, green, leaves | 44.0 ± 6.9 | 67.6 ± 8.8 | 69.3 ± 3.5 °° | 68.1 ± 3.4 ° | 48.1 ± 23.9 | |
| Weight of assimilating, green, leaves (g) | ° | 12.4 ± 2.7 ° | 15.9 ± 2.9 | 13.9 ± 1.6 °° | 10.5 ± 3.0 | 7.4 ± 4.9 |
| Weight of a single assimilating, green, leaf (g) | 0.3 ± 0.02 °° | 0.2 ± 0.02 ° | 0.2 ± 0.02 | 0.2 ± 0.05 | 0.2 ± 0.04 | |
| Relative water content of assimilating, green, leaves (%) | 75.4 ± 1.4 | 67.1 ± 1.7 | 63.1 ± 1.7 | 61.8 ± 3.4 | 64.2 ± 2.4 | |
| Number of non-assimilating, brown, leaves | °° | 7.2 ± 0.5 | 21.9 ± 4.4 ° | 19.9 ± 9.3 ° | 11.8 ± 9.1 | 20.3 ± 13.9 |
| Weight of non-assimilating, brown, leaves (g) | ° | 0.7 ± 0.3 ° | 1.3 ± 0.1 °° | 1.6 ± 0.9 ° | 1.04 ± 0.7 | 1.5 ± 1.2 |
| Weight of single non-assimilating, brown, leaf (g) | 0.09 ± 0.03 ° | 0.06 ± 0.01 ° | 0.08 ± 0.03 | 0.10 ± 0.03 | 0.07 ± 0.01 | |
| Relative water content of non-assimilating, brown, leaves (%) | 65.9 ± 8.7 | 42.5 ± 8.5 | 44.2 ± 11.4 | 49.5 ± 9.6 | 39.7 ± 1.3 | |
| Inflorescence length (cm) | - | - | 35.2 ± 5.6 | 31.4 ± 7.8 | 30.6 ± 7.3 | |
| Inflorescence weight (g) | - | - | 13.7 ± 8.3 | 18.5 ± 10.2 | 12.1 ± 2.9 | |
| Inflorescence relative water content (%) | - | - | 66.5 ± 2.5 | 64.9 ± 2.5 | 62.4 ± 3.6 | |
| Stem EO yield (mg/kg) | 1.6 ± 2.2 | 11.5 ± 26.1 | 4.7 ± 2.6 | 4.6 ± 3.8 | 8.8 ± 6.5 °° | |
| Leaf EO yield (mg/kg) | 4.4 ± 6.7 | 14.4 ± 31.7 | 11.7 ± 8.3 | 12.1 ± 10.5 | 7.2 ± 5.2 | |
| Inflorescence EO yield (mg/kg) | - | - | 25.2 ± 31.4 | 15.5 ± 11.7 | 9.3 ± 7.8 | |
| Morphological Traits | C Category 25–50% (Mild Degree of Invasion) | ||||
|---|---|---|---|---|---|
| May/June | June/July | August | September | October | |
| Plant height (cm) | 78.9 ± 16.1 | 93.7 ± 31.4 | 123.9 ± 31.4 | 129.7 ± 39.5 | 119.9 ± 35.3 |
| Entire plant’s weight (g) | 19.5 ± 7.1 | 22.7 ± 15.4 | 33.7 ± 22.7 | 52.3 ± 41.2 | 44.5 ± 26.7 |
| Relative water content of entire plant (%) | 70.9 ± 4.7 | 62.6 ± 2.8 | 57.6 ± 2.2 | 60.1 ± 7.9 | 59.1 ± 3.6 |
| Stem length (cm) | 78.9 ± 16.1 | 93.7 ± 31.4 | 95.8 ± 21.3 | 96.2 ± 27.5 | 93.5 ± 25.2 |
| Stem weight (g) | 11.0 ± 5.0 | 12.4 ± 9.3 | 9.2 ± 3.3 | 6.5 ± 4.3 | 5.2 ± 3.4 |
| Stem relative water content (%) | 68.8 ± 6.4 | 58.9 ± 2.9 | 53.2 ± 0.9 | 54.0 ± 16.3 | 51.2 ± 2.3 |
| Number of all leaves | 48.4 ± 3.4 | 70.1 ± 21.0 | 68.6 ± 6.8 | 63.6 ± 7.8 | 60.1 ± 10.9 |
| Weight of all leaves (g) | 8.3 ± 2.1 | 9.9 ± 5.9 | 7.9 ± 3.0 | 9.5 ± 3.0 | 10.7 ± 3.6 |
| Weight of a single leaf (g) | 0.2 ± 0.03 | 0.1 ± 0.03 | 0.1 ± 0.06 | 0.2 ± 0.07 | 0.2 ± 0.05 |
| Relative water content of all leaves (%) | 19.5 ± 7.1 | 22.7 ± 15.4 | 33.7 ± 22.7 | 52.3 ± 41.2 | 44.5 ± 26.7 |
| Number of assimilating, green, leaves | 41.7 ± 2.9 | 59.0 ± 13.5 | 61.9 ± 9.1 | 58.4 ± 5.9 | 46.7 ± 17.8 |
| Weight of assimilating, green, leaves (g) | 8.0 ± 2.1 | 9. 5 ± 5.5 | 7.7 ± 2.9 | 9.1 ± 3.0 | 9.2 ± 4.6 |
| Weight of a single assimilating, green, leaf (g) | 0.2 ± 0.04 | 0.2 ± 0.05 | 0.1 ± 0.07 | 0.2 ± 0.07 | 0.2 ± 0.05 |
| Relative water content of assimilating, green, leaves (%) | 73.8 ± 2.7 | 67.4 ± 3.4 | 61.3 ± 4.8 | 59.3 ± 1.5 | 52.8 ± 14.3 |
| Number of non-assimilating, brown, leaves | 6.6 ± 2.5 | 11.1 ± 7.6 | 6.7 ± 2.5 | 5.1 ± 4.6 | 13.4 ± 7.8 |
| Weight of non-assimilating, brown, leaves (g) | 0.3 ± 0.1 | 0.5 ± 3.8 | 0.3 ± 0.2 | 0.4 ± 0.4 | 4.5 ± 1.9 |
| Weight of single non-assimilating, brown, leaf (g) | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.07 ± 0.03 | 0.09 ± 0.07 |
| Relative water content of non-assimilating, brown, leaves (%) | 52.8 ± 11.2 | 50.9 ± 11.4 | 41.4 ± 10.4 | 43.9 ± 11.3 | 34.7 ± 11.9 |
| Inflorescence length (cm) | - | - | 28.1 ± 11.8 | 33.5 ± 15.7 | 26.4 ± 11.1 |
| Inflorescence weight (g) | - | - | 8.1 ± 8.0 | 17.9 ± 17.4 | 13.9 ± 11.4 |
| Inflorescence relative water content (%) | - | - | 67.3 ± 0.9 | 62.2 ± 2.9 | 51.6 ± 13.7 |
| Stem EO yield (mg/kg) | 1.7 ± 3.0 | 2.5 ± 3.2 | 4.3 ± 4.6 | 3.5 ± 2.0 | 3.3 ± 2.2 |
| Leaf EO yield (mg/kg) | 3.9 ± 3.6 | 3.6 ± 4.0 | 8.1 ± 5.5 | 6.0 ± 8.0 | 3.9 ± 3.1 |
| Inflorescence EO yield (mg/kg) | - | - | 12.1 ± 17.8 | 19.5 ± 28.4 | 8.4 ± 8.3 |
| n. | Category | GPS | r.a. (%) | pH | W (%) | Area (ha) | int. | |
|---|---|---|---|---|---|---|---|---|
| 1 | A | 49°0′30.89″ N | 21°20′6.69″ E | 75–100 | 6.7 | 29.66 | 0.342 | 0 |
| 2 | A | 49°0′18.67″ N | 21°16′13.28″ E | 75–100 | 6.9 | 20.75 | 0.439 | 0 |
| 3 | A | 49°0′50.51″ N | 21°12′43.90″ E | 75–100 | 6.6 | 24.25 | 0.322 | 0 |
| 4 | A | 48°57′1.67″ N | 21°21′19.30″ E | 75–100 | 6.6 | 31.75 | 0.164 | 1 |
| 5 | B | 48°53′46.87″ N | 21°17′55.67″ E | 50–75 | 6.5 | 18.96 | 0.190 | 0 |
| 6 | B | 49°1′56.26″ N | 21°15′13.30″ E | 50–75 | 6.7 | 23.32 | 0.275 | 1 |
| 7 | B | 48°58′0.13″ N | 21°17′2.22″ E | 50–75 | 6.6 | 22.75 | 8.408 | 0 |
| 8 | B | 48°59′33.18″ N | 21°12′38.96″ E | 50–75 | 6.5 | 28.20 | 0.674 | 0 |
| 9 | C | 48°59′34.40″ N | 21°12′53.02″ E | 25–50 | 6.3 | 20,91 | 2.913 | 1 |
| 10 | C | 48°58′12.88″ N | 21°18′58.71″ E | 25–50 | 6.3 | 23.76 | 25.91 | 0 |
| 11 | C | 48°58′4.97″ N | 21°19′25.90″ E | 25–50 | 5.6 | 39.03 | 0.661 | 0 |
| 12 | C | 49°0′26.85″ N | 21°20′13.25″ E | 25–50 | 6.6 | 34.67 | 0.660 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranová, B.; Troščáková-Kerpčárová, E.; Gruľová, D. Survey of the Solidago canadensis L. Morphological Traits and Essential Oil Production: Aboveground Biomass Growth and Abundance of the Invasive Goldenrod Appears to Be Reciprocally Enhanced within the Invaded Stands. Plants 2022, 11, 535. https://doi.org/10.3390/plants11040535
Baranová B, Troščáková-Kerpčárová E, Gruľová D. Survey of the Solidago canadensis L. Morphological Traits and Essential Oil Production: Aboveground Biomass Growth and Abundance of the Invasive Goldenrod Appears to Be Reciprocally Enhanced within the Invaded Stands. Plants. 2022; 11(4):535. https://doi.org/10.3390/plants11040535
Chicago/Turabian StyleBaranová, Beáta, Eva Troščáková-Kerpčárová, and Daniela Gruľová. 2022. "Survey of the Solidago canadensis L. Morphological Traits and Essential Oil Production: Aboveground Biomass Growth and Abundance of the Invasive Goldenrod Appears to Be Reciprocally Enhanced within the Invaded Stands" Plants 11, no. 4: 535. https://doi.org/10.3390/plants11040535