Warmer Temperature and Spatiotemporal Dynamics during Primary Succession on Tropical Coastal Dunes
Abstract
:1. Introduction
2. Results
2.1. Temporal Changes during Succession
2.1.1. Bare Sand, Local Weather, and Plant Cover
2.1.2. Successional Groups
2.1.3. Species Turnover
2.2. Spatial-Temporal Changes during Succession
2.3. Species Interactions: Facilitators and Sand Binders vs. Species Richness
3. Discussion
- (a)
- Temporal trends—As expected during any successional process on coastal dunes, the percentage of bare sand declined over time. We also observed changes in the local weather regime with an increased mean yearly temperature during the last decades. In turn, plant cover and species richness changed in a hump-backed manner. The increment in plant cover and species richness coincided partially with the increased temperature.
- (b)
- Spatial patterns —The facilitator, colonizers, and sand binders grew first on the arms, slope, and crest. In turn, the competitors and nucleators grew on well-developed dune ridges and near the slack, with dense plant cover. In addition, the dispersion index showed that the spatial patterns of plant species distribution were aggregated during different periods of the 25-year successional sequence.
- (c)
- Species interactions —The statistical correlations between the most dominant plant species and species richness was used as an indirect estimation of species interactions. Species richness increased linearly with increasing plant cover of the facilitator, showing indirect evidence of facilitation. In turn, the grassy competitor seemed to inhibit the colonization of new species beyond a threshold plant cover.
3.1. Temporal Trends
3.2. Spatial Patterns
3.3. Successional Groups and Species Interactions
4. Materials and Methods
4.1. Study Site
4.2. Environmental Variables
4.3. Vegetation Sampling
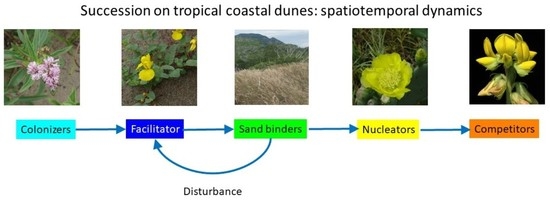
4.4. Successional Groups
| Successional Group | Plant Species | Attributes | Typical Location |
|---|---|---|---|
| Facilitator | Chamaecrista chamaecristoides | Ameliorates harsh conditions | Mobile dunes |
| Colonizers | Palafoxia lindenii, Croton punctatus, Cyperus articulatus, Pappophorum vaginatum | Tolerant to burial by sand | Mobile dunes |
| Sand binders | Schizachyrium scoparium, Trachypogon plumosus, Pectis saturejoides, Aristida adscensionis, Bidens pilosa, Bouteloa repens, Centrosema virginiana, Cnidosculus texanus, Commelina erecta, Euphorbia dioica, Macroptilium atropurpureum, Metastelma pringlei, Porophyllum nummularium, Rhynchelytrum sp., Triplasis purpurea, Walteria indica | Less tolerant to burial; better competitors | Semi-mobile dunes |
| Nucleators | Randia laetevirens Opuntia stricta | Provide shade, organic matter | Stabilized dunes |
| Competitors | Florestina liebmannii Tecoma stans Trixis inula, Amphilopium paniculatum, Cardiospermum halicacabum, Crotalaria incana, Iresine celosia, Mimosa chaetorcarpa, Passiflora foetida, Tecoma stans, Vitis sp. | Efficient competitors, shade tolerance | Stabilized dunes Coastal thickets |
4.5. Data Analyses
4.5.1. Bare Sand, Temperature, Precipitation, Species Richness, and Plant Cover
4.5.2. Successional Sequence and Species Interactions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van der Maarel, E. Dry coastal ecosystems. In Ecosystems of the World; Elsevier: Amsterdam, The Netherlands, 1993; p. 600. [Google Scholar]
- Martínez, M.L.; Castillo-Campos, G.; García-Franco, J.G.; Pérez-Maqueo, O.; Mendoza-González, G.; Pale-Pale, J.J. Mexican coastal dunes: Recipients and donors of alien flora. Diversity 2021, 13, 530. [Google Scholar] [CrossRef]
- Maun, M.A. Adaptations of plants to burial in coastal sand dunes. Can. J. Bot. 1998, 76, 713–738. [Google Scholar]
- Espejel, I.; Jiménez-Orocio, O.; Castillo-Campos, G.; Garcillán, P.P.; Álvarez, L.; Castillo-Argüero, S.; Durán, R.; Ferrer, M.; Infante-Mata, D.; Iriarte, S.; et al. Flora on beaches and coastal sand dunes of Mexico. Acta Bot. Mex. 2017, 121, 39–81. [Google Scholar] [CrossRef] [Green Version]
- Everard, M.; Jones, L.; Watts, B. Have we neglected the societal importance of sand dunes? An ecosystem services perspective. Aquat. Conserv. Mar. Freshw. Ecosyst. 2010, 20, 476–487. [Google Scholar] [CrossRef]
- Mendoza-González, G.; Martínez, M.L.; Lithgow, D.; Pérez-Maqueo, O.; Simonin, P. Land use change and its effects on the value of ecosystem services along the coast of the Gulf of Mexico. Ecol. Econ. 2012, 82, 23–32. [Google Scholar] [CrossRef]
- Martínez, M.L.; Hesp, P.A.; Gallego-Fernández, J.B. Coastal dune restoration: Trends and perspectives. In Restoration of Coastal Dunes. Springer Series on Environmental Management; Martínez, M., Gallego-Fernández, J., Hesp, P., Eds.; Springer: Berlin, Heidelberg, 2013; pp. 323–339. [Google Scholar]
- Martínez, M.L.; Vázquez, G.; Favila, M.E.; Álvarez-Molina, L. Variation in different measures of diversity during primary succession on a tropical coastal dune. Plant Ecol. Divers. 2019, 12, 489–505. [Google Scholar] [CrossRef]
- Álvarez-Molina, L.L.; Martínez, M.L.; Pérez-Maqueo, O.; Gallego-Fernández, J.B.; Flores, P. Richness, diversity, and rate of primary succession over 20 year in tropical coastal dunes. Plant Ecol. 2012, 213, 1597–1608. [Google Scholar] [CrossRef]
- Feagin, R.A.; Wu, X.B. The spatial patterns of functional groups and successional direction in a coastal dune community. Rangel. Ecol. Manag. 2007, 60, 417–425. [Google Scholar] [CrossRef]
- Martínez, M.L.; Vázquez, G.; Sánchez Colón, S. Spatial and tenporal variability during primary succession on tropical coastal sand dunes. J. Veg. Sci. 2001, 12, 361–372. [Google Scholar] [CrossRef]
- Cowles, H.C. The Ecological Relations of the Vegetation on the Sand Dunes of Lake Michigan. Bot. Gaz. 1899, 27, 95–117. [Google Scholar] [CrossRef] [Green Version]
- Feagin, R.A.; Wu, X.B.; Smeins, F.E.; Whisenant, S.G.; Grant, W.E. Individual versus community level processes and pattern formation in a model of sand dune plant succession. Ecol. Modell. 2005, 183, 435–449. [Google Scholar] [CrossRef]
- Martínez, M.L. Facilitation of seedling establisment by an endemic shrub in tropical coastal dunes. Plant Ecol. 2003, 168, 333–345. [Google Scholar] [CrossRef]
- Martínez, M.L.; Pérez-Maqueo, O.; Vásquez, V.M. Facilitative interactions on coastal dunes in response to seasonal weather fluctuations and benefactor size. Ecoscience 2004, 11, 390–398. [Google Scholar] [CrossRef]
- Yarranton, G.A.; Morrison, R.G. Spatial Dynamics of a Primary Succession: Nucleation. J. Ecol. 1974, 62, 417–428. [Google Scholar] [CrossRef]
- Callaway, R.M.; Walker, L.R. Competition and facilitation: A synthetic approach to interactions in plant communities. Ecology 1997, 78, 1958–1965. [Google Scholar] [CrossRef]
- Prach, K.; Ujházy, K.; Knopp, V.; Fanta, J. Two centuries of forest succession, and 30 years of vegetation changes in permanent plots in an inland sand dune area, the Netherlands. PLoS ONE 2021, 16, e0250003. [Google Scholar] [CrossRef]
- Ajedegba, J.O.; Perotto-Baldivieso, H.L.; Jones, K.D. Coastal Dune Vegetation Resilience on South Padre Island, Texas: A Spatiotemporal Evaluation of the Landscape Structure. J. Coast. Res. 2019, 35, 534–544. [Google Scholar] [CrossRef]
- Muñoz-Reinoso, J.C.; Jordán Villafuerte, R.; Tejada-Tejada, M. Analysis of Spatio-Temporal Changes in the Vegetation Cover of the Coastal Landscape of Doñana. J. Coast. Res. 2020, 95, 113–117. [Google Scholar] [CrossRef]
- Salgado, K.; Martínez, M.L.; Álvarez-Molina, L.L.; Hesp, P.; Equihua, M.; Mariño-Tapia, I. Impact of urbanization and landscape changes on the vegetation of coastal dunes along the Gulf of Mexico. Ecoscience 2021, 29, 103–116. [Google Scholar] [CrossRef]
- Sciandrello, S.; Tomaselli, G.; Minissale, P. The role of natural vegetation in the analysis of the spatio-temporal changes of coastal dune system: A case study in Sicily. J. Coast. Conserv. 2015, 19, 199–212. [Google Scholar] [CrossRef]
- Dubroeucq, D.; Geissert, D.; Moreno, P.; Millot, G. Soil evolution and plant communities in coastal dunes near Veracruz, Mexico. Cah. ORSTOM Série Pédol. 1992, 27, 237–250. [Google Scholar]
- Martinez, M.L.; Taramelli, A.; Silva, R. Resistance and Resilience: Facing the Multidimensional Challenges in Coastal Areas. J. Coast. Res. 2017, 77, 1–6. [Google Scholar] [CrossRef]
- Martínez, M.L.; Landgrave, R.; Silva, R.; Hesp, P. Shoreline Dynamics and Coastal Dune Stabilization in Response to Changes in Infrastructure and Climate. J. Coast. Res. 2019, 92, 6–12. [Google Scholar] [CrossRef]
- Borregaard, M.K.; Hendrichsen, D.K.; Nachman, G. Spatial Distribution Patterns; Elsevier: Amsterdam, The Netherlands, 2008; pp. 3304–3310. [Google Scholar]
- Moreno-Casasola, P.; Espejel, I. Classification and ordination of coastal sand dune vegetation along the Gulf and Caribbean Sea of Mexico. Vegetatio 1986, 66, 147–182. [Google Scholar] [CrossRef]
- Jackson, D.W.T.; Costas, S.; González-Villanueva, R.; Cooper, A. A global ‘greening’ of coastal dunes: An integrated consequence of climate change? Glob. Planet. Chang. 2019, 182, 103026. [Google Scholar] [CrossRef]
- Mendes, R.V.; Fonseca Giannini, P.C. Coastal dunefields of south Brazil as a record of climatic changes in the Outh American Monsoon System. Geomorphology 2015, 246, 22–34. [Google Scholar] [CrossRef]
- Miot da Silva, G.; Martinho, C.T.; Hesp, P.; Keim, B.D.; Ferligoj, Y. Changes in dunefield geomorphology and vegetation cover as a response to local and regional climate variations. J. Coast. Res. 2013, 165, 1307–1312. [Google Scholar] [CrossRef]
- Hugenholtz, C.H.; Wolfe, S.A. Recent stabilization of active sand dunes on the Canadian prairies and relation to recent climate variations. Geomorphology 2005, 68, 131–147. [Google Scholar] [CrossRef]
- Tsoar, H.; Levin, N.; Porat, N.; Maia, L.P.; Herrmann, H.J.; Tatumi, S.H.; Claudino-Sales, V. The effect of climate change on the mobility and stability of coastal sand dunes in Ceará State (NE Brazil). Quat. Res. 2009, 71, 217–226. [Google Scholar] [CrossRef]
- Levin, N.; Ben-Dor, E. Monitoring sand dune stabilization along the coastal dunes of Ashdod-Nizanim, Israel, 1945-1999. J. Arid Environ. 2004, 58, 335–355. [Google Scholar] [CrossRef]
- Jackson, D.W.T.; Cooper, J.A.G. Coastal dune fields in Ireland:rapid regional response to climate change. J. Coast. Res. 2011, 293–297. [Google Scholar]
- Provoost, S.; Laurence, M.; Jones, M.; Edmonondson, S.E. Changes in landscape and vegetation of coastal dunes in northwest Europe: A review. J. Coast. Conserv. 2011, 15, 207–226. [Google Scholar] [CrossRef]
- Pye, K.; Blott, S.J.; Howe, M.A. Coastal dune stabilization in Wales and requirements for rejuvenation. J. Coast. Conserv. 2014, 18, 27–54. [Google Scholar] [CrossRef]
- Bachle, S.; Nippert, J.B. Physiological and anatomical trait variability of dominant C4 grasses. Acta Oecologica 2018, 93, 14–20. [Google Scholar] [CrossRef]
- Teeri, J.A.; Stowe, L.G. Climatic patterns and the distribution of C4 grasses in North America. Oecologia 1976, 23, 1–12. [Google Scholar] [CrossRef]
- Sage, R.F.; Monson, R.K.; Ehleringer, J.R.; Adachi, S.; Pearcy, R.W. Some like it hot: The physiological ecology of C4 plant evolution. Oecologia 2018, 187, 941–966. [Google Scholar] [CrossRef]
- WFO. Randia laetevirens Standl. Available online: http://www.worldfloraonline.org/taxon/wfo-0000294553 (accessed on 3 October 2022).
- Llifle Opuntia stricta var. Dillenii. Available online: Encyclopedia/CACTI/Family/Cactaceae/16271/Opuntia_stricta_var._dillenii (accessed on 3 October 2022).
- Moreno-Casasola, P. Patterns of Plant Species Distribution on Coastal Dunes Along the Gulf of Mexico. J. Biogeogr. 1988, 15, 787–806. [Google Scholar] [CrossRef]
- Pakeman, R.J.; Alexander, J.; Beaton, J.; Brooker, R.; Cummins, R.; Eastwood, A.; Fielding, D.; Fisher, J.; Gore, S.; Hewison, R.; et al. Species composition of coastal dune vegetation in Scotland has proved resistant to climate change over a third of a century. Glob. Chang. Biol. 2015, 21, 3738–3747. [Google Scholar] [CrossRef]
- Bar Kutiel, P.; Katz, O.; Ziso-Cohen, V.; Divinsky, I.; Katra, I. Water availability in sand dunes and its implications for the distribution of Artemisia monosperma. Catena 2016, 137, 144–151. [Google Scholar] [CrossRef]
- Moreno-Casasola, P. Ecología de la Vegetación de Dunas Costeras: Factores Físicos; National Autonomous University of Mexico: Mexico City, Mexico, 1982; Volume 61. [Google Scholar]
- Zunzunegui, M.; Esquivias, M.P.; Oppo, F.; Gallego-Fernández, J.B. Interspecific competition and livestock disturbance control the spatial patterns of two coastal dune shrubs. Plant Soil 2012, 354, 299–309. [Google Scholar] [CrossRef]
- Cutler, N.A.; Belyea, L.R.; Dugmore, A.J. The spatiotemporal dynamics of a primary succession. J. Ecol. 2008, 96, 231–246. [Google Scholar] [CrossRef]
- Simpson, E.H. The Interpretation of Interaction in Contingency Tables. J. R. Stat. Soc. Ser. B 1951, 13, 238–241. [Google Scholar] [CrossRef]
- Callaway, R.M.; Pennings, S.C. Facilitation may buffer competitive effects: Indirect and diffuse interactions among salt marsh plants. Am. Nat. 2000, 156, 416–424. [Google Scholar] [CrossRef]
- Valiente-Banuet, A.; Verdú, M. Temporal shifts from facilitation to competition occur between closely related taxa. J. Ecol. 2008, 96, 489–494. [Google Scholar] [CrossRef]
- Wright, A.; Schnitzer, S.A.; Reich, P.B. Living close to your neighbors: The importance of both competition and facilitation in plant communities. Ecology 2014, 95, 2213–2223. [Google Scholar] [CrossRef] [Green Version]
- Michalet, R.; Le Bagousse-Pinguet, Y.; Maalouf, J.P.; Lortie, C.J. Two alternatives to the stress-gradient hypothesis at the edge of life: The collapse of facilitation and the switch from facilitation to competition. J. Veg. Sci. 2014, 25, 609–613. [Google Scholar] [CrossRef]
- Gallego-Fernández, J.B.; Martínez, M.L. Environmental filtering and plant functional types on mexican foredunes along the gulf of Mexico. Ecoscience 2011, 18, 52–62. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties; John Wiley: New York, NY, USA, 2001. [Google Scholar]
- McCune, B.; Mefford, M.J. PC-ORD, version 6.21. In Multivariate Analysis of Ecological Data; MjM Software: Gleneden Beach, OR, USA, 2011. [Google Scholar]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, M.L.; Pérez-Maqueo, O.; Vázquez, G.; Landgrave, R. Warmer Temperature and Spatiotemporal Dynamics during Primary Succession on Tropical Coastal Dunes. Plants 2022, 11, 3029. https://doi.org/10.3390/plants11223029
Martínez ML, Pérez-Maqueo O, Vázquez G, Landgrave R. Warmer Temperature and Spatiotemporal Dynamics during Primary Succession on Tropical Coastal Dunes. Plants. 2022; 11(22):3029. https://doi.org/10.3390/plants11223029
Chicago/Turabian StyleMartínez, M. Luisa, Octavio Pérez-Maqueo, Gabriela Vázquez, and Rosario Landgrave. 2022. "Warmer Temperature and Spatiotemporal Dynamics during Primary Succession on Tropical Coastal Dunes" Plants 11, no. 22: 3029. https://doi.org/10.3390/plants11223029