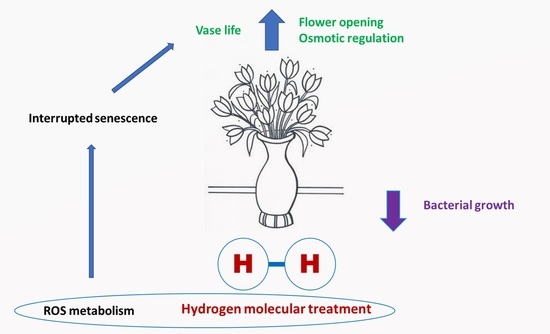
Is It a Challenge to Use Molecular Hydrogen for Extending Flower Vase Life?
Abstract
:1. Introduction
2. The Impact of Hydrogen Solution in Floral Preharvest and Postharvest
3. The Potential Observation Using Hydrogen Tools in Floral Preservative Solution
4. Further Prospects for Hydrogen Treatment in the Floral Industry in Korea
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, W.-B.; Zhang, M.-L.; Yu, J.-H. Role of nitric oxide in delaying senescence of cut rose flowers and its interaction with ethylene. Sci. Hortic. 2013, 155, 30–38. [Google Scholar] [CrossRef]
- In, B.-C.; Seo, J.Y.; Lim, J.H. Preharvest environmental conditions affect the vase life of winter-cut roses grown under different commercial greenhouses. Hortic. Environ. Biotechnol. 2016, 57, 27–37. [Google Scholar] [CrossRef]
- Onozaki, T.; Ikeda, H.; Yamaguchi, T. Genetic improvement of vase life of carnation flowers by crossing and selection. Sci. Hortic. 2001, 87, 107–120. [Google Scholar] [CrossRef]
- Dolan, C.; Humphrey, J. Governance and trade in fresh vegetables: The impact of UK supermarkets on the African horticulture industry. J. Dev. Stud. 2000, 37, 147–176. [Google Scholar] [CrossRef]
- Pompodakis, N.; Terry, L.; Joyce, D.; Lydakis, D.; Papadimitriou, M. Effect of seasonal variation and storage temperature on leaf chlorophyll fluorescence and vase of cut roses. Postharvest Biol. Technol. 2005, 36, 1–8. [Google Scholar] [CrossRef]
- Azad, A.K.; Ishikawa, T.; Ishikawa, T.; Sawa, Y.; Shibata, H. Intracellular energy depletion triggers programmed cell death during petal senescence in tulip. J. Exp. Bot. 2008, 59, 2085–2095. [Google Scholar] [CrossRef]
- Fanourakis, D.; Pieruschka, R.; Savvides, A.; Macnish, A.J.; Sarlikioti, V.; Woltering, E.J. Sources of vase life variation in cut roses: A review. Postharvest Biol. Technol. 2013, 78, 1–15. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Lim, J.H. Do eco-friendly floral preservative solutions prolong vase life better than chemical solutions? Horticulturae 2021, 7, 415. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Jung, Y.O.; Lim, J.H. Tools for cut flower for export: Is it a genuine challenge from growers to customers? Flower Res. J. 2020, 28, 241–249. [Google Scholar] [CrossRef]
- Mohd Rafdi, H.; Joyce, D.; Lisle, A.; Li, X.; Irving, D.; Gupta, M. A retrospective study of vase life determinants for cut Acacia holosericea foliage. Sci. Hortic. 2014, 180, 254–261. [Google Scholar] [CrossRef]
- Arrom, L.; Munné-Bosch, S. Sucrose accelerates flower opening and delays senescence through a hormonal effect in cut lily flowers. Plant Sci. 2012, 188–189, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Zamani, S.; Kazemi, M.; Aran, M. Postharvest life of cut rose flowers as affected by salicylic acid and glutamin. World Appl. Sci. J. 2011, 12, 1621–1624. [Google Scholar]
- Saeed, T.; Hassan, I.; Abbasi, N. Effect of gibberellic acid on the vase life and oxidative activities in senescing cut gladiolus flowers. Plant Growth Regul. 2014, 72, 89–95. [Google Scholar] [CrossRef]
- Fan, H.-m.; Li, T.; Sun, X.; Sun, X.-z.; Zheng, C.-s. Effects of humic acid derived from sediments on the postharvest vase life extension in cut chrysanthemum flowers. Postharvest Biol. Technol. 2015, 101, 82–87. [Google Scholar] [CrossRef]
- Nergi, M.; Ahmadi, N. Effects of 1-MCP and ethylene on postharvest quality and expression of senescence-associated genes in cut rose cv. Sparkle. Sci. Hortic. 2014, 166, 78–83. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.-i.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Xie, Y.; Mao, Y.; Lai, D.; Zhang, W.; Shen, W. H2 Enhances Arabidopsis salt tolerance by manipulating ZAT10/12-mediated antioxidant defence and controlling sodium exclusion. PLoS ONE 2012, 7, e49800. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.; Zhang, M.; Sun, X. Molecular hydrogen is involved in phytohormone signaling and stress responses in plants. PLoS ONE 2013, 8, e71038. [Google Scholar] [CrossRef]
- Wu, Y.; Yuan, M.; Song, J.; Chen, X.; Yang, H. Hydrogen gas from inflammation treatment to cancer therapy. ACS Nano 2019, 13, 8505–8511. [Google Scholar] [CrossRef]
- Fang, H.; Wang, C.; Wang, S.; Liao, W. Hydrogen gas increases the vase life of cut rose ‘Movie star’ by regulating bacterial community in the stem ends. Postharvest Biol. Technol. 2021, 181, 111685. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Wang, S.; Liu, Y.; Zou, J.; Ding, W.; Du, H.; Shen, W. Magnesium hydride acts as a convenient hydrogen supply to prolong the vase life of cut roses by modulating nitric oxide synthesis. Postharvest Biol. Technol. 2021, 177, 111526. [Google Scholar] [CrossRef]
- Kumar, N.; Srivastava, G.C.; Dixit, K. Senescence in rose (Rosa hybrida L.): Role of the endogenous anti-oxidant system. J. Hortic. Sci. Technol. 2008, 83, 125–131. [Google Scholar] [CrossRef]
- van Doorn, W.G.; Woltering, E.J. Physiology and molecular biology of petal senescence. J. Exp. Bot. 2008, 59, 453–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naing, A.H.; Lee, K.; Arun, M.; Lim, K.B.; Kim, C.K. Characterization of the role of sodium nitroprusside (SNP) involved in long vase life of different carnation cultivars. BMC Plant Biol. 2017, 17, 149. [Google Scholar] [CrossRef]
- Ren, P.-J.; Jin, X.; Liao, W.-B.; Wang, M.; Niu, L.-J.; Li, X.-P.; Xu, X.-T.; Zhu, Y.-C. Effect of hydrogen-rich water on vase life and quality in cut lily and rose flowers. Hortic. Environ. Biotechnol. 2017, 58, 576–584. [Google Scholar] [CrossRef]
- Su, J.; Nie, Y.; Zhao, G.; Cheng, D.; Wang, R.; Chen, J.; Zhang, S.; Shen, W. Endogenous hydrogen gas delays petal senescence and extends the vase life of lisianthus cut flowers. Postharvest Biol. Technol. 2019, 147, 148–155. [Google Scholar] [CrossRef]
- Wang, C.; Fang, H.; Gong, T.; Zhang, J.; Niu, L.; Huang, D.; Huo, J.; Liao, W. Hydrogen gas alleviates postharvest senescence of cut rose ‘Movie star’ by antagonizing ethylene. Plant Mol. Biol. 2020, 102, 271–285. [Google Scholar] [CrossRef]
- Huo, J.; Huang, D.; Zhang, J.; Fang, H.; Wang, B.; Wang, C.; Ma, Z.; Liao, W. Comparative proteomic analysis during the involvement of nitric oxide in hydrogen gas-improved postharvest freshness in cut lilies. Int. J. Mol. Sci. 2018, 19, 3955. [Google Scholar] [CrossRef] [Green Version]
- Ha, S.T.T.; Lim, J.H.; In, B.-C. Extension of the vase life of cut roses by both improving water relations and repressing ethylene responses. J. Hortic. Sci. Technol. 2019, 37, 65–77. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yin, Q.; Zhang, T.; Cheng, P.; Xu, S.; Shen, W. Hydrogen nanobubble water delays petal senescence and prolongs the vase life of cut carnation (Dianthus caryophyllus L.) flowers. Plants 2021, 10, 1662. [Google Scholar] [CrossRef]
- Hu, H.; Li, P.; Shen, W. Preharvest application of hydrogen-rich water not only affects daylily bud yield but also contributes to the alleviation of bud browning. Sci. Hortic. 2021, 287, 110267. [Google Scholar] [CrossRef]
- Zhu, Y.; Liao, W. The metabolic constituent and rooting-related enzymes responses of marigold explants to hydrogen gas during adventitious root development. Theor. Exp. Plant Physiol. 2017, 29, 77–85. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Wang, S.; Zou, J.; Ding, W.; Shen, W. Magnesium hydride-mediated sustainable hydrogen supply prolongs the vase life of cut carnation flowers via hydrogen sulfide. Front. Plant Sci. 2020, 11, 595376. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.T.T.; Kim, Y.-T.; Jeon, Y.H.; Choi, H.W.; In, B.-C. Regulation of Botrytis cinerea infection and gene expression in cut roses by using nano silver and salicylic acid. Plants 2021, 10, 1241. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.T.T.; Jung, Y.-O.; Lim, J.-H. Pretreatment with Scutellaria baicalensis Georgi extract improves the postharvest quality of cut roses (Rosa hybrida L.). Hortic. Environ. Biotechnol. 2020, 61, 511–524. [Google Scholar] [CrossRef]
- Reid, M. Handling of Cut Flowers for Export. 2009. Available online: https://ucanr.edu/sites/Postharvest_Technology_Center_/files/231308.pdf (accessed on 3 May 2022).
- Hancock, J.T.; Russell, G. Downstream signaling from molecular hydrogen. Plants 2021, 10, 367. [Google Scholar] [CrossRef]
- Cai, M.; Du, H.-m. Effects of hydrogen-rich water pretreatment on vase life of carnation (Dianthus caryophyllus) cut flowers. J. Shanghai Jiaotong Univ. (Agric. Sci.) 2015, 33, 41–45. [Google Scholar]
- Temesgen, T.; Bui, T.T.; Han, M.; Kim, T.-i.; Park, H. Micro and nanobubble technologies as a new horizon for water-treatment techniques: A review. Adv. Colloid Interface Sci. 2017, 246, 40–51. [Google Scholar] [CrossRef]
- Li, L.; Zeng, Y.; Cheng, X.; Shen, W. The applications of molecular hydrogen in horticulture. Horticulturae 2021, 7, 513. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, H.; Huo, J.; Huang, D.; Wang, B.; Liao, W. Involvement of calcium and calmodulin in nitric oxide-regulated senescence of cut lily flowers. Front. Plant Sci. 2018, 9, 1284. [Google Scholar] [CrossRef]
- Lee, Y.B.; Kim, W.S. Improving vase life and keeping quality of cut rose flowers using a chlorine dioxide and sucrose holding solution. Hortic. Sci. Technol. 2018, 36, 380–387. [Google Scholar] [CrossRef]
- Hancock, J.T.; LeBaron, T.W.; May, J.; Thomas, A.; Russell, G. Molecular hydrogen: Is this a viable new treatment for plants in the UK? Plants 2021, 10, 2270. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Park, P.H.; Jung, J.A.; Park, K.Y.; Suh, J.-N.; Kwon, O.K.; Yoo, B.S.; Lee, S.Y.; Park, P.M.; Choi, Y.J.; et al. Achievement of flower breeding in Korea and its prospects. Korean J. Breed. Sci. 2020, 52, 161–169. (In Korean) [Google Scholar] [CrossRef]
- Hu, H.; Zhao, S.; Li, P.; Shen, W. Hydrogen gas prolongs the shelf life of kiwifruit by decreasing ethylene biosynthesis. Postharvest Biol. Technol. 2018, 135, 123–130. [Google Scholar] [CrossRef]
- Singh, S.; Jain, S.; Ps, V.; Tiwari, A.K.; Nouni, M.R.; Pandey, J.K.; Goel, S. Hydrogen: A sustainable fuel for future of the transport sector. Renew. Sustain. Energy Rev. 2015, 51, 623–633. [Google Scholar] [CrossRef]


| Hydrogen Forms | Floral Treatments |
Utilization
Treatment Parameters | Results | References |
|---|---|---|---|---|
| Hydrogen rich water (HRW) | Cut rose (Rosa hybrida ‘Movie star’) Daylily (Hemerocallis fulva L.) cultivar ‘Dawuzui’ Marigold (Tagetes erecta L.) explants | 1% HRW Preharvest: 0.8 μmol L−1 H2 50% HRW | Development of beneficial bacteria abundances on the stem-end cut surface. Improvement of yield and quality. Induced root development | [20] [31] [32] |
| Hydrogen nanobubble water(HNW) | Carnation (Dianthus caryophyllus L.) cultivar ‘Pink Diamond’ | 5% HNW | Development of the effective concentration and residence time of H2 in water for extending vase life. | [30] |
| Magnesium hydride(MgH2) | Carnation (Dianthus caryophyllus L.) cultivar ‘Pink Diamond’ Cut rose (Rosa hybrida ‘Carola’) | MgH2 (0.1 g L–1) with citrate MgH2 (0.001 g L–1) with H2-releasing donor | MgH2-prolonged vase life of cut carnation flowers via increasing GST expression. Re-establishing redox homeostasis to extend vase life | [33] [21] |
| Flower Investigation | Treatment | Result | Reference |
|---|---|---|---|
| Rose ‘Movie star’ | 1% HRW (best in 0.00235 mM H2) | Less flower senescence. Investigated by ethylene metabolism. | [27] |
| Lily (Lilium spp.) and rose (Rosa hybrid L.) | Lily: 0.5% HRW (2.25 µM H2) and 1% (4.5 µM H2); Rose: 50% HRW (0.225 mM H2) | Extended vase life. Greater flower diameter. Reduced oxidative stress. | [25] |
| Lily (Lilium ‘Manissa’) | 1% HRW (0.0022 mM H2) and 150 μM sodium nitroprusside (SNP) | Improved flower freshness. ATP synthase CF1 alpha subunit (AtpA) up-regulated. | [28] |
| Lisianthus (Eustoma grandiflorum) | HRW (0.078 mM H2) | Vase life prolonged. Redox maintained as reducing oxidative stress. | [26] |
| Carnation (Dianthus caryophyllus L.). | Hydrogen nanobubble water (5% HNW): best in 0.025 mM H2 | Less senescence leading to prolonged vase life. Minimized oxidative stress. | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.K.; Lim, J.H. Is It a Challenge to Use Molecular Hydrogen for Extending Flower Vase Life? Plants 2022, 11, 1277. https://doi.org/10.3390/plants11101277
Nguyen TK, Lim JH. Is It a Challenge to Use Molecular Hydrogen for Extending Flower Vase Life? Plants. 2022; 11(10):1277. https://doi.org/10.3390/plants11101277
Chicago/Turabian StyleNguyen, Toan Khac, and Jin Hee Lim. 2022. "Is It a Challenge to Use Molecular Hydrogen for Extending Flower Vase Life?" Plants 11, no. 10: 1277. https://doi.org/10.3390/plants11101277