Pharmacological Efficacy of Tamarix aphylla: A Comprehensive Review
Abstract
:1. Introduction
2. Material Methods
3. Ethnopharmacology: Traditional Practices
4. Phytochemistry
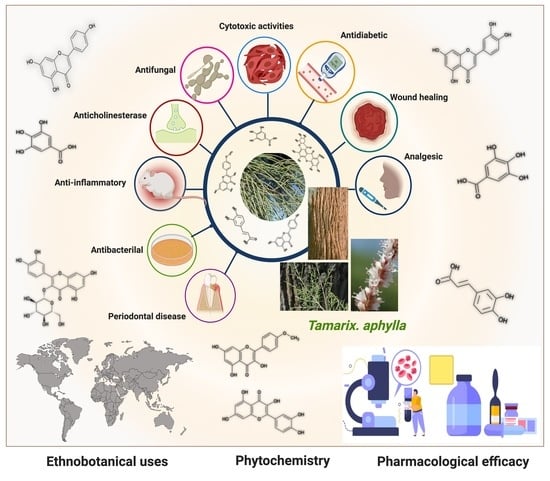
5. Pharmacological Efficacy of Tamarix aphylla
5.1. Anti-Inflammatory Activity
5.2. Wound-Healing Activity
5.3. Antibacterial Activity
5.4. Antifungal Activity
5.5. Periodontal Disease
5.6. Anticholinesterase Activity
5.7. Analgesic Activity
5.8. Antidiabetic Activity
5.9. Cytotoxic Activity
6. Toxicity
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Narendhirakannan, R.T.; Subramanian, S.; Kandaswamy, M. Biochemical evaluation of antidiabetogenic properties of some commonly used Indian plants on streptozotocin-induced diabetes in experimental rats. Clin. Exp. Pharmacol. Physiol. 2006, 33, 1150–1157. [Google Scholar] [CrossRef]
- Hussain, A.; Ahmad, M.; Wahab, S.; Hussain, M.; Ali, M. A review on pharmacological and phytochemical profile of Asparagus racemosus Willd. Pharmacologyonline 2011, 3, 1353–1364. [Google Scholar]
- Alsayari, A.; Muhsinah, A.B.; Almaghaslah, D.; Annadurai, S.; Wahab, S. Pharmacological efficacy of ginseng against respiratory tract infections. Molecules 2021, 26, 4095. [Google Scholar] [CrossRef]
- Ali, M.; Alhazmi, H.A.; Ansari, S.H.; Hussain, A.; Ahmad, S.; Alam, M.S.; Ali, M.S.; El-Sharkawy, K.A.; Hakeem, K.R. Tamarix aphylla (L.) Karst. Phytochemical and Bioactive Profile Compilations of Less Discussed but Effective Naturally Growing Saudi Plant. In Plant and Human Health, Volume 3; Springer: Cham, Switzerland, 2019; Volume 3, pp. 343–352. ISBN 9783030044084. [Google Scholar]
- Ahmad, M.F.; Ahmad, F.A.; Ashraf, S.A.; Saad, H.H.; Wahab, S.; Khan, M.I.; Ali, M.; Mohan, S.; Hakeem, K.R.; Athar, M.T. An updated knowledge of Black seed (Nigella sativa Linn.): Review of phytochemical constituents and pharmacological properties. J. Herb. Med. 2021, 25, 100404. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Ahmad, I.; Irfan, S.; Siddiqua, A.; Usmani, S.; Ahmad, M.P. Pharmacological Efficacy and Safety of Glycyrrhiza glabra in the treatment of respiratory tract infections. Mini-Rev. Med. Chem. 2021, 21. [Google Scholar] [CrossRef]
- Pradeepa, S.; Subramanian, S.; Kaviyarasan, V. Biochemical evaluation of antidiabetic properties of Pithecellobium dulce fruits studied in streptozotocin induced experimental diabetic rats. Int. J. Herb. Med. 2013, 1, 21–28. [Google Scholar]
- Ullah, R.; Tariq, S.A.; Khan, N.; Sharif, N.; Ud Din, Z.; Mansoor, K. Antihyperglycemic effect of methanol extract of Tamarix aphylla L. Karst (Saltcedar) in streptozocin–nicotinamide induced diabetic rats. Asian Pac. J. Trop. Biomed. 2017, 7, 619–623. [Google Scholar] [CrossRef]
- Gaskin, J.F. Tamaricaceae. In Flowering Plants Dicotyledons; Springer: Berlin/Heidelberg, Germany, 2003; pp. 363–368. [Google Scholar]
- Baum, B.R. The Genus Tamarix.—Jerusalem: The Israel Academy of Sciences and Humanities; Publications Jerusalem; Israel Academy of Sciences and Humanities: West Jerusalem, Israel, 1978. [Google Scholar]
- Alnuqaydan, A.M.; Rah, B. Tamarix articulata (T. articulata)—An Important Halophytic Medicinal Plant with Potential Pharmacological Properties. Curr. Pharm. Biotechnol. 2019, 20, 285–292. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Kalkhorani, M.; Abbas Zaidi, S.M.; Farzaei, M.H.; Rahimi, R. The genus Tamarix: Traditional uses, phytochemistry, and pharmacology. J. Ethnopharmacol. 2020, 246, 112245. [Google Scholar] [CrossRef]
- Samadi, N.; Ghaffari, S.M.; Akhani, H. Meiotic behaviour, karyotype analyses and pollen viability in species of Tamarix (Tamaricaceae). Willdenowia 2013, 43, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Mustafa Akhlaq, A.M. New phenolic acids from the galls of Tamarix aphylla (L.) Karst. Int. Res. J. Pharm. 2011, 2, 222–225. [Google Scholar]
- Barakat, H.H.; Nada, S.A. Chemical and Biological Investigations of the Constitutive Phenolics of Two Egyptian Folk-Medicinal Plants; A Novel Phenolic from the Galls of Tamarix aphylla. Nat. Prod. Sci. 1996, 2, 96–101. [Google Scholar]
- Nawwar, M.A.; Ayoub, N.A.; El-Rai, M.A.; Bassyouny, F.; Mostafa, E.S.; Al-Abd, A.M.; Harms, M.; Wende, K.; Lindequist, U.; Linscheid, M.W. Cytotoxic ellagitannins from Reaumuria vermiculata. Fitoterapia 2012, 83, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Mahfoudhi, A.; Prencipe, F.P.; Mighri, Z.; Pellati, F. Metabolite profiling of polyphenols in the tunisian plant tamarix aphylla (L.) Karst. J. Pharm. Biomed. Anal. 2014, 99, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Nawwar, M.A.M.M.; Hussein, S.A.M.M.; Ayoub, N.A.; Hofmann, K.; Linscheid, M.; Harms, M.; Wende, K.; Lindequist, U. Aphyllin, the first isoferulic acid glycoside and other phenolics from Tamarix aphylla flowers. ChemInform 2009, 40, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Majumder, P.; Paridhavi, M. A comprehensive ethno-phyto-pharmacological review on novel Indian medicinal plants used in polyherbal formulations. Int. J. Phytomed. 2013, 5, 394–414. [Google Scholar]
- Ahmad, M.; Zafar, M.; Sultana, S. Salvadora persica, Tamarix aphylla and Zizyphus mauritiana-Three Woody Plant Species Mentioned in Holy Quran and Ahadith and Their Ethnobotanical Uses in North Western Part (D.I. Khan) of Pakistan. Pak. J. Nutr. 2009, 8, 542–547. [Google Scholar] [CrossRef] [Green Version]
- Qadir, M.I.; Abbas, K.; Hamayun, R.; Ali, M. Analgesic, anti-inflammatory and anti-pyretic activities of aqueous ethanolic extract of Tamarix aphylla L. (Saltcedar) in mice. Pak. J. Pharm. Sci. 2014, 27, 1985–1988. [Google Scholar] [PubMed]
- Laaroussi, I. Natural Product Temporarily Tightening the Mucous Membranes of the Vagina. Patent Application No. WO/2012/052627, 26 April 2012. [Google Scholar]
- Marwat, S.K.; Rehman, F.U.; Khan, M.A.; Ahmad, M.; Zafar, M.; Ghulam, S. Medicinal folk recipes used as traditional phytotherapies in district Dera Ismail Khan, KPK, Pakistan. Pak. J. Bot. 2011, 43, 1453–1462. [Google Scholar]
- Ullah, S.; Rashid Khan, M.; Ali Shah, N.; Afzal Shah, S.; Majid, M.; Asad Farooq, M. Ethnomedicinal plant use value in the Lakki Marwat District of Pakistan. J. Ethnopharmacol. 2014, 158, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yin, L.; Pan, B. Biological and ecological characteristics ofTamarix L. and its effect on the ecological environment. Sci. China Ser. D Earth Sci. 2002, 45, 18–22. [Google Scholar] [CrossRef]
- Umair, M.; Altaf, M.; Bussmann, R.W.; Abbasi, A.M. Ethnomedicinal uses of the local flora in Chenab riverine area, Punjab province Pakistan. J. Ethnobiol. Ethnomed. 2019, 15, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalid, M.; Hassani, D.; Bilal, M.; Butt, Z.A.; Hamayun, M.; Ahmad, A.; Huang, D.; Hussain, A. Identification of oral cavity biofilm forming bacteria and determination of their growth inhibition by Acacia arabica, Tamarix aphylla L. and Melia azedarach L. medicinal plants. Arch. Oral Biol. 2017, 81, 175–185. [Google Scholar] [CrossRef]
- Uzair, M.; Ijaz, A.S.; Khan, T.R. Survey of Ethno-Medicinal Weeds of District Rajhan Pur. Indian Res. J. Pharm. Sci. 2014, 1, 38–45. [Google Scholar]
- Suleiman, M.H.A. Ethnobotanical, Phytochemical, and Biological Study of Tamarix aphylla and Aerva javanica Medicinal Plants Growing in the Asir Region, Saudi Arabia. Trop. Conserv. Sci. 2019, 12, 1940082919869480. [Google Scholar] [CrossRef] [Green Version]
- Kamal, M.; Wazir, S.M.; Hassan, M.; Saad, M.S.; Khan, U.; Muhammad, A.; Taj, S. Ethnobotanically Important Plants of District Bannu, Pakistan. J. Plant Sci. 2009, 15, 87–93. [Google Scholar]
- Eddouks, M.; Maghrani, M.; Lemhadri, A.; Ouahidi, M.-L.; Jouad, H. Ethnopharmacological survey of medicinal plants used for the treatment of diabetes mellitus, hypertension and cardiac diseases in the south-east region of Morocco (Tafilalet). J. Ethnopharmacol. 2002, 82, 97–103. [Google Scholar] [CrossRef]
- Merzouki, A.; Ed-derfoufi, F.; Molero Mesa, J. Contribution to the knowledge of Rifian traditional medicine. II: Folk medicine in Ksar Lakbir district (NW Morocco). Fitoterapia 2000, 71, 278–307. [Google Scholar] [CrossRef]
- Pittler, M. A Guide to Medicinal Plants. Focus Altern. Complementary Ther. 2010, 14, 354. [Google Scholar] [CrossRef]
- Tahraoui, A.; El-Hilaly, J.; Israili, Z.H.; Lyoussi, B. Ethnopharmacological survey of plants used in the traditional treatment of hypertension and diabetes in south-eastern Morocco (Errachidia province). J. Ethnopharmacol. 2007, 110, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Al-Fatimi, M. Ethnobotanical survey of medicinal plants in central Abyan governorate, Yemen. J. Ethnopharmacol. 2019, 241, 111973. [Google Scholar] [CrossRef]
- Shah, A.; Rahim, S.; Bhatti, K.H.; Khan, A.; Din, N.; Imran, M.; Mohsin, M.; Ishtiaq, M.; Nabila, A.; Ansari, A.; et al. Ethnobotanical study and conservation status of trees in the district Sargodha, Punjab, Pakistan. Phyton 2015, 84, 34–44. [Google Scholar] [CrossRef]
- Bahadur, S.; Khan, M.S.; Shah, M.; Shuaib, M.; Ahmad, M.; Zafar, M.; Begum, N.; Gul, S.; Ashfaq, S.; Mujahid, I.; et al. Traditional usage of medicinal plants among the local communities of Peshawar valley, Pakistan. Acta Ecol. Sin. 2020, 40, 1–29. [Google Scholar] [CrossRef]
- Saeed Khattak, N.; Nouroz, F.; Ur Rahman, I.; Noreen, S. Ethno veterinary uses of medicinal plants of district Karak, Pakistan. J. Ethnopharmacol. 2015, 171, 273–279. [Google Scholar] [CrossRef]
- Yaseen, G.; Ahmad, M.; Zafar, M.; Sultana, S.; Kayani, S.; Cetto, A.A.; Shaheen, S. Traditional management of diabetes in Pakistan: Ethnobotanical investigation from Traditional Health Practitioners. J. Ethnopharmacol. 2015, 174, 91–117. [Google Scholar] [CrossRef] [PubMed]
- Hammiche, V.; Maiza, K. Traditional medicine in Central Sahara: Pharmacopoeia of Tassili N’ajjer. J. Ethnopharmacol. 2006, 105, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Alzweiri, M.; Al Sarhan, A.; Mansi, K.; Hudaib, M.; Aburjai, T. Ethnopharmacological survey of medicinal herbs in Jordan, the Northern Badia region. J. Ethnopharmacol. 2011, 137, 27–35. [Google Scholar] [CrossRef]
- Brock, J.H. Tamarix spp. (Salt Cedar), an invasive exotic woody plant in arid and semi-arid riparian habitats of western USA. Ecol. Manag. Invasive Riverside Plants 1994, 4, 27–44. [Google Scholar]
- Horton, J.S. Notes on the Introduction of Deciduous Tamarisk. Available online: https://gifiloqokawom.pdfleadership.icu/notes-on-the-introduction-of-deciduous-tamarisk-book-21438vb.php (accessed on 9 August 2021).
- Jasiem, T.M.; Nasser, N.M.; Al-Bazaz, H.K. Tamarix aphylla L.: A review. Res. J. Pharm. Technol. 2019, 12, 3219–3222. [Google Scholar] [CrossRef]
- Nawwar, M.A.M.; Hussein, S.A.M. Gall polyphenolics of Tamarix aphylla. Phytochemistry 1994, 36, 1035–1037. [Google Scholar] [CrossRef]
- Nag, G.; De, B. Acetylcholinesterase inhibitory activity of Terminalia chebula, Terminalia bellerica and Emblica officinalis and some phenolic compounds. Int. J. Pharm. Pharm. Sci. 2011, 3, 121–124. [Google Scholar]
- Sharma, S.K.; Parmar, V.S.; Shanna, S.K.; Parmar, V.S. Novel constituents of Tamarix species. J. Sci. Ind. Res. 1998, 57, 873–890. [Google Scholar]
- Souliman, A.M.A.; Barakat, H.H.; El-Mousallamy, A.M.D.; Marzouk, M.S.A.; Nawwar, M.A.M. Phenolics from the bark ofTamarix aphylla. Phytochemistry 1991, 30, 3763–3766. [Google Scholar] [CrossRef]
- Nawwar, M.A.M.; Hussein, S.A.M.; Buddrus, J.; Linscheid, M. Tamarixellagic acid, an ellagitannin from the galls of Tamarix aphylla. Phytochemistry 1994, 35, 1349–1354. [Google Scholar] [CrossRef]
- Shafaghat, A. Phytochemical investigation of quranic fruits and plants. J. Med. Plants 2010, 9, 61–66. [Google Scholar]
- Orabi, M.A.A.; Taniguchi, S.; Sakagami, H.; Yoshimura, M.; Yoshida, T.; Hatano, T. Hydrolyzable tannins of tamaricaceous plants. V. Structures of monomeric-trimeric tannins and cytotoxicity of macrocyclic-type tannins isolated from tamarix nilotica (1). J. Nat. Prod. 2013, 76, 947–956. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Falleh, H.; Trabelsi, N.; Boulaaba, M.; Smaoui, A.; Abdelly, C. Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. Comptes Rendus—Biol. 2008, 331, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Ishak, M.S.; El-Sissi, H.I.; El-Sherbieny, A.E.; Nawwar, M.A. Tannins and polyphenolics of the galls of Tamarix aphylla. II. Planta Med. 1972, 21, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Mahfoudhi, A.; Grosso, C.; Gonçalves, R.F.; Khelifi, E.; Hammami, S.; Achour, S.; Trabelsi-Ayadi, M.; Valentão, P.; Andrade, P.B.; Mighri, Z. Evaluation of Antioxidant, Anticholinesterase, and Antidiabetic Potential of Dry Leaves and Stems in Tamarix aphylla Growing Wild in Tunisia. Chem. Biodivers. 2016, 13, 1747–1755. [Google Scholar] [CrossRef]
- Baaka, N.; Mahfoudhi, A.; Haddar, W.; Mhenni, M.F.; Mighri, Z. Green dyeing process of modified cotton fibres using natural dyes extracted from Tamarix aphylla (L.) Karst. leaves. Nat. Prod. Res. 2017, 31, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Orfali, R.S.; Ebada, S.S.; El-Shafae, A.M.; Al-Taweel, A.M.; Lin, W.H.; Wray, V.; Proksch, P. 3-O-trans-caffeoylisomyricadiol: A new triterpenoid from Tamarix nilotica growing in Saudi Arabia. Z. Nat.—Sect. C J. Biosci. 2009, 64, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Nawwar, M.A.M.; El Sherbeiny, A.E.A.; El Ansari, M.A. Plant constitutents ofTamarix aphylla flowers (Tamaricaceae). Experientia 1975, 31, 1118. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [Green Version]
- Wahab, S.; Ahmad, M.F.; Hussain, A.; Usmani, S.; Shoaib, A.; Ahmad, W. Effectiveness of Azithromycin as add-on Therapy in COVID-19 Management. Mini-Rev. Med. Chem. 2021, 21, 2860–2873. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Clevers, H. Reparative inflammation takes charge of tissue regeneration. Nature 2016, 529, 307–315. [Google Scholar] [CrossRef]
- Leonardi, G.C.; Accardi, G.; Monastero, R.; Nicoletti, F.; Libra, M. Ageing: From inflammation to cancer. Immun. Ageing 2018, 15, 1. [Google Scholar] [CrossRef]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis. 2018, 9, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadallah, A.S.; Mujeeb-Ur-Rehman; Atta-Ur-Rahman; Yousuf, S.; Atia-Tul-Waha; Jabeen, A.; Swilam, M.M.; Khalifa, S.A.M.; El-Seedi, H.R.; Iqbal Choudhary, M. Anti-inflammatory principles from Tamarix aphylla L.: A bioassay-guided fractionation study. Molecules 2020, 25, 2994. [Google Scholar] [CrossRef]
- Alsayari, A.; Wahab, S. Genus Ziziphus for the treatment of chronic inflammatory diseases. Saudi J. Biol. Sci. 2021, 28, 6897–6914. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; DiPietro, L.A. Critical review in oral biology & medicine: Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Gallelli, G.; Cione, E.; Serra, R.; Leo, A.; Citraro, R.; Matricardi, P.; Di Meo, C.; Bisceglia, F.; Caroleo, M.C.; Basile, S.; et al. Nano-hydrogel embedded with quercetin and oleic acid as a new formulation in the treatment of diabetic foot ulcer: A pilot study. Int. Wound J. 2020, 17, 485–490. [Google Scholar] [CrossRef]
- Pereira, R.F.; Bártolo, P.J. Traditional Therapies for Skin Wound Healing. Adv. Wound Care 2016, 5, 208–229. [Google Scholar] [CrossRef] [Green Version]
- Abbas, B.; Al-Qarawi, A.A.; Al-Hawas, A. The ethnoveterinary knowledge and practice of traditional healers in Qassim region, Saudi Arabia. J. Arid. Environ. 2002, 50, 367–379. [Google Scholar] [CrossRef]
- Sajid Ali, M.; Sarfaraz Alam, M.; Ahmad, S.; Ali, M.; Ahsan, W.; Siddiqui, M.R.; Salahuddin Ansari, M.; Shamim, M.; Ali, M.D. Wound healing activity of alcoholic extract of Tamarixaphylla L. On animal models. Biomed. Pharmacol. J. 2019, 12, 41–48. [Google Scholar] [CrossRef]
- Soliman Yu, H.; Ibrahim Al, S. Anti-inflammatory and Wound Healing Activities of Herbal Gel Containing an Antioxidant Tamarix aphylla Leaf Extract. Int. J. Pharmacol. 2011, 7, 829–835. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.; Rizvi, A.; Wahab, S.; Ansari, S.; Hussain, S.; Zareen, I. Antibacterial Screening of the Bark of Adenanthera pavonina (L.). Int. J. Biomed. Res. 2011, 2, 110–122. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.; Wahab, S.; Zarin, I.; Hussain, M.D.S. Antibacterial Activity of the Leaves of Coccinia indica (W. and A) Wof India. Biol. Res. 2010, 4, 241–248. [Google Scholar]
- Ahmad, M.D.F.; Wahab, S.; Ali Ahmad, F.; Intakhab Alam, M.; Ather, H.; Siddiqua, A.; Amir Ashraf, S.; Abu Shaphe, M.; Idreesh Khan, M.; Ali Beg, R. A novel perspective approach to explore pros and cons of face mask in prevention the spread of SARS-CoV-2 and other pathogens. Saudi Pharm. J. 2021, 29, 121–133. [Google Scholar] [CrossRef]
- El-Zayat, M.M.; Eraqi, M.M.; Alfaiz, F.A.; Elshaer, M.M. Antibacterial and antioxidant potential of some Egyptian medicinal plants used in traditional medicine. J. King Saud Univ.-Sci. 2021, 33, 101466. [Google Scholar] [CrossRef]
- Verma, R.; Pavithra, P.; Janani, V.; Charumathi, K.; Indumathy, R.; Potala, S. Antibacterial activity of plants used in Indian herbal medicine. Int. J. Green Pharm. 2010, 4, 22. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Adnan, M.; Tariq, A.; Bibi, R.; AbdElsalam, N.; Rehman, H.; Murad, W.; Ahmad, S.; Israr, M.; Sabahat, S.; Ullah, R.; et al. Antimicrobial potential of alkaloids and flavonoids extracted from Tamarix aphylla leaves against common human pathogenic bacteria. Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 27. [Google Scholar] [CrossRef] [Green Version]
- Taghipour, M.T.; Nameni, R.; Taghipour, M.; Ghorat, F. Phytochemical Analysis and Antimicrobial Activity of Ziziphus spina-christi and Tamarix aphylla Leaves’ Extracts as Effective Treatment for Coronavirus Disease 2019 (COVID-19). Thrita 2020, 9, e107776. [Google Scholar] [CrossRef]
- Meot-Duros, L.; Le Floch, G.; Magné, C. Radical scavenging, antioxidant and antimicrobial activities of halophytic species. J. Ethnopharmacol. 2008, 116, 258–262. [Google Scholar] [CrossRef] [Green Version]
- Al Sobeai, S.M. Anticancer, Cytotoxic Effect of Tamarix Aphylla, and Antibacterial Screening Efficiency against Multidrug-Resistant Human Pathogens. Asian J. Pharm. Clin. Res. 2018, 11, 241. [Google Scholar] [CrossRef]
- Iqbal, H.; Ishfaq, M.; Abbas, M.N.; Ahmad, I.; Rehman, A.; Amin, S.B.; Shagufta, B.I.; Ullah, M. In vitro Antimicrobial Study of Tamarix aphylla in View of Phytochemical Constituents. Pharmacologia 2015, 6, 333–336. [Google Scholar] [CrossRef] [Green Version]
- Saini, R.; Saini, S.; Sharma, S. Biofilm: A dental microbial infection. J. Nat. Sci. Biol. Med. 2011, 2, 71–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diao, M.; Liang, Y.; Zhao, J.; Zhao, C.; Zhang, J.; Zhang, T. Enhanced cytotoxicity and antioxidant capacity of kaempferol complexed with α-lactalbumin. Food Chem. Toxicol. 2021, 153, 112265. [Google Scholar] [CrossRef]
- del Valle, P.; García-Armesto, M.R.; de Arriaga, D.; González-Donquiles, C.; Rodríquez-Fernández, P.; Rúa, J. Antimicrobial activity of kaempferol and resveratrol in binary combinations with parabens or propyl gallate against Enterococcus faecalis. Food Control 2016, 61, 213–220. [Google Scholar] [CrossRef]
- Sanglard, D. Clinical relevance of mechanisms of antifungal drug resistance in yeasts. Enferm. Infecc. Microbiol. Clínica 2002, 20, 462–470. [Google Scholar] [CrossRef]
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.W.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.K.; et al. The Global Burden of Skin Disease in 2010: An Analysis of the Prevalence and Impact of Skin Conditions. J. Investig. Dermatol. 2014, 134, 1527–1534. [Google Scholar] [CrossRef] [Green Version]
- Ghazwani, M.; Hani, U.; Osmani, R.A.M.; Rahamathulla, M.; Begum, M.Y.; Wahab, S.; Gowda, D.V.; Ravikumar, A.A.; Kumar, H.Y.; Ather, H.; et al. An Efficient Herbal Approach for Treating Fungal Infection in Cervical Cancer Patients by Developing and Optimizing a Vaginal Suppository. Int. J. Polym. Sci. 2021, 2021, 9198387. [Google Scholar] [CrossRef]
- Kumar Mishra, K.; Deep Kaur, C.; Kumar Sahu, A.; Panik, R.; Kashyap, P.; Prasad Mishra, S.; Dutta, S. Medicinal Plants Having Antifungal Properties. In Medicinal Plants—Use in Prevention and Treatment of Diseases [Working Title]; IntechOpen: Rijeka, Croatia, 2020; ISBN 978-1-78985-888-4. [Google Scholar]
- Arif, T.; Bhosale, J.D.; Kumar, N.; Mandal, T.K.; Bendre, R.S.; Lavekar, G.S.; Dabur, R. Natural products—Antifungal agents derived from plants. J. Asian Nat. Prod. Res. 2009, 11, 621–638. [Google Scholar] [CrossRef]
- Bibi, S.; Afzal, M.; Khan, M.B. Antifungal Activity of Tamarix aphylla (L.) Karst. Stem-bark Extract Against Some Pathogenic Fungi. Am.-Eurasian J. Agric. Environ. Sci. 2015, 5, 44–48. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45, S1–S8. [Google Scholar] [CrossRef]
- Mann, J.; Bernstein, Y.; Findler, M. Periodontal disease and its prevention, by traditional and new avenues (Review). Exp. Ther. Med. 2019, 19, 1504. [Google Scholar] [CrossRef]
- Huang, R.; Li, M.; Gregory, R.L. Bacterial interactions in dental biofilm. Virulence 2011, 2, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Wahab, S.; Nisar, N.; Dera, A.A.; Alshahrani, M.Y.; Abullias, S.S.; Irfan, S.; Alam, M.M.; Srivastava, S. Evaluation of antibacterial properties of Matricaria aurea on clinical isolates of periodontitis patients with special reference to red complex bacteria. Saudi Pharm. J. 2020, 28, 1203–1209. [Google Scholar] [CrossRef]
- Könönen, E.; Gursoy, M.; Gursoy, U. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef] [Green Version]
- Marchesan, J.T.; Girnary, M.S.; Moss, K.; Monaghan, E.T.; Egnatz, G.J.; Jiao, Y.; Zhang, S.; Beck, J.; Swanson, K.V. Role of inflammasomes in the pathogenesis of periodontal disease and therapeutics. Periodontology 2000 2020, 82, 93–114. [Google Scholar] [CrossRef]
- Orban, B. Gingivitis. J. Periodontol. 1955, 26, 173–179. [Google Scholar] [CrossRef]
- Trombelli, L.; Farina, R.; Silva, C.O.; Tatakis, D.N. Plaque-induced gingivitis: Case definition and diagnostic considerations. J. Clin. Periodontol. 2018, 45, S44–S67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.H. (Ed.) Nutritional Aspects of Aging; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781351075145. [Google Scholar]
- Anticholinesterase|Drug|Britannica. Available online: https://www.britannica.com/science/anticholinesterase (accessed on 4 September 2021).
- Merfort, I.; Buddrus, J.; Nawwar, M.A.M.; Lambert, J. A triterpene from the bark of Tamarix aphylla. Phytochemistry 1992, 31, 4031–4032. [Google Scholar] [CrossRef]
- Szwajgier, D.; Borowiec, K. Phenolic acids from malt are efficient acetylcholinesterase and butyrylcholinesterase inhibitors. J. Inst. Brew. 2012, 118, 40–48. [Google Scholar] [CrossRef]
- Khan, H.; Pervaiz, A.; Intagliata, S.; Das, N.; Nagulapalli Venkata, K.C.; Atanasov, A.G.; Najda, A.; Nabavi, S.M.; Wang, D.; Pittalà, V.; et al. The analgesic potential of glycosides derived from medicinal plants. DARU J. Pharm. Sci. 2020, 28, 387–401. [Google Scholar] [CrossRef]
- Abo-Dola, M.; Lutfi, M.; Bakhiet, A.; Mohamed, A. Anti-Inflammatory, Analgesic, Antipyretic and the Membrane-Stabilizing Effects of Tamarix aphylla Ethanolic Extract. Eur. J. Med. Plants 2015, 5, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Balamurugan, R.; Duraipandiyan, V.; Ignacimuthu, S. Antidiabetic activity of γ-sitosterol isolated from Lippia nodiflora L. in streptozotocin induced diabetic rats. Eur. J. Pharmacol. 2011, 667, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Nakrani, M.N.; Wineland, R.H.; Anjum, F. Physiology, Glucose Metabolism; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Mergenthaler, P.; Lindauer, U.; Dienel, G.A.; Meisel, A. Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends Neurosci. 2013, 36, 587–597. [Google Scholar] [CrossRef] [Green Version]
- Bhagavan, N.V.; Ha, C.-E. Carbohydrate Metabolism II. Essent. Med. Biochem. 2015, 2, 205–225. [Google Scholar] [CrossRef]
- Association, A.D. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009, 32, S62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrah, T.E.; Dhillon, B.; Keane, P.A.; Webb, D.J.; Dhaun, N. The eye, the kidney, and cardiovascular disease: Old concepts, better tools, and new horizons. Kidney Int. 2020, 98, 323–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eizirik, D.L.; Colli, M.L.; Ortis, F. The role of inflammation in insulitis and Β-cell loss in type 1 diabetes. Nat. Rev. Endocrinol. 2009, 5, 219–226. [Google Scholar] [CrossRef]
- Wright, E.; Scism-Bacon, J.L.; Glass, L.C. Oxidative stress in type 2 diabetes: The role of fasting and postprandial glycaemia. Int. J. Clin. Pract. 2006, 60, 308–314. [Google Scholar] [CrossRef] [Green Version]
- Kooti, W.; Farokhipour, M.; Asadzadeh, Z.; Ashtary-Larky, D.; Asadi-Samani, M. The role of medicinal plants in the treatment of diabetes: A systematic review. Electron. Physician 2016, 8, 1832–1842. [Google Scholar] [CrossRef] [Green Version]
- Balamurugan, R.; Ignacimuthu, S. Antidiabetic and hypolipidemic effect of methanol extract of Lippia nodiflora L. in streptozotocin induced diabetic rats. Asian Pac. J. Trop. Biomed. 2011, 1, S30–S36. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, V.; Prakash, O. Antidiabetic, hypolipidemic and histopathological analysis of Dillenia indica (L.) leaves extract on alloxan induced diabetic rats. Asian Pac. J. Trop. Med. 2011, 4, 347–352. [Google Scholar] [CrossRef] [Green Version]
- Jolles, J.W.; de Visser, L.; van den Bos, R. Male Wistar rats show individual differences in an animal model of conformity. Anim. Cogn. 2011, 14, 769–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daisy, P.; Eliza, J.; Mohamed Farook, K.A.M. A novel dihydroxy gymnemic triacetate isolated from Gymnema sylvestre possessing normoglycemic and hypolipidemic activity on STZ-induced diabetic rats. J. Ethnopharmacol. 2009, 126, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Garud, M.S.; Kulkarni, Y.A. Gallic acid attenuates type I diabetic nephropathy in rats. Chem.-Biol. Interact. 2018, 282, 69–76. [Google Scholar] [CrossRef]
- Obafemi, T.O.; Jaiyesimi, K.F.; Olomola, A.A.; Olasehinde, O.R.; Olaoye, O.A.; Adewumi, F.D.; Afolabi, B.A.; Adewale, O.B.; Akintayo, C.O.; Ojo, O.A. Combined effect of metformin and gallic acid on inflammation, antioxidant status, endoplasmic reticulum (ER) stress and glucose metabolism in fructose-fed streptozotocin-induced diabetic rats. Toxicol. Rep. 2021, 8, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.S.; Ismail, H.F.; Hashim, Z.; Majid, F.A.A. Synergistic antihyperglycaemic effect of combination therapy with gallic acid and andrographolide in streptozotocin-induced diabetic rats. Biocatal. Agric. Biotechnol. 2019, 18, 101048. [Google Scholar] [CrossRef]
- Arfin, S.; Siddiqui, G.A.; Naeem, A.; Moin, S. Inhibition of advanced glycation end products by isoferulic acid and its free radical scavenging capacity: An in vitro and molecular docking study. Int. J. Biol. Macromol. 2018, 118, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Ranilla, L.G.; Kwon, Y.-I.; Apostolidis, E.; Shetty, K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour. Technol. 2010, 101, 4676–4689. [Google Scholar] [CrossRef]
- Verma, N.; Behera, B.C.; Sharma, B.O. Glucosidase Inhibitory and Radical Scavenging Properties of Lichen Metabolites Salazinic Acid, Sekikaic Acid and Usnic Acid Liken Metabolitleri Salazinik, Sekikaik ve Usnik Asitin Glikosidaz Engelleyici ve Radikal Süpürücü Özelliği Research Article. J. Biol. Chem. J. Biol. Chem. 2012, 40, 7–21. [Google Scholar]
- Ramkumar, K.M.; Thayumanavan, B.; Palvannan, T.; Rajaguru, P. Inhibitory effect of Gymnema Montanum leaves on α-glucosidase activity and α-amylase activity and their relationship with polyphenolic content. Med. Chem. Res. 2010, 19, 948–961. [Google Scholar] [CrossRef]
- Li, D.Q.; Qian, Z.M.; Li, S.P. Inhibition of three selected beverage extracts on α-glucosidase and rapid identification of their active compounds using HPLC-DAD-MS/MS and biochemical detection. J. Agric. Food Chem. 2010, 58, 6608–6613. [Google Scholar] [CrossRef] [PubMed]
- Önal, S.; Timur, S.; Okutucu, B.; Zihnioǧlu, F. Inhibition of α-glucosidase by aqueous extracts of some potent antidiabetic medicinal herbs. Prep. Biochem. Biotechnol. 2005, 35, 29–36. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Chen, F.; Wang, X.; Jiang, Y.; Lin, S. Anti-diabetic activities of phenolic compounds in muscadine against alpha-glucosidase and pancreatic lipase. LWT—Food Sci. Technol. 2012, 46, 164–168. [Google Scholar] [CrossRef]
- Yao, X.; Zhu, L.; Chen, Y.; Tian, J.; Wang, Y. In vivo and in vitro antioxidant activity and α-glucosidase, α-amylase inhibitory effects of flavonoids from Cichorium glandulosum seeds. Food Chem. 2013, 139, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, P.; Wahab, S.; Dera, A.; Chandramoorthy, H.; Irfan, S.; Patel, A.; Abullias, S.; Zaman, G.; Ahmad, I. Anti-cancer activity of ethanolic leaf extract of Salvia officinalis against oral squamous carcinoma cells in vitro via caspase mediated mitochondrial apoptosis. Pharmacogn. Mag. 2020, 16, 554. [Google Scholar] [CrossRef]
- Abaza, M.S.I.; Afzal, M.; Al-Attiyah, R.J.; Guleri, R. Methylferulate from Tamarix aucheriana inhibits growth and enhances chemosensitivity of human colorectal cancer cells: Possible mechanism of action. BMC Complement. Altern. Med. 2016, 16, 384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Wahab, S.; Alshahrani, M.Y.; Ahmad, M.F.; Abbas, H. Current trends and future perspectives of nanomedicine for the management of colon cancer. Eur. J. Pharmacol. 2021, 910, 174464. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, M.F.; Kandasamy, G.; Vasudevan, R.; Ali, M.S.; Amir, M. Glycyrrhiza glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology. Plants 2021, 10, 2751. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.R.; Kucuk, O.; Khuri, F.R.; Shin, D.M. Perspectives for cancer prevention with natural compounds. J. Clin. Oncol. 2009, 27, 2712–2725. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics for Hispanics/Latinos, 2012. CA Cancer J. Clin. 2012, 62, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namasivayam, N. Chemoprevention in experimental animals. Ann. N. Y. Acad. Sci. 2011, 1215, 60–71. [Google Scholar] [CrossRef]
- Romeilah, R.M.; El-Beltagi, H.S.; Shalaby, E.A.; Younes, K.M.; EL Moll, H.; Rajendrasozhan, S.; Mohamed, H. Antioxidant and cytotoxic activities of Artemisia monosperma L. and Tamarix aphylla L. essential oils. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12233. [Google Scholar] [CrossRef]
- Alhourani, N.; Kasabri, V.; Bustanji, Y.; Abbassi, R.; Hudaib, M. Potential Antiproliferative Activity and Evaluation of Essential Oil Composition of the Aerial Parts of Tamarix aphylla (L.) H. Karst: A Wild Grown Medicinal Plant in Jordan. Evid.-Based Complement. Altern. Med. 2018, 2018, 9363868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, H.; Wazir, S.M.; Khan, R.A. In Vitro Antioxidant, Antifungal and Cytotoxic Activities of Selected Medicinal Plants of District Bannu and Lakki Marwat. Stud. Ethno-Med. 2017, 11, 226–232. [Google Scholar] [CrossRef]
- Khalid, M.; Bilal, M.; Munir, H.; Shah, S.Z.H.; Khurshid, M.; El-Shazly, M.; Iqbal, H.M.N. In-vitro Evaluation of Anti-Bacterial, Anti-biofilm and Cytotoxic Activity of Naturally Inspired Juglans regia, Tamarix aphylla L., and Acacia modesta with Medicinal Potentialities. J. Pure Appl. Microbiol. 2020, 14, 1133–1142. [Google Scholar] [CrossRef]
- El Ansari, M.A.; Nawwar, M.A.M.; El Dein, A.; El Sherbeiny, A.; El Sissi, H.I. A sulphated kaempferol 7,4′-dimethyl ether and a quercetin isoferulylglucuronide from the flowers of Tamarix aphylla. Phytochemistry 1976, 15, 231–232. [Google Scholar] [CrossRef]
- Yusufoglu, H.S.; Alam, A.; Al-Howeemel, A. Pharmacognostie and preliminary phytochemical standardization of Tainarix aphylla and Ziziphus nummularia growing in Saudi Arabia. Asian J. Biol. Life Sci. 2015, 4, 42–46. [Google Scholar]
- Orabi, M.A.A.; Yoshimura, M.; Amakura, Y.; Hatano, T. Ellagitannins, gallotannins, and gallo-ellagitannins from the galls of Tamarix aphylla. Fitoterapia 2015, 104, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Nawwar, M.; Swilam, N.; Hashim, A.; Al-Abd, A.; Abdel-Naim, A.; Lindequist, U. Cytotoxic isoferulic acidamide from Myricaria germanica (Tamaricaceae). Plant Signal. Behav. 2013, 8, e22642. [Google Scholar] [CrossRef] [Green Version]
- Nicolini, F.; Burmistrova, O.; Marrero, M.T.; Torres, F.; Hernández, C.; Quintana, J.; Estévez, F. Induction of G 2 /M phase arrest and apoptosis by the flavonoid tamarixetin on human leukemia cells. Mol. Carcinog. 2014, 53, 939–950. [Google Scholar] [CrossRef]
- Orabi, K.Y.; Abaza, M.S.; El Sayed, K.A.; Elnagar, A.Y.; Al-Attiyah, R.; Guleri, R.P. Selective growth inhibition of human malignant melanoma cells by syringic acid-derived proteasome inhibitors. Cancer Cell Int. 2013, 13, 82. [Google Scholar] [CrossRef] [Green Version]
- AMA, A. Tamarix nilotica (Ehrenb) Bunge: A Review of Phytochemistry and Pharmacology. J. Microb. Biochem. Technol. 2017, 9, 544–553. [Google Scholar] [CrossRef] [Green Version]
- Martins, J.; Brijesh, S. Phytochemistry and pharmacology of anti-depressant medicinal plants: A review. Biomed. Pharmacother. 2018, 104, 343–365. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Hedayatollah, S. Toxicity and safety of medicinal plants. J. HerbMed. Pharmacol. 2013, 2, 21–22. [Google Scholar]
- Wahab, S.; Ahmad, I.; Irfan, S.; Baig, M.H.; Farouk, A.-E.; Dong, J.-J. Use of Natural Compounds as a Potential Therapeutic Agent Against COVID-19. Curr. Pharm. Des. 2021, 27, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, M.Y.; Rafi, Z.; Alabdallah, N.M.; Shoaib, A.; Ahmad, I.; Asiri, M.; Zaman, G.S.; Wahab, S.; Saeed, M.; Khan, S. A comparative antibacterial, antioxidant, and antineoplastic potential of rauwolfia serpentina (L.) leaf extract with its biologically synthesized gold nanoparticles (r-aunps). Plants 2021, 10, 2278. [Google Scholar] [CrossRef]
- Ashour, O.M.; Abdel-Naim, A.B.; Abdallah, H.M.; Nagy, A.A.; Mohamadin, A.M.; Abdel-Sattar, E.A. Evaluation of the Potential Cardioprotective Activity of Some Saudi Plants against Doxorubicin Toxicity. Z. Nat. C 2012, 67, 297–307. [Google Scholar] [CrossRef]







| Geographical Location | Parts of the Plant Used | Indication | Route of Administration | Results | References |
|---|---|---|---|---|---|
| Southeastern Morocco | Leaf | Hypertension | S/Decoction | Information was collected from the respondents of Errachidia province regarding the plants used for hypertension, one of which was T. aphylla. | [34] |
| The central region of Abyan governorate, Yemen | Bark and leaf | Abdominal pain, hair loss, cough, and asthma | S, Lo/Infusion, decoction | Ethnobotanical survey of medicinal plants showed that residents of Yemen use T. aphylla for abdominal pain, hair loss, cough, and asthma. | [35] |
| Northwestern part of Pakistan | Whole plant | Abscesses and wounds, rheumatism, jaundice, bad evils | S, Lo/Decoction of ash, ash, boiled leaves | T. aphylla is one of the plants enlisted in the Holy Quran, Islamic literature, and Ahadith with ethnobotanical relevance. | [20] |
| District Sargodha, Punjab, Pakistan | Bark | Measles, aphrodisiac | S/Powdered with oil, smoke | T. aphylla is one of the most used timber species in district Sargodha. | [36] |
| Peshawar Valley of Pakistan | Bark and leaf | Paralysis, abdominal pain, tetanus, rheumatism, and wound healing | S, Lo/Powder extract | Ethnobotanical study on medicinal plants exhibited that residents of Peshawar use T. aphylla in various diseases. | [37] |
| District Karak, Pakistan | Leaf | Animal pain killer for wounds, in bird flu | S/Smoke | This study showed the ethnoveterinary use of T. aphylla. | [38] |
| Pakistan | Fruit | Diabetes | S/Decoction | T. aphylla can be used as antidiabetic medication. | [39] |
| Karamar Valley, Swabi, Pakistan | Bark | Jaundice, rheumatism, infection of gums and teeth | ND | T. aphylla’s extract showed activity against the biofilm-causing bacteria in periodontal diseases. | [27] |
| Chenab riverine area, Punjab province, Pakistan | Leaf and bark | Cough and cold, eye infection, wounds and boils, febricity | S, Lo/ Poultice, paste, decoction, ash | Local people employ T. aphylla to cure various ailments in numerous regions of Pakistan. | [26] |
| Rajhan Pur, Punjab, Pakistan | Root, leaf | All contagious diseases, jaundice, smallpox, leprosy, tuberculosis | ND | A field survey showed various uses of T. aphylla in multiple diseases. | [28] |
| Central Sahara | Shoots | Aid to menstruation, postpartum care, fever | S/Decoction | Results showed that people traditionally use T. aphylla as a potential traditional healer. | [40] |
| Jordan, North Badia | Leaf | Fever | S/Decoction | T. aphylla is an effective medication in pain and inflammations. | [41] |
| Phytochemical Category | Phytochemical Name | Structure | Part/Extract | References |
|---|---|---|---|---|
| Aromatic hydrocarbon Carbohydrate | Fructose |  | G, Gu/EtAc | [53] |
| Glucose |  | G, Gu/EtAc | [53] | |
| Raffinose |  | G/EtAc | [53] | |
| Ribose |  | G/EtAc | [53] | |
| Sucrose |  | G/EtAc | [53] | |
| Xylose |  | G, Gu/EtAc | [53] | |
| Flavonoids | Apigenin |  | SS, L, B, EtOH | [54] |
| Isoquercetin |  | G/EtAc | [53] | |
| Isorhamnetin |  | L, S, B/EtOH, aqueous | [17,55] | |
| Juglanin |  | G/EtAc | [53] | |
| Kaempferide |  | Ap/EtOH | [17,55,56] | |
| Kaempferol |  | L and S/MeOH, EtOH | [17,55] | |
| Luteolin |  | L and S, EtOH | [17] | |
| Quercetin |  | SS, L/EtOH | [17,56] | |
| Quercetin dimethyl ether |  | L/Aqueous | [55] | |
| Quercetin-3-rhamnoside |  | F/EtOH | [57] | |
| Quercetin 3-O-galactoside |  | L and S/MeOH, EtOH | [54] | |
| Phenolic acid | Caffeic acid |  | L and S, EtOH | [17] |
| Dehydrodigallic acid |  | G, EtAc | [53] | |
| Dehydrotrigallic acid |  | G/aqueous ethanolic acid | [49] | |
| Ellagic acid |  | G, debarked heartwood | [53] | |
| Gallic acid |  | L, debarked heartwood | [55] | |
| Isoferulic acid |  | Debarked heartwood, G, EtAc | [53] | |
| p-Coumaric acid |  | L and S/EtOH, aqueous | [17,55] | |
| Syringic acid |  | L, debarked heartwood/aqueous | [55] | |
| Phenolic glycoside | Dehydrodigallic-xanthone |  | G/Aq-EtOH | [49] |
| Dehydrotrigallic-xanthone |  | G/Aq-EtOH | [49] |
| Pharmacological Activity | Plant Part | Solvent for Extraction | Model/Induction/Agent/ Species | Dosage or Concentration | Sample Size/Pathogens | Results | References |
|---|---|---|---|---|---|---|---|
| Antidiabetic | Leaf | MeOH | In vivo study, diabetic Wistar rats (induced by nicotinamide + STZ) | 100, 250, 400 mg/kg/ day | 50 male Wistar rats | Outcomes showed nontoxic and antidiabetic properties | [8] |
| Antidiabetic | Leaf, stem | MeOH | In vitro study, α-glucosidase inhibitory assay | 1–1000 μg/mL | - | Antidiabetic properties were shown | [54] |
| Antipyretic, analgesic, and anti-inflammatory properties | Aerial parts | Aqueous ethanolic extract | In vivo, edema and yeast-induced, carrageenan-induced paw edema | 100 mg/kg/day | Swiss albino male mice (20–30 g), three groups of six mice | Outcomes revealed antipyretic and analgesic activities with lessened anti-inflammatory action | [21] |
| Anti-inflammatory and wound healing | Leaf | EtOH | In vivo, carrageenan-induced paw edema | 15%, 25% in gel base | Wistar rats (180–200 g) | Outcomes showed gel usefulness | [70] |
| Antimicrobial | Leaf, stem, bark | Diverse extracts | In vitro study, micro-titer assay, agar well diffusion method | 1 mg/mL | - | Helpful against 11 biofilm-forming strains | [27] |
| Leaf | MeOH extract | In vitro: antibacterial activity against Escherichia coli and Staphylococcus aureus, agar dilution method against three Aspergillus species | 0.86–30 mg/ mL | - | No considerable antibacterial effect | [141] | |
| Antifungal | Leaf | MeOH extract | In vitro: dilution of agar tubes | 67 μL (200 μg/mL) | - | T. aphylla showed significant antifungal activity: 70%, 55%, and 54% against Aspergillus flavius, Aspergillus niger, and Aspergillus fumigatus, respectively | [141] |
| Stem, bark | Crude ethanolic extracts | In vitro | Concentrations 500 ppm, 1000 ppm, and 2000 ppm | Six pathogenic fungi | Maximum inhibition has been shown by T. aphylla | [90] | |
| Bark | MeOH | In vitro: disc diffusion assay | 25, 50, 75, and 100 mg/mL | Aspergillus flavus and Candida albicans | T. aphylla inhibited most fungal strains at higher concentrations | [81] | |
| Toxicity | Leaf | MeOH | In vivo, toxicity study in mice | 500–2100 mg/kg/day | 50 male, Wistar rats (220–320) g | Nontoxic | [8] |
| Toxicity | Leaf | EtOH | In vivo, toxicity study in rats | Doses administered: 50, 100, 300, 1000, and 2000 mg/kg body weight | Wistar rats | No toxicity was reported up to 2000 mg/kg body weight | [70] |
| Toxicity | Leaf | MeOH | In vivo | 50–500 mg/ mL | Brine shrimp | Brine shrimp showed a 70% mortality rate | [141] |
| Wound healing | Leaf | EtOH | In vivo, excision wound and induced paw edema model | 15%, 25% in gel base | Wistar rats | T. aphylla leaf extract possesses wound-healing properties | [70] |
| Periodontal disease | Bark, stem, leaves | Methanol, ethanol, acetone, and water extracts | In vitro, phylogenetic analysis | - | Pathogenic bacteria from dental biofilms. | T. aphylla showed potent antibiofilm activity | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshehri, S.A.; Wahab, S.; Abullais, S.S.; Das, G.; Hani, U.; Ahmad, W.; Amir, M.; Ahmad, A.; Kandasamy, G.; Vasudevan, R. Pharmacological Efficacy of Tamarix aphylla: A Comprehensive Review. Plants 2022, 11, 118. https://doi.org/10.3390/plants11010118
Alshehri SA, Wahab S, Abullais SS, Das G, Hani U, Ahmad W, Amir M, Ahmad A, Kandasamy G, Vasudevan R. Pharmacological Efficacy of Tamarix aphylla: A Comprehensive Review. Plants. 2022; 11(1):118. https://doi.org/10.3390/plants11010118
Chicago/Turabian StyleAlshehri, Saad Ali, Shadma Wahab, Shahabe Saquib Abullais, Gotam Das, Umme Hani, Wasim Ahmad, Mohd Amir, Ayaz Ahmad, Geetha Kandasamy, and Rajalakshimi Vasudevan. 2022. "Pharmacological Efficacy of Tamarix aphylla: A Comprehensive Review" Plants 11, no. 1: 118. https://doi.org/10.3390/plants11010118