A DPPH· Kinetic Approach on the Antioxidant Activity of Various Parts and Ripening Levels of Papaya (Carica papaya L.) Ethanolic Extracts
Abstract
:1. Introduction
2. Results and Discussion
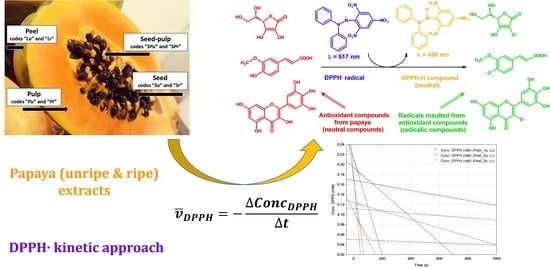
2.1. Radical Scavenging Capacity of Unripe and Ripe Papaya Extracts
2.2. DPPH· Reaction Kinetics in the Presence of the Unripe and Ripe Papaya Extracts
3. Materials and Methods
3.1. Materials
3.2. Obtaining Papaya Fruit Extracts
3.3. Evaluation of the Antioxidant Activity by DPPH· Method
3.4. Evaluation of the DPPH· Reaction Rates
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calvache, J.N.; Cueto, M.; Farroni, A.; Pla, M.d.E.; Gerschenson, L.N. Antioxidant characterization of new dietary fiber concentrates from papaya pulp and peel (Carica papaya L.). J. Funct. Foods 2016, 27, 319–328. [Google Scholar] [CrossRef]
- Galang, M.G.M.; Macabeo, A.P.G.; Chang, W.-C.; Isobe, M.; Aguinaldo, M.A.M. Glucosides from the unripe fruit juice of Carica papaya Linn. (Caricaceae) cultivar ‘Red Lady’ with antioxidant activity. J. Funct. Foods 2016, 22, 358–362. [Google Scholar] [CrossRef]
- Gironés-Vilaplana, A.; Baenas, N.; Villaño, D.; Speisky, H.; García-Viguera, C.; Moreno, D.A. Evaluation of Latin-American fruits rich in phytochemicals with biological effects. J. Funct. Foods 2014, 7, 599–608. [Google Scholar] [CrossRef]
- Hall, R.M.; Mayer, D.A.; Mazzutti, S.; Ferreira, S.R.S. Simulating large scale SFE applied to recover bioactive compounds from papaya seeds. J. Supercrit. Fluids 2018, 140, 302–309. [Google Scholar] [CrossRef]
- Ikram, E.H.K.; Stanley, R.; Netzel, M.; Fanning, K. Phytochemicals of papaya and its traditional health and culinary uses—A review. J. Food Compos. Anal. 2015, 41, 201–211. [Google Scholar] [CrossRef]
- Samaram, S.; Mirhosseini, H.; Tan, C.P.; Ghazali, H.M.; Bordbar, S.; Serjouie, A. Optimisation of ultrasound-assisted extraction of oil from papaya seed by response surface methodology: Oil recovery, radical scavenging antioxidant activity, and oxidation stability. Food Chem. 2015, 172, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Bachheti, A.; Sharma, P.; Bachheti, R.K.; Husen, A. Phytochemistry, pharmacological activities, nanoparticle fabrication, commercial products and waste utilization of Carica papaya L.: A comprehensive review. Curr. Res. Biotechnol. 2020, 2, 145–160. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Stucken, K.; Cantuarias, C.; Lamas, F.; García, V.; Pastén, A. Antimicrobial properties of papaya (Vasconcellea pubescens) subjected to low-temperature vacuum dehydration. Innov. Food Sci. Emerg. Technol. 2021, 67, 102563. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Wang, X.-M.; Han, Z.-P.; Zhao, M.-X.; Yin, L. Purification, antioxidant and moisture-preserving activities of polysaccharides from papaya. Carbohydr. Polym. 2012, 87, 2332–2337. [Google Scholar] [CrossRef]
- Osato, J.A.; Santiago, L.A.; Remo, G.M.; Cuadra, M.S.; Mori, A. Antimicrobial and antioxidant activities of unripe papaya. Life Sci. 1993, 53, 1383–1389. [Google Scholar] [CrossRef]
- Mishra, B.B.; Gautamn, S.; Chander, R.; Sharma, A. Characterization of nutritional, organoleptic and functional properties of intermediate moisture shelf stable ready-to-eat Carica papaya cubes. Food Biosci. 2015, 10, 69–79. [Google Scholar] [CrossRef]
- Chielle, D.P.; Bertuol, D.A.; Meili, L.; Tanabe, E.H.; Dotto, G.L. Convective drying of papaya seeds (Carica papaya L.) and optimization of oil extraction. Ind. Crop. Prod. 2016, 85, 221–228. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Kaur, P.; Ghoshal, G.; Jain, A. Bio-utilization of fruits and vegetables waste to produce β-carotene in solid state fermentation: Characterization and antioxidant activity. Process Biochem. 2019, 76, 155–164. [Google Scholar] [CrossRef]
- Martins, G.F.; Fabi, J.P.; Mercadante, A.Z.; de Rosso, V.V. The ripening influence of two papaya cultivars on carotenoid biosynthesis and radical scavenging capacity. Food Res. Int. 2016, 81, 197–202. [Google Scholar] [CrossRef]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Schmidt, E.M.; Bonafe, E.G.; Eberlin, M.N.; Sawaya, A.C.H.F.; Visentainer, J.V. Antioxidant activity, phenolics and UPLC-ESI(-)-MS of extracts from different tropical fruits parts and processed peels. Food Res. Int. 2015, 77, 392–399. [Google Scholar] [CrossRef]
- Pavan, V.; Sancho, R.A.S.; Pastore, G.M. The effect of in vitro digestion on the antioxidant activity of fruit extracts (Carica papaya, Artocarpus heterophillus and Annona marcgravii). LWT Food Sci. Technol. 2014, 59, 1247–1251. [Google Scholar] [CrossRef] [Green Version]
- Vuong, Q.V.; Hirun, S.; Chuen, T.L.K.; Goldsmith, C.D.; Murchie, S.; Bowyer, M.C.; Phillips, P.A.; Scarlett, C.J. Antioxidant and anticancer capacity of saponin-enriched Carica papaya leaf extracts. Int. J. Food Sci. Technol. 2015, 50, 169–177. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Hirun, S.; Roach, P.D.; Bowyer, M.C.; Phillips, P.A.; Scarlett, C.J. Effect of extraction conditions on total phenolic compounds and antioxidant activities of Carica papaya leaf aqueous extracts. J. Herb. Med. 2013, 3, 104–111. [Google Scholar] [CrossRef]
- Faller, A.L.K.; Fialho, E. Polyphenol content and antioxidant capacity in organic and conventional plant foods. J. Food Compos. Anal. 2010, 23, 561–568. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Lim, T.T.; Tee, J.J. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007, 103, 1003–1008. [Google Scholar] [CrossRef]
- Rodrigues, L.G.G.; Mazzutti, S.; Vitali, L.; Micke, G.A.; Ferreira, S.R.S. Recovery of bioactive phenolic compounds from papaya seeds agroindustrial residue using subcritical water extraction. Biocatal. Agric. Biotechnol. 2019, 22, 101367. [Google Scholar] [CrossRef]
- Salla, S.; Sunkara, R.; Ogutu, S.; Walker, L.T.; Verghese, M. Antioxidant activity of papaya seed extracts against H2O2 induced oxidative stress in HepG2 cells. LWT Food Sci. Technol. 2016, 66, 293–297. [Google Scholar] [CrossRef]
- Udomkun, P.; Nagle, M.; Argyropoulos, D.; Mahayothee, B.; Latif, S.; Müller, J. Compositional and functional dynamics of dried papaya as affected by storage time and packaging material. Food Chem. 2016, 196, 712–719. [Google Scholar] [CrossRef]
- Lako, J.; Trenerry, V.C.; Wahlqvist, M.; Wattanapenpaiboon, N.; Sotheeswaran, S.; Premier, R. Phytochemical flavonols, carotenoids and the antioxidant properties of a wide selection of Fijian fruit, vegetables and other readily available foods. Food Chem. 2007, 101, 1727–1741. [Google Scholar] [CrossRef]
- Sancho, L.E.G.-G.; Yahia, E.M.; González-Aguilar, G.A. Identification and quantification of phenols, carotenoids, and vitamin C from papaya (Carica papaya L., cv. Maradol) fruit determined by HPLC-DAD-MS/MS-ESI. Food Res. Int. 2011, 44, 1284–1291. [Google Scholar] [CrossRef]
- Rivera-Pastrana, D.M.; Yahia, E.M.; González-Aguilara, G.A. Phenolic and carotenoid profiles of papaya fruit (Carica papaya L.) and their contents under low temperature storage. J. Sci. Food Agric. 2010, 90, 2358–2365. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Husin, F.; Ya’akob, H.; Rashid, S.N.A.; Shahar, S.; Soib, H.H. Cytotoxicity study and antioxidant activity of crude extracts and SPE fractions from Carica papaya leaves. Biocatal. Agric. Biotechnol. 2019, 19, 101130. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity using the DPPH• Free Radical Method. LWT Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Chat, O.A.; Najar, M.H.; Dar, A.A. Evaluation of reduction kinetics of 2,2-diphenyl-1-picrylhydrazyl radical by flavonoid glycoside Rutin in mixed solvent based micellar media. Colloids Surf. A Phys. Eng. Asp. 2013, 436, 343–353. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Wianowska, D.; Olszowy, M. On practical problems in estimation of antioxidant activity of compounds by DPPH method (Problems in estimation of antioxidant activity). Food Chem. 2012, 131, 1037–1043. [Google Scholar] [CrossRef]
- Fadda, A.; Serra, M.; Molinu, M.G.; Azara, E.; Barberis, A.; Sanna, D. Reaction time and DPPH concentration influence antioxidant activity and kinetic parameters of bioactive molecules and plant extracts in the reaction with the DPPH radical. J. Food Compos. Anal. 2014, 35, 112–119. [Google Scholar] [CrossRef]
- Jabbari, M.; Jabbari, A. Antioxidant potential and DPPH radical scavenging kinetics of water-insoluble flavonoid naringenin in aqueous solution of micelles. Colloids Surf. A Phys. Eng. Asp. 2013, 489, 392–399. [Google Scholar] [CrossRef]
- Mira-Sánchez, M.D.; Castillo-Sánchez, J.; Morillas-Ruiz, J.M. Comparative study of rosemary extracts and several synthetic and natural food antioxidants. Relevance of carnosic acid/carnosol ratio. Food Chem. 2020, 309, 125688. [Google Scholar] [CrossRef]
- Hădărugă, D.I.; Pantea, C.; Hădărugă, N.G. Antioxidant activity and kinetics on kiwi fruit (Actinidia deliciosa) ethanolic extracts by 2,2-diphenyl-1-picrylhydrazyl (DPPH·) method. J. Agroaliment. Process. Technol. 2016, 22, 207–211. [Google Scholar]
- Ivanovici, M.; Sicoe, G.; Hădărugă, D.I. Kinetics and antiradical activity of natural and synthetic phenolic compounds by DPPH method: A comparative study. J. Agroaliment. Process. Technol. 2018, 24, 97–103. [Google Scholar]
- Sicoe, G.; Oprinescu, C.I.; Golea, G.M.; Riviş, A.; Hădărugă, N.G. Kinetics on the DPPH· reaction with hydroalcoholic extracts from various pomegranate parts. J. Agroaliment. Process. Technol. 2017, 23, 271–280. [Google Scholar]
- Zuhair, R.A.; Aminah, A.; Sahilah, A.M.; Eqbal, D. Antioxidant activity and physicochemical properties changes of papaya (Carica papaya L. cv. Hongkong) during different ripening stage. Int. Food Res. J. 2013, 20, 1653–1659. [Google Scholar]
- Zhou, K.; Wang, H.; Mei, W.; Li, X.; Luo, Y.; Dai, H. Antioxidant Activity of Papaya Seed Extracts. Molecules 2011, 16, 6179–6192. [Google Scholar] [CrossRef] [Green Version]
- Celiz, G.; Renfige, M.; Finetti, M. Spectral analysis allows using the DPPH* UV–Vis assay to estimate antioxidant activity of colored compounds. Chem. Pap. 2020, 74, 3101–3109. [Google Scholar] [CrossRef]
- de Menezes, B.B.; Frescura, L.M.; Duarte, R.; Villetti, M.A.; da Rosa, M.B. A critical examination of the DPPH method: Mistakes and inconsistencies in stoichiometry and IC50 determination by UV-Vis spectroscopy. Anal. Chim. Acta 2021, 1157, 338398. [Google Scholar] [CrossRef]
- Yao, Y.; Lin, G.; Xie, Y.; Ma, P.; Li, G.; Meng, Q.; Wu, T. Preformulation studies of myricetin: A natural antioxidant flavonoid. Pharmazie 2014, 69, 19–26. [Google Scholar] [CrossRef]
- Kalinowska, M.; Piekut, J.; Bruss, A.; Follet, C.; Sienkiewicz-Gromiuk, J.; Świsłocka, R.; Rzączyńska, Z.; Lewandowski, W. Spectroscopic (FT-IR, FT-Raman, 1H, 13C NMR, UV/VIS), thermogravimetric and antimicrobial studies of Ca(II), Mn(II), Cu(II), Zn(II) and Cd(II) complexes of ferulic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 122, 631–638. [Google Scholar] [CrossRef]
- Ruiz, B.G.; Roux, S.; Courtois, F.; Bonazzi, C. Spectrophotometric method for fast quantification of ascorbic acid and dehydroascorbic acid in simple matrix for kinetics measurements. Food Chem. 2016, 211, 583–589. [Google Scholar] [CrossRef]






| No | Code | RSC (5 s) (%) | RSC (1 min) (%) | RSC (5 min) (%) | RSC (15 min) (%) |
|---|---|---|---|---|---|
| 1 | Lu * | 2.43 a | 15.57 a | 29.66 a | 39.95 a |
| 2 | Lr | 35.21 (±3.17) b | 55.12 (±15.40) b | 65.66 (±19.65) b | 68.10 (±16.88) b |
| 3 | Pu | 33.16 (±4.76) b | 35.21 (±4.87) c | 38.56 (±4.30) a | 41.31 (±4.17) a |
| 4 | Pr * | 84.97 c | 85.74 d | 86.23 bc | 86.44 bc |
| 5 | Su | 23.96 (±8.80) bd | 29.00 (±9.51) e | 31.39 (±9.67) a | 32.76 (±9.96) a |
| 6 | Sr | 59.31 (±1.84) e | 68.98 (±0.25) bf | 73.63 (±0.36) b | 74.76 (±0.94) b |
| 7 | SPu | 35.06 (±8.23) b | 37.71 (±8.14) c | 39.61 (±8.26) a | 41.05 (±8.23) a |
| 8 | SPr | 56.08 (±13.21) e | 58.17 (±10.39) b | 60.01 (±8.53) b | 60.66 (±7.91) b |
| No | Code | DPPH· Reaction Rate on t1 Time Range, v1 (μM/s) | DPPH· Reaction Rate on t2 Time Range, v2 (μM/s) | DPPH· Reaction Rate on t3 Time Range, v3 (μM/s) |
|---|---|---|---|---|
| 1 | Lu * | 1.00 * a | 0.20 * | 0.050 *a |
| 2 | Lr | 2.70 (±0.85) b | 0.34 * | 0.017 (±0.008) b |
| 3 | Pu | 1.65 (±0.21) a | - | 0.013 (±0.002) b |
| 4 | Pr * | 4.00 *bc | - | 0.001 *c |
| 5 | Su | 1.40 (±0.14) a | 0.10 * | 0.008 (±0.004) b |
| 6 | Sr | 3.25 (±0.07) b | 0.15 (±0.07) | 0.009 (±0.004) b |
| 7 | SPu | 1.80 (±0.42) a | 0.07 * | 0.007 *b |
| 8 | SPr | 2.75 (±0.35) b | - | 0.004 *bc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iordănescu, O.A.; Băla, M.; Gligor, D.; Zippenfening, S.E.; Cugerean, M.I.; Petroman, M.I.; Hădărugă, D.I.; Hădărugă, N.G.; Riviş, M. A DPPH· Kinetic Approach on the Antioxidant Activity of Various Parts and Ripening Levels of Papaya (Carica papaya L.) Ethanolic Extracts. Plants 2021, 10, 1679. https://doi.org/10.3390/plants10081679
Iordănescu OA, Băla M, Gligor D, Zippenfening SE, Cugerean MI, Petroman MI, Hădărugă DI, Hădărugă NG, Riviş M. A DPPH· Kinetic Approach on the Antioxidant Activity of Various Parts and Ripening Levels of Papaya (Carica papaya L.) Ethanolic Extracts. Plants. 2021; 10(8):1679. https://doi.org/10.3390/plants10081679
Chicago/Turabian StyleIordănescu, Olimpia Alina, Maria Băla, Dina Gligor (Pane), Simelda Elena Zippenfening, Marius Ioan Cugerean, Mircea Ionuţ Petroman, Daniel Ioan Hădărugă, Nicoleta Gabriela Hădărugă, and Mircea Riviş. 2021. "A DPPH· Kinetic Approach on the Antioxidant Activity of Various Parts and Ripening Levels of Papaya (Carica papaya L.) Ethanolic Extracts" Plants 10, no. 8: 1679. https://doi.org/10.3390/plants10081679