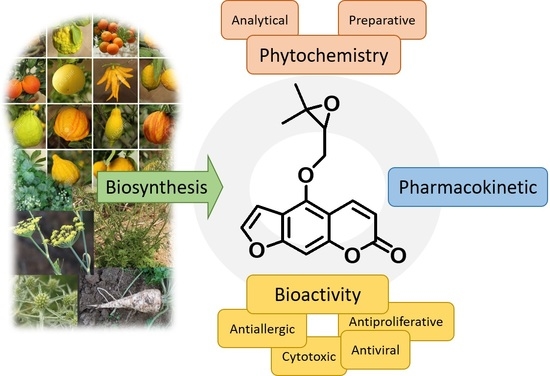
Oxypeucedanin: Chemotaxonomy, Isolation, and Bioactivities
Abstract
:1. Introduction
2. Phytochemical Studies of Oxypeucedanin
2.1. Preparative Analysis of Oxypeucedanin
2.1.1. Isolation and Purification of Oxypeucedanin from the Apiaceae Family
2.1.2. Isolation and Purification of Oxypeucedanin from the Rutaceae Family
| Plant Family | Plant Species | Subjected Plant Part/Soluble Extract | Method of Isolation/Purification | Reference |
|---|---|---|---|---|
| Apiaceae | Anethum graveolens | WP/NHEX | VLC [NHEX‒EtOAc 100:0 to 90:10], VLC [NHEX–EtOAc 50:50], RP-PTLC [NHEX–EtOAc 60:40] | [12] |
| Angelica archangelica | R/CHCl3 | MPLC [butanone‒CHCl3‒DEE‒NHEX 6.9:1:1.4;90.7% to 48:6.8:10:35.2%], MPLC [THF–Propanol–MeCN–H2O 7.5:1.3:1.7:89.5 to 45.1:7.5:10:37.4] | [13] | |
| Angelica dahurica | R/MeOH | CC [NHEX–EtOH–MeOH], CC [NHEX–EtOH], CC[NHEX–EtOH– MeOH 5:1:1], CC [NHEX–EtOH 1:1] | [16] | |
| R/MeOH | CC [NHEX–EtOAc–MeOH], CC [NHEX–EtOAc–MeOH 5:1:1], CC [NHEX–EtOAc 1:1] | [17] | ||
| R/MeOH | CC [NHEX–EtOAc 5:1, 1:1, 0:1], CC [NHEX–EtOAc 5:1] | [18] | ||
| R/EtOH | CC [NHEX–Me2CO 20:1, 15:1, 10:1, 7:1, 4:1, 2:1, 1:1; CHCl3–MeOH 10:1, 8:1, 6:1, 4:1, 2:1, 1:1], CC [NHEX–Me2CO 20:1, 15:1, 10:1, 8:1, 6:1, 4:1, 2:1], CC [NHEX–Me2CO 14:1, 4:1] | [19] | ||
| R/n-BuOH | CC [NHEX‒Me2CO 20:1 to 2:1], RP-CC [MeOH‒H2O 75:25], RP-HPLC [MeCN‒H2O 40:60], recrys. | [20] | ||
| R/CHCl3 | CC | [21] | ||
| R/CHCl3 | CC [CHCl3‒Me2CO 1:0, 0:1], CC [NHEX-EtOAc], recrys. [DEE‒CH2Cl2] | [22] | ||
| R/CH2Cl2 | CC [NHEX–EtOAc 5:1 to 0:1] | [23] | ||
| R/CH2Cl2 | CC [NHEX–EtOAc], CC [NHEX–EtOAc 3:1] | [24] | ||
| R/EtOAc | CC [NHEX–EtOAc 3:2] | [26,27] | ||
| R/EtOAc | CC [CH2Cl2‒MeOH 100:0 to 0:100], ODS-CC [MeOH‒H2O 50:50 to 95:5], SLH [MeOH] | [28] | ||
| R/EtOAc | HSCCC [NHEX–EtOAc–MeOH–H2O 5:5:4:6] | [31] | ||
| R/EtOAc | HSCCC [NHEX–EtOAc–MeOH–H2O 1:1:1:1, 5:5:4.5:5.5] | [32] | ||
| R/EtOAc | HSCCC [NHEX–EtOAc–MeOH–H2O 1:1:1:1, 5:5:4:6] | [33] | ||
| R/EtOAc | CC [NHEX–EtOAc 20:80 to 0:100], CC [EtOAc–MeOH 100:0 to 0:100], recrys. | [29] | ||
| R/PET | CCC [NHEX–MeOAc–MeCN–H2O 4:3:4:4], HPLC | [34] | ||
| R/NHEX | CC [NHEX–EtOAc], ODS-CC [MeOH–H2O 80:20] | [35] | ||
| R/nd | nd | [15] | ||
| Angelica furcijuga | Fl/EtOAc | CC [NHEX–EtOAc 10:1 to 5:1 to 1:1, to MeOH 100%], RP-CC [MeOH–H2O 70:30 to 80:20 to 90:10 to MeOH 100%], RP-CC [MeOH–H2O 60:40 to 75:25, 0:100], HPLC [MeOH–H2O 75:25] | [36] | |
| Angelica koreana | R/EtOAc | HPLC [MeCN–H2O 30:70] | [37] | |
| Angelica pancicii | R/MeOH R/CH2Cl2 | FC [petrol; DEE], HPLC [MeCN–H2O (HCO2H 2%) 50:50, 65:35] HPLC [MeCN–H2O (HCO2H 2%) 50:50, 65:35] | [38] | |
| Angelica purpurascens | Fr/NHEX | CC [NHEX–EtOAc 100:0 to 0:100], CC [NHEX–EtOAc 80:20] | [39] | |
| Diplolophium buchanani | L/CH2Cl2 | CPC [NHEX–EtOAc–MeOH–H2O 10:5:5:1], recrys. [NHEX–EtOAc] | [40] | |
| Ducrosia anethifolia | AP/CHCl3 | CC [CHCl3–MeOH 100:0 to 20:80], MPLC [NHEX–CH2Cl2 50:50 to 0:100, CH2Cl2–MeOH 100:0 to 100:0], MPLC [NHEX–EtOAc 95:5 to 0:100], CPTLC [NHEX–EtOAc 95:5 to 0:100] | [41] | |
| Ferulago angulate (syn. F. trifida) | R/NHEX | PTLC [CHCl3–Me2CO 95:5] | [44] | |
| R/CHCl3 | CC [CHCl3‒EtOAc 10:0, 5:5], CC [CHCl3‒EtOAc 9:1] | [45,46] | ||
| Ferulogo bernardii | AP/NHEX | CC [PET‒EtOAc‒MeOH 60:40], recrys. [EtOH‒H2O] | [47] | |
| Ferulago capillaris | AP, R/NHEX | CC [NHEX–EtOAc] | [48] | |
| Ferulago humulis | Rhizome/PET | CC [NHEX‒EtOAc; EtOAc‒MeOH], PTLC [cyclohexane‒EtOAc 2:1] | [42] | |
| Ferulago subvelutina | R/EtOAc | CC [NHEX‒CHCl3, CHCl3, CHCl3‒EtOAc], CC [CHCl3‒EtOAc 9:1, EtOAc], recrys. | [49] | |
| Harbouria trachypleura | AP/MeOH | VLC [NHEX‒EtOAc], FC [CH2Cl2–Me2CO 97:3], CC [CH2Cl2–Me2CO 97:3 to 4:1], RP-VLC [MeOH‒H2O 50:50 to 100:0], PTLC [CH2Cl2–Me2CO 97:3] | [50] | |
| Levisticum officinale | R/EtOAc | CC [NHEX–EtOAc 100:0 to 0:100; EtOAc–MeOH 100:0 to 80:20] | [42] | |
| Ostericum koreanum | R/CHCl3 | CC [Bz‒EtOAc 9:1] | [51] | |
| R/EtOAc | CC [NHEX–EtOAc] | [52,53] | ||
| Petroselinurn crispurn | flake/DEE | RP-CC [MeCN–H2O 3:2], HPLC [MeCN–H2O 37:63], HPLC [CHCl3‒MeOH 99.9:0.1] | [54] | |
| L/DEE | CC [NHEX–DEE 1:1], HPLC [MeCN–H2O 35:65] | [55] | ||
| L/EtOAc | nd | [57] | ||
| L/CH2Cl2 | CC [PET–EtOAc 1:0, 9:1, 8:2], CC [CH2Cl2–EtOAc 9:1], CC [CH2Cl2–MeOH 9:1, 8:2, 0:1], SLH [PE–CH2Cl2–MeOH 3:2:1], SLH [PET–CH2Cl2–MeOH 4:2:1], PTLC [PET–EtOAc 7:3] | [56] | ||
| Peucedanum cervaria | Fr/CH2Cl2 | CCC [heptane–EtOAc–MeOH–H2O 3:2:3:2] | [58] | |
| Peucedanum ostruthium | R/EtOAc | HPLC [MeOH–H2O (HOAc 0.1%) 0:100 to 100:0] | [59] | |
| Prangos ferulacea | R/Me2CO | VLC [heptane–EtOAc 10:0 to 0:10], MPLC [heptane–EtOAc 7:3 to 5:5] | [61] | |
| Prangos pabularia | R/CHCl3 | CC [NHEX–EtOAc 20:1; 10:1 to 0:1; EtOAc–MeOH 15:1 to 2:1], CC [NHEX–EtOAc 15:1] | [62] | |
| Prangos uloptera | L/NHEX | CC [NHEX–EtOAc 100:0, 1:99, 5:95, 10:99, 20:80, 40:60, 60:40, 80:20, 100:0, MeOH 100], CC [NHEX–EtOAc 20:80, 0:100, MeOH 100%], PTLC [Me2CO–CHCl3 5:95] | [63] | |
| Rutaceae | Citrus hystrix | Fr/EtOAc | ODS-CC [MeOH–H2O 50:50], PTLC [EtOAc–NHEX 4:1] | [64] |
| Citrus limon | peel/EO | PTLC [EtOAc–Me2CO 95:5], HPLC | [65] | |
| peel/EO | CC [PET–EtOAc 80:20], recrys. | [66] | ||
| Citrus aurantifolia & C. latifolia | nd/EO | HSCCC [NHEX–EtOAc–MeOH–H2O 6:4:5:5] | [67] | |
| Skimmia japonica | L/EtOAc | PTLC [CHCl3–EtOAc 8:2], PTLC [NHEX–CHCl3–EtOAc 7:2:1], CC [NHEX–CHCl3–EtOAc 8:1:1, 7:2:1, 6:3:1] | [68] | |
| Zanthoxylum flavum | R/MeOH | RP-SPE [MeOH–H2O 6:4] | [69] |
2.2. Structural Identification of Oxypeucedanin
2.3. Analytical Investigations of Oxypeucedanin
2.3.1. Identification of Oxypeucedanin in the Apiaceae Family
| Plant Family/Herbal Product | Plant Species | Subjected Plant Part/Soluble Extract | Analytical Instrument | Eluent System for Chromatography | Quantity | Reference |
|---|---|---|---|---|---|---|
| Apiaceae | Angelica archangelica | Fr/hydro-ethanolic (96%) | HPLC-UV | H2O‒MeOH [40:60 to 5:95] | 0‒6.45 mg/g | [71] |
| R, L/MeOH | HPLC-MS | H2O (1% HCO2H)‒MeOH [40:60]; H2O (0.1% HCO2H)‒MeOH [100:0 to 70:30, 40:60, 0:100, 100:0] | na | [72] | ||
| Angelica dahurica | Radix/MeOH | RRLC | H2O‒MeOH [55:45, 50:50, 42:58, 36:64, 30:70, 20:80] | 0.066‒1.45 mg/g | [14] | |
| Radix/MeOH | HPLC-UV | H2O (0.2% H3PO4)‒MeOH [52:48, 40:60, 55:45, 48:52, 25:75] | 0.816 µg/mL (by IL-DLLME) | [73] | ||
| Radix/MeOH | HPLC-UV | H2O‒MeCN [70:30, 40:60, 30:70, 40:60, 70:30] | 22.30 µg/g | [74] | ||
| R/MeOH | HPLC-DAD | H2O‒MeOH [40:60, 20:80, 10:90, 0:100] | 1.5–3.0 mg/g | [75] | ||
| R/MeOH | HPLC-DAD | H2O‒MeOH [40:60, 20:80, 10:90, 0:100] | 36.95 ± 1.45 (freeze dried) 24.96 ± 0.75 (shade dried) 24.22 ± 1.75 (40 °C) 32.13 ± 1.42 (70 °C) | [76] | ||
| R/MeOH | SFC | CO2‒MeOH (0.1% DEA) [100:0, 97:3, 90:10] | 1.54–2.93 g/100 g (0.16‒0.77%) | [77] | ||
| nd/MeOH | 1H-qNMR | solvent: DMSO-d6 | 6.38–6.39 ppm (0.17–0.35%) | [78] | ||
| Radix/hydro-ethanolic (70%) | HPLC-DAD | H2O (0.1% HCO2H)‒MeOH [95:5, 35:65, 5:95] (for qualification) H2O (0.1% HCO2H)‒MeOH [70:30, 40:60, 40, 5:95] (for quantification) | 1.24–4.98 mg/g | [79] | ||
| Radix/hydro-ethanolic (70%) | HPLC-UV | H2O‒MeCN [30:70] | 0.063 ± 0.01 % (collected from Korea) 0.024 ± 0.02 % (collected from China) | [80] | ||
| R/EtOH | LC-NMR-MS | H2O‒MeCN [40:60] | na | [81] | ||
| R/H2O (for boiled sample) and soybean oil, MeOH (for fried sample) | HPLC-DAD-ESI-MS | H2O (0.1% HCO2H)‒MeOH [for boiled sample: 95:5, 75:25, 26:64, 95:5] [for fried sample: 95:5, 85:15, 77:23, 47:53, 20:80, 95:5] | 15 µg/mL (for boiled sample) 8 µg/mL (for fried sample) | [82] | ||
| Ostericum koreanum | R/MeOH | HPLC-UV | H2O‒MeCN [65:35 to 25:75] | 0.57 ± 0.26 0.99 ± 0.89 | [83] | |
| nd/hydro-ethanolic (70%) | HPLC-UV | H2O‒MeCN [40:60] | 0.70 ± 0.02–21.11 ± 0.07% (Korean samples) 1.02 ± 0.01–12.60 ± 0.10% (Chinese samples) | [53] | ||
| Petroselinum crispum | AP/MeOH | HPLC-QTOF-MS | H2O (0.1% HCO2H)‒MeCN [90:10, 60:40, 20:80, 10:90, 0:100, 90:10] | 46.04 ± 5.50 mg/kg | [84] | |
| L, R, flake/EtOAc | HPLC-UV | cyclohexane‒isopropyl ether‒n-amyl alcohol [15:4:0.5] | 102.87 ± 14.08 µg/g (curled L) 88.68 ± 6.04 µg/g (curled R) 88.60 ± 17.90 µg/g (flake) | [55] | ||
| Peucedanum alsaticum | Fr/PET | UPLC | H2O‒MeCN [74:26 to 55:45] | na | [85] | |
| Peucedanum ostruthium | Rh/CH2Cl2 | HPLC-DAD-MS | H2O (0.01% HOAc)‒MeCN (0.01% HOAc) [75:25 to 63:37 to 55:45 to 35:65 to 5:95] | 1.58 ± 0.03–25.05 ± 0.11 mg/g | [86] | |
| R/EtOAc | HPLC-DAD (RP-C30) HPLC-UV-ESI-MS | H2O (0.1% HOAc)‒MeOH [100:0 to 0:100] | na | [59] | ||
| Peucedanum palustre | R, St, L, umbel/MeOH | HPLC-DAD-ESI-HR-MS | H2O (0.01 M HCO2H)‒MeCN [100:0 to 40:60, 10:90] | 24.3 ± 14.0 mg/g (in R) 2.62 ± 1.56 mg/g (in St) 2.25 ± 1.28 mg/g (in L) 22.8 ± 30.9 mg/g (in umbel) | [87] | |
| R, umbel/MeOH | HPLC-MS | H2O (1% HCO2H)‒MeOH [40:60] H2O‒MeOH (1% HCO2H) [71:29, 0:100], H2O‒MeOH (1% HCO2H) [100:0 to 70:30, 40:60, 0:100, 100:0] | nd | [72] | ||
| R/NHEX | HPLC-DAD | THF‒MeCN‒MeOH‒H2O [3.1:35:5.4:56.5] | 0.16–0.44 mg/100 g | [88] | ||
| Prangos ferulacea | R/NHEX, hydro-ethanolic (95%), MeOH | HPLC-UV | H2O‒MeOH [30:70] | 59.38 ± 0.007 mg/g (extraction with Soxhlet, NHEX) 79.27 ± 0.22 mg/g (extraction with UAE, hydro-ethanolic 95%) 55.29 ± 0.01 mg/g (extraction with maceration, MeOH) | [89] | |
| Rutaceae | Atalantia ceylanica | Se/MeOH | HPLC-UV | nd | na | [90] |
| Citrus spp. | EO | HPLC-UV | NHEX‒isopropanol [98:2] | 0.7–1.65 g/L (Lemon EO from Sicily) 0.95 g/L (Lemon EO from Spain) 2.02 g/L (Lemon EO from Argentina) 0.49 g/L (Lime EO from Mexican 1) 0.96 g/L (Lime EO from Mexican 2) 0.68 g/L (Lime EO from Iran) | [91] | |
| EO | HPTLC | CH2Cl2‒DEE [100:3], CHCl3‒heptane [95:5] | na | [92] | ||
| EO | HPLC-UV | NHEX + EtOAc (92:8)‒NHEX + EtOH (90:10) | 1.55 ± 0.38 g/kg (extracted EO by Sfumatrice technology) 2.2 ± 0.41 g/kg (extracted EO by Pelatrice technology) 1.9 ± 0.45 g/kg (extracted EO by FMC technology) 0.86 ± 0.26 g/kg (extracted EO by Torchi technology) | [93] | ||
| Citrus aurantifolia & C. latifolia | EO | HPLC-DAD | H2O + MeOH + THF (85:10:5)‒MeOH + THF (95:5) [100:0, 60:40, 10:90, 100:0] | 4.09–10.54 g/L (C. aurantifolia) 0.27–10.72 g/L (C. latifolia) | [94] | |
| EO | HPLC-UV | NHEX + EtOAc (93:7)‒NHEX + EtOH (90:10) [100:0 to 5:95, 100:0] | 144 mg/100 g (C. aurantifolia) 210–328 mg/100 g (C. latifolia) | [95] | ||
| Citrus aurantifolia & C. latifolia & C. paradisi | EO | HPLC-DAD | H2O‒MeCN [70:30 to 60:40] | na | [67] | |
| Citrus limon | Wax/EO | UHPLC-DAD | H2O (0.1% HCO2H)‒MeCN [25:75, 100:0, 50:50, 30:70] | 62 ± 0.8 mg/kg | [96] | |
| EO | HPLC-DAD | NHEX + EtOAc (92:8)‒NHEX + EtOH (90:10) [100:0 to 0:100] | 89–157 mg/100 g | [66] | ||
| Citrus medica | Fr/EO | HPLC-DAD | H2O‒MeCN [70:30, 40:60, 0:100, 70:30] | 2.03–21.30 g/100 g | [97] | |
| Eureka limon | peel/EO | GC-MS | - | na | [98] | |
| Yuanhu zhitong (Chinese herbal drug) | Hydro-methanolic (75%) | UPLC-Q-TOF-MS | H2O (0.2% HCO2H)‒MeCN | na | [99] | |
| Hydro-methanolic (75%) | RRLC-QQQ | H2O (0.3% HCO2H)‒MeCN [80:20, 60:40, 20:80] | 0.12–4.01 µg/g | [100] |
2.3.2. Characterization of Oxypeucedanin in the Rutaceae Family
2.3.3. Identification of Oxypeucedanin from Other Natural Sources
3. Biological Activities of Oxypeucedanin
3.1. Antiallergic Activity
3.2. Antiarrhythmic Activity
3.3. Anticonvulsant Activity
3.4. Antifeedant Activity
3.5. Antigenotoxic Activity
3.6. Anti-Inflammatory Activity
3.7. Antimalarial Activity
3.8. Antimicrobial Activity
3.9. Antioxidant Activity
3.10. Antiproliferative Activity
3.11. Antiviral Activity
3.12. Calcium Antagonistic Activity
3.13. Cytotoxic Activity
3.14. Enzyme Inhibitory Activity
3.15. Insecticidal Activity
3.16. Phytotoxic Activity
4. Pharmacokinetic Analysis of Oxypeucedanin
5. Conclusions and Perspectives
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bruni, R.; Barreca, D.; Protti, M.; Brighenti, V.; Righetti, L.; Anceschi, L.; Mercolini, L.; Benvenuti, S.; Gattuso, G.; Pellati, F. Botanical sources, chemistry, analysis, and biological activity of furanocoumarins of pharmaceutical interest. Molecules 2019, 24, 2163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, L.P. Polyphenols and polyphenol-derived compounds and contact dermatitis. In Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2013; Volume 1, pp. 793–818. ISBN 9780123984562. [Google Scholar]
- Richard, E.G. The science and (Lost) art of psoralen plus UVA phototherapy. Dermatol. Clin. 2020, 38, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Berakha, G.J.; Lefkovits, G. Psoralen phototherapy and phototoxicity. Ann. Plast. Surg. 1985, 14, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Melough, M.M.; Cho, E.; Chun, O.K. Furocoumarins: A review of biochemical activities, dietary sources and intake, and potential health risks. Food Chem. Toxicol. 2018, 113, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Bourgaud, F.; Olry, A.; Hehn, A. Recent advances in molecular genetics of furanocoumarin synthesis in higher plants. In Recent Advances in Redox Active Plant and Microbial Products: From Basic Chemistry to Widespread Applications in Medicine and Agriculture; Springer: Dordrecht, The Netherlands, 2014; pp. 363–375. ISBN 9789401789530. [Google Scholar]
- National Center for Biotechnology Information PubChem Compound Summary for CID 5359227. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Pentyl-acetate#section=Names-and-Identifiers%0Ahttps://pubchem.ncbi.nlm.nih.gov/compound/alpha-Mangostin%0Ahttps://pubchem.ncbi.nlm.nih.gov/compound/5359227 (accessed on 31 December 2020).
- Schulzová, V.; Hajšlová, J.; Botek, P.; Peroutka, R. Furanocoumarins in vegetables: Influence of farming system and other factors on levels of toxicants. J. Sci. Food Agric. 2007, 87, 2763–2767. [Google Scholar] [CrossRef]
- Hung, W.L.; Suh, J.H.; Wang, Y. Chemistry and health effects of furanocoumarins in grapefruit. J. Food Drug Anal. 2017, 25, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Dugrand-Judek, A.; Olry, A.; Hehn, A.; Costantino, G.; Ollitrault, P.; Froelicher, Y.; Bourgaud, F. The distribution of coumarins and furanocoumarins in Citrus species closely matches Citrus phylogeny and reflects the organization of biosynthetic pathways. PLoS ONE 2015, 10, e0142757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mottaghipisheh, J.; Kiss, T.; Tóth, B.; Csupor, D. The Prangos genus: A comprehensive review on traditional use, phytochemistry, and pharmacological activities. Phytochem. Rev. 2020, 19, 1449–1470. [Google Scholar] [CrossRef]
- Stavri, M.; Gibbons, S. The antimycobacterial constituents of dill (Anethum graveolens). Phyther. Res. 2005, 19, 938–941. [Google Scholar] [CrossRef]
- Härmälä, P.; Vuorela, H.; Hiltunen, R.; Nyiredy, S.; Sticher, O.; Törnquist, K.; Kaltia, S. Strategy for the isolation and identification of coumarins with calcium antagonistic properties from the roots of Angelica archangelica. Phytochem. Anal. 1992, 3, 42–48. [Google Scholar] [CrossRef]
- Fan, G.; Deng, R.; Zhou, L.; Meng, X.; Kuang, T.; Lai, X.; Zhang, J.; Zhang, Y. Development of a rapid resolution liquid chromatographic method combined with chemometrics for quality control of Angelicae dahuricae radix. Phytochem. Anal. 2012, 23, 299–307. [Google Scholar] [CrossRef]
- Wang, C.C.; Lai, J.E.; Chen, L.G.; Yen, K.Y.; Yang, L.L. Inducible nitric oxide synthase inhibitors of Chinese herbs. Part 2: Naturally occurring furanocoumarins. Bioorganic Med. Chem. 2000, 8, 2701–2707. [Google Scholar] [CrossRef]
- Eun, J.S.; Park, J.A.; Choi, B.H.; Cho, S.K.; Kim, D.K.; Kwak, Y.G. Effects of oxypeucedanin on hKv1.5 and action potential duration. Biol. Pharm. Bull. 2005, 28, 657–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dae, K.K.; Jong, P.L.; Jae, H.Y.; Dong, O.E.; Jae, S.E.; Kang, H.L. Acetylcholinesterase inhibitors from the roots of Angelica dahurica. Arch. Pharm. Res. 2002, 25, 856–859. [Google Scholar] [CrossRef]
- Oh, H.; Lee, H.S.; Kim, T.; Chai, K.Y.; Chung, H.T.; Kwon, T.O.; Jun, J.Y.; Jeong, O.S.; Kim, Y.C.; Yun, Y.G. Furocoumarins from Angelica dahurica with hepatoprotective activity on tacrine-induced cytotoxicity in Hep G2 cells. Planta Med. 2002, 68, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.D.; Kim, J.Y.; Ryu, H.W.; Kim, J.H.; Han, S.I.; Ra, J.E.; Seo, K.H.; Jang, K.C.; Lee, J.H. Identification and characterisation of coumarins from the roots of Angelica dahurica and their inhibitory effects against cholinesterase. J. Funct. Foods 2013, 5, 1421–1431. [Google Scholar] [CrossRef]
- Lee, B.W.; Ha, T.K.Q.; Cho, H.M.; An, J.P.; Kim, S.K.; Kim, C.S.; Kim, E.; Oh, W.K. Antiviral activity of furanocoumarins isolated from Angelica dahurica against influenza a viruses H1N1 and H9N2. J. Ethnopharmacol. 2020, 259, 112945. [Google Scholar] [CrossRef]
- Park, S.H.; Hong, J.Y.; Park, H.J.; Lee, S.K. The antiproliferative activity of oxypeucedanin via induction of G2/M phase cell cycle arrest and p53-dependent MDM2/p21 expression in human hepatoma cells. Molecules 2020, 25, 501. [Google Scholar] [CrossRef] [Green Version]
- Marumoto, S.; Miyazawa, M. β-secretase inhibitory effects of furanocoumarins from the root of Angelica dahurica. Phyther. Res. 2010, 24, 510–513. [Google Scholar] [CrossRef]
- Piao, X.L.; Park, I.H.; Baek, S.H.; Kim, H.Y.; Park, M.K.; Park, J.H. Antioxidative activity of furanocoumarins isolated from Angelicae dahuricae. J. Ethnopharmacol. 2004, 93, 243–246. [Google Scholar] [CrossRef]
- Thanh, P.N.; Jin, W.Y.; Song, G.Y.; Bae, K.H.; Kang, S.S. Cytotoxic coumarins from the root of Angelica dahurica. Arch. Pharm. Res. 2004, 27, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Mottaghipisheh, J.; Iriti, M. Sephadex® LH-20, Isolation, and purification of flavonoids from plant species: A comprehensive review. Molecules 2020, 25, 4146. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Okuda, H.; Baba, K. Histamine-release effectors from Angelica dahurica var. dahurica root. J. Nat. Prod. 1997, 60, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Sumiyoshi, M.; Sakanaka, M.; Taniguchi, M.; Baba, K. In vitro and in vivo antiproliferative effect of a combination of ultraviolet-A and alkoxy furocoumarins isolated from umbelliferae medicinal plants, in melanoma cells. Photochem. Photobiol. 2013, 89, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, L. Coumarins from the roots of Angelica dahurica cause anti-allergic inflammation. Exp. Ther. Med. 2017, 14, 874–880. [Google Scholar] [CrossRef] [Green Version]
- Song, D.K.; Kim, J.Y.; Li, G.; Lee, K.S.; Seo, C.S.; Yan, J.J.; Jung, J.S.; Kim, H.J.; Chang, H.W.; Son, J.K. Agents protecting against sepsis from the roots of Angelica dahurica. Biol. Pharm. Bull. 2005, 28, 380–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, B.M.; Liu, Y. High speed counter current chromatography: Overview of solvent-system and elution-mode. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 629–636. [Google Scholar] [CrossRef]
- Wei, Y.; Ito, Y. Isolation of imperatorin, oxypeucedanin, and isoimperatorin from Angelica dahurica (Fisch. ex Hoffm) Benth. et Hook by stepwise flow rate high-speed countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 1609–1618. [Google Scholar] [CrossRef]
- Wei, Y.; Ito, Y. Preparative isolation of imperatorin, oxypeucedanin and isoimperatorin from traditional Chinese herb “bai zhi” Angelica dahurica (Fisch. ex Hoffm) Benth. et Hook using multidimensional high-speed counter-current chromatography. J. Chromatogr. A 2006, 1115, 112–117. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Xie, Q.; Fisher, D.; Sutherland, I.A. Preparative isolation of imperatorin, oxypeucedanin and isoimperatorin from a traditional Chinese herb using a HSCCC strategy based on optimization of rapid flow rate. Chromatographia 2009, 70, 1185–1189. [Google Scholar] [CrossRef]
- Wu, X.; Chao, Z.; Wang, C.; Yu, L. Separation of chemical constituents from three plant medicines by counter-current chromatography using a three-phase solvent system at a novel ratio. J. Chromatogr. A 2015, 1384, 107–114. [Google Scholar] [CrossRef]
- Kim, Y.K.; Young, S.K.; Shi, Y.R. Antiproliferative effect of furanocoumarins from the root of Angelica dahurica on cultured human tumor cell lines. Phyther. Res. 2007, 21, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Morikawa, T.; Ohgushi, T.; Ishiwada, T.; Nishida, N.; Yoshikawa, M. Inhibitors of nitric oxide production from the flowers of Angelica furcijuga: Structures of hyuganosides IV and V. Chem. Pharm. Bull. 2005, 53, 387–392. [Google Scholar] [CrossRef] [Green Version]
- Seo, E.K.; Kyeong, H.K.; Min, K.K.; Cho, M.H.; Choi, E.W.; Kim, K.N.; Mar, W. Inhibitors of 5α-reductase type I in LNCaP cells from the roots of Angelica koreana. Planta Med. 2002, 68, 162–163. [Google Scholar] [CrossRef] [PubMed]
- Mileski, K.S.; Trifunović, S.S.; Ćirić, A.D.; Šakić, Ž.M.; Ristić, M.S.; Todorović, N.M.; Matevski, V.S.; Marin, P.D.; Tešević, V.V.; Džamić, A.M. Research on chemical composition and biological properties including antiquorum sensing activity of Angelica pancicii Vandas aerial parts and roots. J. Agric. Food Chem. 2017, 65, 10933–10949. [Google Scholar] [CrossRef] [Green Version]
- Karakaya, S.; Bingol, Z.; Koca, M.; Dagoglu, S.; Pınar, N.M.; Demirci, B.; Gulcin, İ.; Brestic, M.; Sytar, O. Identification of non-alkaloid natural compounds of Angelica purpurascens (Avé-Lall.) Gilli. (Apiaceae) with cholinesterase and carbonic anhydrase inhibition potential. Saudi Pharm. J. 2020, 28, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Marston, A.; Hostettmann, K.; Msonthi, J.D. Isolation of antifungal and larvicidal constituents of Diplolophium buchanani by centrifugal partition chromatography. J. Nat. Prod. 1995, 58, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Mottaghipisheh, J.; Nové, M.; Spengler, G.; Kúsz, N.; Hohmann, J.; Csupora, D. Antiproliferative and cytotoxic activities of furocoumarins of Ducrosia anethifolia. Pharm. Biol. 2018, 56, 658–664. [Google Scholar] [CrossRef] [Green Version]
- Monsefesfahani, H.; Farimani, M.M.; Ebrahimi, S.N.; Jung, J.H.; Aliahmadi, A.; Abbas-Mohammadi, M.; Skropeta, D.; Kazemian, H.; Feizabadi, M.; Miran, M. Antibacterial components of Levisticum officinale koch against multidrug-resistant Mycobacterium tuberculosis. Pharm. Sci. 2020, 26, 441–447. [Google Scholar] [CrossRef]
- Bagherifar, S.; Sourestani, M.M.; Zolfaghari, M.; Mottaghipisheh, J.; Zomborszki, Z.P.; Csupor, D. Variation of chemical constituents and antiradical capacity of nine Ferulago angulata populations from Iran. Chem. Biodivers. 2019, 16, 21–25. [Google Scholar] [CrossRef] [Green Version]
- Razavi, S.M.; Ravansalar, A.; Mirinejad, S. The investigation on phytochemicals from Ferulago angulata (Schlecht) Boiss, indigenous to central parts of Iran. Nat. Prod. Res. 2015, 29, 2037–2040. [Google Scholar] [CrossRef]
- Goodarzi, S.; Tavakoli, S.; Abai, M.R.; Amini, Z.; Vatandoost, H.; Yassa, N.; Hadjiakhoondi, A.; Tofighi, Z. Strong insecticidal potential of methanol extract of Ferulago trifida fruits against Anopheles stephensi as malaria vector. Environ. Sci. Pollut. Res. 2019, 26, 7711–7717. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Delnavazi, M.R.; Hadjiaghaee, R.; Jafari-Nodooshan, S.; Khalighi-Sigaroodi, F.; Akhbari, M.; Hadjiakhoondi, A.; Yassa, N. Bioactive coumarins from the roots and fruits of Ferulago trifida Boiss., an endemic species to Iran. Nat. Prod. Res. 2018, 32, 2724–2728. [Google Scholar] [CrossRef] [PubMed]
- Khalighi-Sigaroodi, F.; Hadjiakhoondi, A.; Shafiee, A.; Mozaffarian, V.A.; Shahverdi, A.R.; Alavi, S.H.R. Phytochemical analysis of Ferulogo bernardii Tomk & M.Pimen. Daru 2006, 14, 214–221. [Google Scholar]
- Jiménez, B.; Grande, M.C.; Anaya, J.; Torres, P.; Grande, M. Coumarins from Ferulago capillaris and F. brachyloba. Phytochemistry 2000, 53, 1025–1031. [Google Scholar] [CrossRef]
- Süzgeç-Selçuk, S.; Anıl, S.; Rayaman, E.; Gürer, S.; Özsoy, N.; Uruşak, E.A. The biological activities and phytochemical content of Ferulago humulis boiss. Indian J. Tradit. Knowl. 2020, 19, 728–735. [Google Scholar]
- Gohari, A.R.; Naseri, M.; Monsef-Esfehani, H.R.; Saeidnia, S.; Dastan, D. Antioxidative coumarins from the roots of Ferulago subvelutina. Asian J. Chem. 2013, 25, 1875–1878. [Google Scholar] [CrossRef]
- Guz, N.R.; Lorenz, P.; Stermitz, F.R. New coumarins from Harbouria trachypleura: Isolation and synthesis. Tetrahedron Lett. 2001, 42, 6491–6494. [Google Scholar] [CrossRef]
- Choi, J.S.; Shin, H.Y.; Kwon, K.S.; Shin, S.; Choung, S.Y.; Kwon, Y.S.; Lee, J.W.; Choi, B.H.; Lee, C.K. Effects of oxypeucedanin on global gene expression and MAPK signaling pathway in mouse neuroblastoma neuro-2A cells. Planta Med. 2011, 77, 1512–1518. [Google Scholar] [CrossRef] [Green Version]
- Kang, T.J.; Lee, S.Y.; Singh, R.P.; Agarwal, R.; Yim, D.S. Anti-tumor activity of oxypeucedanin from Ostericum koreanum against human prostate carcinoma DU145 cells. Acta Oncol. 2009, 48, 895–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.K.; Ling, J.H.; Chun, M.H.; Jeong, J.H.; Na, Y.C.; Lee, K.W.; Jung, J.H.; Hong, J. Simultaneous determination of biological marker compounds in Ostericum koreanum by HPLC method and discrimination by principal component analysis. Bull. Korean Chem. Soc. 2008, 29, 2465–2470. [Google Scholar] [CrossRef] [Green Version]
- Beier, R.C.; Ivie, G.W.; Oertli, E.H. Linear furanocoumarins and graveolone from the common herb parsley. Phytochemistry 1994, 36, 869–872. [Google Scholar] [CrossRef]
- Chaudhary, S.K.; Ceska, O.; Tetu, C. Oxypeucedanin, a major furocoumarin in parsley, Petroselinum crispum. Planta Med. 1986, No. 6, 462–464. [Google Scholar] [CrossRef]
- Sbai, H.; Saad, I.; Ghezal, N.; Della Greca, M.; Haouala, R. Bioactive compounds isolated from Petroselinum crispum L. leaves using bioguided fractionation. Ind. Crops Prod. 2016, 89, 207–214. [Google Scholar] [CrossRef]
- Manderfeld, M.M.; Schafer, H.W.; Davidson, P.M.; Zottola, E.A. Isolation and identification of antimicrobial furocoumarins from parsley. J. Food Prot. 1997, 60, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Skalicka-Woźniak, K.; Mroczek, T.; Kozioł, E. High-performance countercurrent chromatography separation of Peucedanum cervaria fruit extract for the isolation of rare coumarin derivatives. J. Sep. Sci. 2015, 38, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Gökay, O.; Kühner, D.; Los, M.; Götz, F.; Bertsche, U.; Albert, K. An efficient approach for the isolation, identification and evaluation of antimicrobial plant components on an analytical scale, demonstrated by the example of Radix imperatoriae. Anal. Bioanal. Chem. 2010, 398, 2039–2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagherifar, S.; Sourestani, M.M.; Zolfaghari, M.; Mottaghipisheh, J.; Zomborszki, Z.P.; Csupor, D. Chemodiversity of volatile oil contents of various parts of 10 Iranian Prangos ferulacea accessions, with analysis of antiradical potential. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- Shokoohinia, Y.; Sajjadi, S.E.; Gholamzadeh, S.; Fattahi, A.; Behbahani, M. Antiviral and cytotoxic evaluation of coumarins from Prangos ferulacea. Pharm. Biol. 2014, 52, 1543–1549. [Google Scholar] [CrossRef] [Green Version]
- Numonov, S.; Bobakulov, K.; Numonova, M.; Sharopov, F.; Setzer, W.N.; Khalilov, Q.; Begmatov, N.; Habasi, M.; Aisa, H.A. New coumarin from the roots of Prangos pabularia. Nat. Prod. Res. 2018, 32, 2325–2332. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.M.; Zahri, S.; Motamed, Z.; Ghasemi, G. Bioscreening of oxypeucedanin, a known furanocoumarin. Iran. J. Basic Med. Sci. 2010, 13, 133–138. [Google Scholar] [CrossRef]
- Murakami, A.; Gao, G.; Kim, O.K.; Omura, M.; Yano, M.; Ito, C.; Furukawa, H.; Jiwajinda, S.; Koshimizu, K.; Ohigashi, H. Identification of coumarins from the fruit of Citrus hystrix DC as inhibitors of nitric oxide generation in mouse macrophage raw 264.7 cells. J. Agric. Food Chem. 1999, 47, 333–339. [Google Scholar] [CrossRef]
- Arimoto, Y.; Sugawara, F.; Yoshida, S.; Yamaguchi, I. Prangolarin is a chemical facilitator for the enhanced development of the infection process in the epicarp of Citrus limon by Penicillium digitatum. J. Agric. Food Chem. 1995, 43, 2283–2285. [Google Scholar] [CrossRef]
- Dugo, P.; Mondello, L.; Cogliandro, E.; Cavazza, A.; Dugo, G. On the genuineness of citrus essential oils. Part LIII. Determination of the composition of the oxygen heterocyclic fraction of lemon essential oils (Citrus limon (L.) Burm. f.) by normal-phase high performance liquid chromatography. Flavour Fragr. J. 1998, 13, 329–334. [Google Scholar] [CrossRef]
- Feger, W.; Brandauer, H.; Gabris, P.; Ziegler, H. Nonvolatiles of commercial lime and grapefruit oils separated by high-speed countercurrent chromatography. J. Agric. Food Chem. 2006, 54, 2242–2252. [Google Scholar] [CrossRef]
- Escoubas, P.; Fukushi, Y.; Lajide, L.; Mizutani, J. A new method for fast isolation of insect antifeedant compounds from complex mixtures. J. Chem. Ecol. 1992, 18, 1819–1832. [Google Scholar] [CrossRef]
- Ross, S.A.; Krishnaven, K.; Radwan, M.M.; Takamatsu, S.; Burandt, C.L. Constituents of Zanthoxylum flavum and their antioxidant and antimalarial activities. Nat. Prod. Commun. 2008, 3, 791–794. [Google Scholar] [CrossRef] [Green Version]
- Sigurdsson, S.; Jonsdottir, S.; Gudbjarnason, S. Geographical variation of the furanocoumarin composition of the fruits of icelandic Angelica archangelica. Zeitschrift fur Naturforsch. Sect. C J. Biosci. 2012, 67, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Eeva, M.; Rauha, J.P.; Vuorela, P.; Vuorela, H. Computer-assisted, high-performance liquid chromatography with mass spectrometric detection for the analysis of coumarins in Peucedanum palustre and Angelica archangelica. Phytochem. Anal. 2004, 15, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Zhang, H.F.; Hu, S.; Bai, X.H.; Li, S. Dispersive liquid-liquid microextraction coupled with high-performance liquid chromatography for determination of coumarin compounds in Radix Angelicae dahuricae. Chromatographia 2012, 75, 131–137. [Google Scholar] [CrossRef]
- Li, F.; Song, Y.; Wu, J.; Chen, X.; Hu, S.; Zhao, H.; Bai, X. Hollow fibre cell fishing and hollow fibre liquid phase microextraction research on the anticancer coumarins of Radix Angelicae dahuricae in vitro and in vivo. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 79–88. [Google Scholar] [CrossRef]
- Liang, W.H.; Chang, T.W.; Charng, Y.C. Influence of harvest stage on the pharmacological effect of Angelica dahurica. Bot. Stud. 2018, 59, 14. [Google Scholar] [CrossRef]
- Liang, W.H.; Chang, T.W.; Charng, Y.C. Effects of drying methods on contents of bioactive compounds and antioxidant activities of Angelica dahurica. Food Sci. Biotechnol. 2018, 27, 1085–1092. [Google Scholar] [CrossRef]
- Pfeifer, I.; Murauer, A.; Ganzera, M. Determination of coumarins in the roots of Angelica dahurica by supercritical fluid chromatography. J. Pharm. Biomed. Anal. 2016, 129, 246–251. [Google Scholar] [CrossRef]
- Yang, L.; Li, Q.; Feng, Y.; Qiu, D. Simultaneous determination of three coumarins in Angelica dahurica by 1H-qNMR method: A fast and validated method for crude drug quality control. J. Anal. Methods Chem. 2020, 2020, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Liu, J.; Cai, H.; Li, S.; Ma, X.; Lou, Y.; Qin, K.; Guan, H.; Cai, B. Novel characterization of Radix Angelicae dahuricae before and after the sulfur-fumigation process by combining high performance liquid chromatographic fingerprint and multi-ingredients determination. Pharmacogn. Mag. 2014, 10, 338–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, A.Y.; Park, S.Y.; Lee, J.; Jung, M.; Kim, J.; Kang, S.S.; Youm, J.R.; Han, S.B. Simultaneous determination of five coumarins in Angelicae dahuricae Radix by HPLC/UV and LC-ESI-MS/MS. Biomed. Chromatogr. 2009, 23, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Kim, C.Y.; Song, D.G.; Pan, C.H.; Cha, K.H.; Lee, D.U.; Um, B.H. Rapid identification of furanocoumarins in Angelica dahurica using the online LC-MMR-MS and their nitric oxide inhibitory activity in RAW 264.7 cells. Phytochem. Anal. 2010, 21, 322–327. [Google Scholar] [CrossRef]
- Yu, M.; Li, T.; Raza, A.; Wang, L.; Song, H.; Zhang, Y.; Li, L.; Hua, Y. Sensory-guided identification of bitter compounds in Hangbaizhi (Angelica dahurica). Food Res. Int. 2020, 129, 108880. [Google Scholar] [CrossRef]
- Kim, S.; Kim, K.Y.; Han, C.S.; Ki, K.S.; Min, K.J.; Zhang, X.; Whang, W.K. Simultaneous analysis of six major compounds in osterici radix and notopterygii rhizoma et radix by HPLC and discrimination of their origins from chemical fingerprint analysis. Arch. Pharm. Res. 2012, 35, 691–699. [Google Scholar] [CrossRef]
- Caboni, P.; Saba, M.; Oplos, C.; Aissani, N.; Maxia, A.; Menkissoglu-Spiroudi, U.; Casu, L.; Ntalli, N. Nematicidal activity of furanocoumarins from parsley against Meloidogyne spp. Pest Manag. Sci. 2015, 71, 1099–1105. [Google Scholar] [CrossRef]
- Skalicka-Woźniak, K.; Markowski, W.; Świeboda, R.; Głowniak, K. Computer-assisted searching for coumarins in Peucedanum alsaticum L. and Peucedanum cervaria (L.) Lap. Acta Chromatogr. 2009, 21, 531–546. [Google Scholar] [CrossRef]
- Vogl, S.; Zehl, M.; Picker, P.; Urban, E.; Wawrosch, C.; Reznicek, G.; Saukel, J.; Kopp, B. Identification and quantification of coumarins in Peucedanum ostruthium (L.) Koch by HPLC-DAD and HPLC-DAD-MS. J. Agric. Food Chem. 2011, 59, 4371–4377. [Google Scholar] [CrossRef]
- Yrjönen, T.; Eeva, M.; Kauppila, T.J.; Martiskainen, O.; Summanen, J.; Vuorela, P.; Vuorela, H. Profiling of coumarins in Peucedanum palustre (L.) Moench populations growing in Finland. Chem. Biodivers. 2016, 13, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Vuorela, H.; Dallenbach-Tölke, K.; Nyiredy, S.; Hiltunen, R.; Sticher, O. HPLC analysis of the main furanocoumarins from Peucedanum palustre. Planta Med. 1989, 55, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Gholivand, M.B.; Yamini, Y.; Dayeni, M.; Shokoohinia, Y. The influence of the extraction mode on three coumarin compounds yield from Prangos ferulacea (L.) Lindl roots. J. Iran. Chem. Soc. 2015, 12, 707–714. [Google Scholar] [CrossRef]
- Bacher, M.; Brader, G.; Hofer, O.; Greger, H. Oximes from seeds of Atalantia ceylanica. Phytochemistry 1999, 50, 991–994. [Google Scholar] [CrossRef]
- Dugo, P.; Russo, M.; Sarò, M.; Carnovale, C.; Bonaccorsi, I.; Mondello, L. Multidimensional liquid chromatography for the determination of chiral coumarins and furocoumarins in Citrus essential oils. J. Sep. Sci. 2012, 35, 1828–1836. [Google Scholar] [CrossRef]
- Le Borgne, E.; Cicchetti, E.; Bertrand, T. HPTLC methods for qualitative and quantitative analysis of selected furocoumarins in essential oils. Flavour Fragr. J. 2017, 32, 330–339. [Google Scholar] [CrossRef]
- Verzera, A.; Dugo, P.; Mondello, L.; Trozzi, A.; Cotroneo, A. Extraction technology and lemon oil composition. Ital. J. Food Sci. 1999, 11, 361–370. [Google Scholar]
- Bonaccorsi, I.; Dugo, P.; Mondello, L.; Sciarrone, D.; Dugo, G.; Haro-Guzman, L. Analytical characterization of industrial essential oils from fruits and leaves of C. Aurantifolia Tan. and C. latifolia Swing. J. Essent. Oil Res. 2011, 23, 68–79. [Google Scholar] [CrossRef]
- Dugo, P.; Mondello, L.; Lamonica, G.; Dugo, G. Characterization of cold-pressed Key and Persian lime oils by gas chromatography, gas chromatography/mass spectroscopy, high-performance liquid chromatography, and physicochemical indices. J. Agric. Food Chem. 1997, 45, 3608–3616. [Google Scholar] [CrossRef]
- Costa, R.; Albergamo, A.; Arrigo, S.; Gentile, F.; Dugo, G. Solid-phase microextraction-gas chromatography and ultra-high performance liquid chromatography applied to the characterization of lemon wax, a waste product from citrus industry. J. Chromatogr. A 2019, 1603, 262–268. [Google Scholar] [CrossRef]
- Gabriele, B.; Fazio, A.; Dugo, P.; Costa, R.; Mondello, L. Essential oil composition of Citrus medica L. Cv diamante (diamante citron) determined after using different extraction methods. J. Sep. Sci. 2009, 32, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Barth, D.; Chouchi, D.; Della Porta, G.; Reverchon, E.; Perrut, M. Desorption of lemon peel oil by supercritical carbon dioxide: Deterpenation and psoralens elimination. J. Supercrit. Fluids 1994, 7, 177–183. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Y.; Tao, Y.; Huang, B.; Shen, D.; Li, G.; Yang, H. Study of chemical fingerprint for yuanhu zhitong tablet by UPLC/Q-TOF-MS. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 807–820. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H.; Chen, X.; Chen, C.; Wang, H.; Meng, F.; Yang, H.; Huang, L. Simultaneous quantification of 17 constituents from Yuanhu Zhitong tablet using rapid resolution liquid chromatography coupled with a triple quadrupole electrospray tandem mass spectrometry. J. Pharm. Biomed. Anal. 2011, 56, 497–504. [Google Scholar] [CrossRef]
- Łuszczki, J.J.; Andres-Mach, M.; Gleńsk, M.; Skalicka-Woźniak, K. Anticonvulsant effects of four linear furanocoumarins, bergapten, imperatorin, oxypeucedanin, and xanthotoxin, in the mouse maximal electroshock-induced seizure model: A comparative study. Pharmacol. Rep. 2010, 62, 1231–1236. [Google Scholar] [CrossRef]
- Singhuber, J.; Baburin, I.; Ecker, G.F.; Kopp, B.; Hering, S. Insights into structure-activity relationship of GABA A receptor modulating coumarins and furanocoumarins. Eur. J. Pharmacol. 2011, 668, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Ballesta-Acosta, M.C.; Pascual-Villalobos, M.J.; Rodríguez, B. Short communication. The antifeedant activity of natural plant products towards the larvae of Spodoptera littoralis. Spanish J. Agric. Res. 2008, 6, 85–91. [Google Scholar] [CrossRef]
- Marumoto, S.; Oda, Y.; Miyazawa, M. Antigenotoxic activity of naturally occurring furanocoumarins. Environ. Mol. Mutagen. 2011, 52, 646–657. [Google Scholar] [CrossRef]
- Buffet, P.A.; Safeukui, I.; Deplaine, G.; Brousse, V.; Prendki, V.; Thellier, M.; Turner, G.D.; Mercereau-Puijalon, O. The pathogenesis of Plasmodium falciparum malaria in humans: Insights from splenic physiology. Blood 2011, 117, 381–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, W.; Liao, Z.G.; Zhao, G.W.; Guan, X.J.; Zhang, J.; Liang, X.L.; Yang, M. Reversal effect of oxypeucedanin on p-glycoprotein-mediated drug transport. Molecules 2018, 23, 1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, A.; Faraoni, M.; Castro, M.; Alza, N.; Cavallaro, V. Natural AChE inhibitors from plants and their contribution to Alzheimer’s disease therapy. Curr. Neuropharmacol. 2013, 11, 388–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa Filho, J.M.; Medeiros, K.C.P.; de Diniz, M.F.F.M.; Batista, L.M.; Athayde-Filho, P.F.; Silva, M.S.; da Cunha, E.V.L.; Almeida, J.R.G.S.; Quintans-Júnior, L.J. Natural products inhibitors of the enzyme acetylcholinesterase. Rev. Bras. Farmacogn. 2006, 16, 258–285. [Google Scholar] [CrossRef] [Green Version]
- Moodie, L.W.K.; Sepcic, K.; Turk, T.; Frangez, R.; Svenson, J. Natural cholinesterase inhibitors from marine organisms. Nat. Prod. Rep. 2019, 36, 1053–1092. [Google Scholar] [CrossRef]
- Dos Santos, T.C.; Gomes, T.M.; Pinto, B.A.S.; Camara, A.L.; De Andrade Paes, A.M. Naturally occurring acetylcholinesterase inhibitors and their potential use for Alzheimer’s disease therapy. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Kumar, T.; Chaiyasut, C.; Rungseevijitprapa, W.; Suttajit, M. Screening of steroid 5α-reductase inhibitory activity and total phenolic content of Thai plants. J. Med. Plants Res. 2011, 5, 1265–1271. [Google Scholar]
- Chen, L.; Yang, H.; Yu, C.; Yuan, M.; Li, H. High hepatic exposure of furanocoumarins in Radix Angelica dahuricae is associated with transporter mediated active uptake. J. Ethnopharmacol. 2018, 212, 74–85. [Google Scholar] [CrossRef]
- Huo, H.; Yu, S.; Liu, X.; Meng, Y.; Ren, Y.; Zhang, L. Simultaneous and sensitive determination of eight coumarins in rat bile and urine after oral administration of Radix Angelicae dahuricae extract by liquid chromatography-electrospray ionization-mass spectrometry. Acta Chromatogr. 2013, 25, 201–219. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Yang, H.J.; Ma, J.Y. Simultaneous determination of three furanocoumarins by UPLC/MS/MS: Application to pharmacokinetic study of Angelica dahurica radix after oral administration to normal and experimental colitis-induced rats. Molecules 2017, 22, 416. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Peng, C.; Du, W.; Wang, S. Pharmacokinetic study of eight coumarins of Radix Angelicae dahuricae in rats by gas chromatography-mass spectrometry. Fitoterapia 2013, 89, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Shi, X.; Yang, W.; Liu, S.; Wang, N.; Shi, R.; Qiao, S.; Wang, Q.; Wang, Y. Quantitative analysis of nine coumarins in rat urine and bile after oral administration of Radix Glehniae extract by high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Biomed. Chromatogr. 2011, 25, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.L.; Zhang, Y.B.; Xu, W.; Kang, T.G.; Yang, X.W. Biotransformation of isoimperatorin by rat liver microsomes and its quantification by LC-MS/MS method. Fitoterapia 2014, 93, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Sharma, R.; Jaswal, V.S.; Nepovimova, E.; Chaudhary, A.; Kuca, K. Psoralen: A Biologically important coumarin with emerging applications. Mini. Rev. Med. Chem. 2020, 20, 1838–1845. [Google Scholar] [CrossRef]

| Plant Species/Purchased | Analytical Instrument | Method of Assessment | Concentration in Living Organisms | Reference |
|---|---|---|---|---|
| Angelica dahurica | UHPLC‒ELSD | AF (0.1% HCO2H)‒MeCN (0.1% HCO2H) [95:5, 86:14, 78:22, 70:30, 66:34, 62:38, 56:44, 16:84, 5:95, 95:5] | C: 0.05 µg/g in lung C: 0.013 µg/g in liver | [113] |
| HPLC–ESI–MS | H2O (0.1% HCO2H)‒MeOH [28:72] | 0.177% (in bile) ‒ 0.082% (in rat urine) | [114] | |
| UHPLC-MS/MS | H2O (0.1% HCO2H)‒MeCN [65:35, 40:60] | 6.67% Cmax: 38.5 ± 1.6 ng/mL at 0.5 g/kg (in normal rats) Cmax: 101.2 ± 21.2 ng/mL at 1.0 g/kg (in normal rats) Cmax: 29.0 ± 4.0 ng/mL at 0.5 g/kg (in TNBS-treated rats) Cmax: 61.2 ± 11.9 ng/mL at 1.0 g/kg (in TNBS-treated rats) | [115] | |
| GC-MS | - | Cmax: 0.46 ± 0.01 µg/mL Tmax: 0.51 h | [116] | |
| LC-MS/MS | H2O (0.1% HCO2H)‒MeCN [65:35, 53:47, 15:85, 65:35] | Cmax: 111 ± 25 ng/mL at 4.5 g/kg extract/5.2 mg/kg OP (in rat plasma) Tmax: 12 ± 0 h | [118] | |
| Glehnia littoralis | HPLC-ESI-MS/MS | H2O (1 mmol AAc)‒MeOH [60:40, 10:90, 60:40] | 19.0 µg/mL (in plant sample) 13 ng (in rat urine after 72 h oral administration) 18 ng (in rat bile after 72 h oral administration) | [117] |
| Purchased | LC-MS/MS | H2O (0.1% HCO2H)‒MeCN [70:30, 52:48, 30:70, 70:30] | Cmax: 118.40 ± 10.93 µg/L (docetaxel in rat plasma) Cmax: 178.80 ± 8.81 µg/L (OP + docetaxel in rat plasma) Tmax: 163.80 ± 11.82 min (docetaxel) Tmax: 104.60 ± 11.68 min (OP + docetaxel) | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mottaghipisheh, J. Oxypeucedanin: Chemotaxonomy, Isolation, and Bioactivities. Plants 2021, 10, 1577. https://doi.org/10.3390/plants10081577
Mottaghipisheh J. Oxypeucedanin: Chemotaxonomy, Isolation, and Bioactivities. Plants. 2021; 10(8):1577. https://doi.org/10.3390/plants10081577
Chicago/Turabian StyleMottaghipisheh, Javad. 2021. "Oxypeucedanin: Chemotaxonomy, Isolation, and Bioactivities" Plants 10, no. 8: 1577. https://doi.org/10.3390/plants10081577