Essential Oil from Zingiber ottensii Induces Human Cervical Cancer Cell Apoptosis and Inhibits MAPK and PI3K/AKT Signaling Cascades
Abstract
:1. Introduction
2. Results
2.1. ZO Essential Oil Stimulates Apoptosis in HeLa Cells
2.2. Effects of ZO Essential Oil on Decreasing IL-6 Production in HeLa Cells
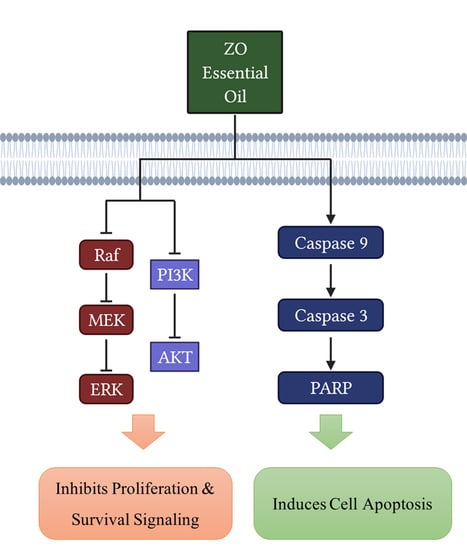
2.3. Effects of ZO Essential Oil on Inducing Apoptosis Signaling in HeLa Cells
2.4. Effects of ZO Essential Oil on the Growth and Survival Signalings in HeLa Cells
3. Discussion
4. Materials and Methods
4.1. Plant Material and Isolation of Zingiber ottensii Essential Oil
4.2. Cell Culture
4.3. Flow Cytometry
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Western Blot Analysis
4.6. Immunofluorescence Study
4.7. Data and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jantrapirom, S.; Piccolo, L.L.; Pruksakorn, D.; Potikanond, S.; Nimlamool, W. Ubiquilin Networking in Cancers. Cancers 2020, 12, 1586. [Google Scholar] [CrossRef] [PubMed]
- Santesso, N.; Mustafa, R.A.; Schunemann, H.J.; Arbyn, M.; Blumenthal, P.D.; Cain, J.; Chirenje, M.; Denny, L.; De Vuyst, H.; Eckert, L.O.; et al. World Health Organization Guidelines for treatment of cervical intraepithelial neoplasia 2-3 and screen-and-treat strategies to prevent cervical cancer. Int. J. Gynaecol. Obstet 2016, 132, 252–258. [Google Scholar] [CrossRef]
- Sherlaw-Johnson, C.; Gallivan, S.; Jenkins, D.; Jones, M.H. Cytological screening and management of abnormalities in prevention of cervical cancer: An overview with stochastic modelling. J. Clin. Pathol. 1994, 47, 430–435. [Google Scholar] [CrossRef] [Green Version]
- Mishra, G.A.; Pimple, S.A.; Shastri, S.S. An overview of prevention and early detection of cervical cancers. Indian J. Med. Paediatr. Oncol. 2011, 32, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Sak, K. Chemotherapy and dietary phytochemical agents. Chemother. Res. Pract. 2012, 2012, 282570. [Google Scholar] [CrossRef] [Green Version]
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hankittichai, P.; Buacheen, P.; Pitchakarn, P.; Na Takuathung, M.; Wikan, N.; Smith, D.R.; Potikanond, S.; Nimlamool, W. Artocarpus lakoocha Extract Inhibits LPS-Induced Inflammatory Response in RAW 264.7 Macrophage Cells. Int. J. Mol. Sci. 2020, 21, 1355. [Google Scholar] [CrossRef] [Green Version]
- Namsen, R.; Rojanasthien, N.; Sireeratawong, S.; Rojsanga, P.; Nimlamool, W.; Potikanond, S. Thunbergia laurifolia Exhibits Antifibrotic Effects in Human Hepatic Stellate Cells. Evid. Based Complement. Alternat. Med. 2017, 2017, 3508569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phaosri, M.; Jantrapirom, S.; Takuathung, M.N.; Soonthornchareonnon, N.; Sireeratawong, S.; Buacheen, P.; Pitchakarn, P.; Nimlamool, W.; Potikanond, S. Salacia chinensis L. Stem Extract Exerts Antifibrotic Effects on Human Hepatic Stellate Cells through the Inhibition of the TGF-beta1-Induced SMAD2/3 Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 6314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nimlamool, W.; Potikanond, S.; Ruttanapattanakul, J.; Wikan, N.; Okonogi, S.; Jantrapirom, S.; Pitchakarn, P.; Karinchai, J. Curcuma amarissima Extract Activates Growth and Survival Signal Transduction Networks to Stimulate Proliferation of Human Keratinocyte. Biology 2021, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Ruttanapattanakul, J.; Wikan, N.; Okonogi, S.; Na Takuathung, M.; Buacheen, P.; Pitchakarn, P.; Potikanond, S.; Nimlamool, W. Boesenbergia rotunda extract accelerates human keratinocyte proliferation through activating ERK1/2 and PI3K/Akt kinases. Biomed. Pharmacother. 2021, 133, 111002. [Google Scholar] [CrossRef]
- Potikanond, S.; Sookkhee, S.; Na Takuathung, M.; Mungkornasawakul, P.; Wikan, N.; Smith, D.R.; Nimlamool, W. Kaempferia parviflora Extract Exhibits Anti-cancer Activity against HeLa Cervical Cancer Cells. Front. Pharmacol. 2017, 8, 630. [Google Scholar] [CrossRef] [Green Version]
- Hooker, J.D. The Flora of British India; Reeve Lovell: London, UK, 1890; Volume 5. [Google Scholar]
- Thitinarongwate, W.; Mektrirat, R.; Nimlamool, W.; Khonsung, P.; Pikulkaew, S.; Okonogi, S.; Kunanusorn, P. Phytochemical and Safety Evaluations of Zingiber ottensii Valeton Essential Oil in Zebrafish Embryos and Rats. Toxics 2021, 9, 102. [Google Scholar] [CrossRef]
- Karnchanatat, A.; Tiengburanatam, N.; Boonmee, A.; Puthong, S.; Sangvanich, P. Zingipain, A cysteine protease from Zingiber ottensii Valeton rhizomes with antiproliferative activities against fungi and human malignant cell lines. Prep. Biochem. Biotechnol. 2011, 41, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Sirat, H.M. Study on the Terpenoids of Zingiber ottensii. Planta Med. 1994, 60, 497. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Nongalleima, K.; Singh, N.I.; Doley, P.; Singh, C.B.; Singh, T.R.; Sahoo, D. Zerumbone reduces proliferation of HCT116 colon cancer cells by inhibition of TNF-alpha. Sci. Rep. 2018, 8, 4090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suradej, B.; Sookkhee, S.; Panyakaew, J.; Mungkornasawakul, P.; Wikan, N.; Smith, D.R.; Potikanond, S.; Nimlamool, W. Kaempferia parviflora Extract Inhibits STAT3 Activation and Interleukin-6 Production in HeLa Cervical Cancer Cells. Int. J. Mol. Sci. 2019, 20, 4226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.W.; Lee, H.; Shiau, M.Y.; Wu, T.C.; Huang, T.T.; Chang, Y.H. Human papillomavirus type 16/18 up-regulates the expression of interleukin-6 and antiapoptotic Mcl-1 in non-small cell lung cancer. Clin. Cancer Res. 2008, 14, 4705–4712. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.H.; Kuo, M.L.; Chen, C.A.; Chou, C.H.; Cheng, W.F.; Chang, M.C.; Su, J.L.; Hsieh, C.Y. The anti-apoptotic role of interleukin-6 in human cervical cancer is mediated by up-regulation of Mcl-1 through a PI3-K/Akt pathway. Oncogene 2001, 20, 5799–5809. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.H.; Kuo, M.L.; Chen, C.A.; Chou, C.H.; Lai, K.B.; Lee, C.N.; Hsieh, C.Y. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene 2003, 22, 1517–1527. [Google Scholar] [CrossRef] [Green Version]
- Ren, C.; Cheng, X.; Lu, B.; Yang, G. Activation of interleukin-6/signal transducer and activator of transcription 3 by human papillomavirus early proteins 6 induces fibroblast senescence to promote cervical tumourigenesis through autocrine and paracrine pathways in tumour microenvironment. Eur. J. Cancer 2013, 49, 3889–3899. [Google Scholar] [CrossRef]
- Hirata, T.; Fujii, M.; Akita, K.; Yanaka, N.; Ogawa, K.; Kuroyanagi, M.; Hongo, D. Identification and physiological evaluation of the components from Citrus fruits as potential drugs for anti-corpulence and anticancer. Bioorg. Med. Chem. 2009, 17, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Maesen, L.J.G.v.d.; Somaatmadja, S. ESCAP Regional Co-ordination Centre for Research and Development of Coarse Grains Pulses Roots and Tuber Crops in the Humid Tropics of Asia and the Pacific. In Plant Resources of South East Asia; ESCAP CGPRT Centre: Bogor, Indonesia, 1990; Volume 32, pp. 1–19. [Google Scholar]
- Rahmani, A.H.; Albutti, A.S.; Aly, S.M. Therapeutics role of olive fruits/oil in the prevention of diseases via modulation of anti-oxidant, anti-tumour and genetic activity. Int. J. Clin. Exp. Med. 2014, 7, 799–808. [Google Scholar]
- Sawadogo, W.R.; Cerella, C.; Al-Mourabit, A.; Moriou, C.; Teiten, M.H.; Guissou, I.P.; Dicato, M.; Diederich, M. Cytotoxic, Antiproliferative and Pro-Apoptotic Effects of 5-Hydroxyl-6,7,3′,4′,5′-Pentamethoxyflavone Isolated from Lantana ukambensis. Nutrients 2015, 7, 10388–10397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chantaranothai, C.; Palaga, T.; Karnchanatat, A.; Sangvanich, P. Inhibition of nitric oxide production in the macrophage-like RAW 264.7 cell line by protein from the rhizomes of Zingiberaceae plants. Prep. Biochem. Biotechnol. 2013, 43, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Phukerd, U.; Soonwera, M. Larvicidal and pupicidal activities of essential oils from Zingiberaceae plants against Aedes aegypti (Linn.) and Culex quinquefasciatus say mosquitoes. Southeast Asian J. Trop Med. Public Health 2013, 44, 761–771. [Google Scholar] [PubMed]
- Chen, C.; Nimlamool, W.; Miller, C.J.; Lou, H.J.; Turk, B.E. Rational Redesign of a Functional Protein Kinase-Substrate Interaction. ACS Chem. Biol. 2017, 12, 1194–1198. [Google Scholar] [CrossRef]
- Deorukhkar, A.; Ahuja, N.; Mercado, A.L.; Diagaradjane, P.; Raju, U.; Patel, N.; Mohindra, P.; Diep, N.; Guha, S.; Krishnan, S. Zerumbone increases oxidative stress in a thiol-dependent ROS-independent manner to increase DNA damage and sensitize colorectal cancer cells to radiation. Cancer Med. 2015, 4, 278–292. [Google Scholar] [CrossRef]
- Yan, H.; Ren, M.Y.; Wang, Z.X.; Feng, S.J.; Li, S.; Cheng, Y.; Hu, C.X.; Gao, S.Q.; Zhang, G.Q. Zerumbone inhibits melanoma cell proliferation and migration by altering mitochondrial functions. Oncol. Lett. 2017, 13, 2397–2402. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.A.; Jantan, I.; Arshad, L.; Bukhari, S.N.A. Exploring the immunomodulatory and anticancer properties of zerumbone. Food Funct. 2017, 8, 3410–3431. [Google Scholar] [CrossRef]
- Kim, M.; Miyamoto, S.; Yasui, Y.; Oyama, T.; Murakami, A.; Tanaka, T. Zerumbone, a tropical ginger sesquiterpene, inhibits colon and lung carcinogenesis in mice. Int. J. Cancer 2009, 124, 264–271. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruttanapattanakul, J.; Wikan, N.; Chinda, K.; Jearanaikulvanich, T.; Krisanuruks, N.; Muangcha, M.; Okonogi, S.; Potikanond, S.; Nimlamool, W. Essential Oil from Zingiber ottensii Induces Human Cervical Cancer Cell Apoptosis and Inhibits MAPK and PI3K/AKT Signaling Cascades. Plants 2021, 10, 1419. https://doi.org/10.3390/plants10071419
Ruttanapattanakul J, Wikan N, Chinda K, Jearanaikulvanich T, Krisanuruks N, Muangcha M, Okonogi S, Potikanond S, Nimlamool W. Essential Oil from Zingiber ottensii Induces Human Cervical Cancer Cell Apoptosis and Inhibits MAPK and PI3K/AKT Signaling Cascades. Plants. 2021; 10(7):1419. https://doi.org/10.3390/plants10071419
Chicago/Turabian StyleRuttanapattanakul, Jirapak, Nitwara Wikan, Kittinan Chinda, Thanathorn Jearanaikulvanich, Napatsorn Krisanuruks, Muantep Muangcha, Siriporn Okonogi, Saranyapin Potikanond, and Wutigri Nimlamool. 2021. "Essential Oil from Zingiber ottensii Induces Human Cervical Cancer Cell Apoptosis and Inhibits MAPK and PI3K/AKT Signaling Cascades" Plants 10, no. 7: 1419. https://doi.org/10.3390/plants10071419