The Genus Plagiothecium Schimp. (Plagiotheciaceae, Bryophyta) in Eurasia: An Annotated Checklist with Distribution and Ecological Data
Abstract
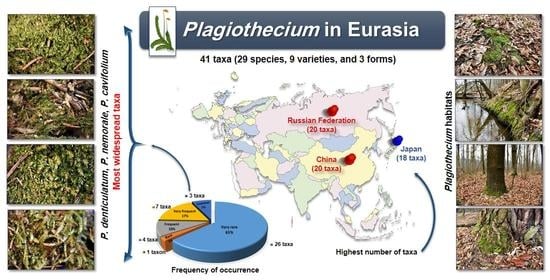
:1. Introduction
2. Results and Discussion
2.1. General Results
2.2. Annotations by Taxon
- Annotations to the following list of Plagiothecium taxa include brief description for each taxon, with comments on nomenclature and taxonomic status (when necessary), ecological preferences, and geographic distribution inside and outside Eurasia (if available).
- Taxon distribution per country in Eurasia is given in detail in Appendix B, with all available literature sources.
- We did not make any broad attempt to provide an exhaustive list of records for all countries in each continent (outside Eurasia), but we gave a general overview of the geographic distribution outside the study area based on the selected references. As far as we know, none of the 41 taxa listed here have been reported from Antarctica [14] or Australia [17].
- Plagiothecium angusticellum G.J. Wolski & P. Nowicka-Krawczyk 2020—a medium-sized plant, with asymmetrical leaves, not shrunken when dry; an acuminate, gently curved, not serrated apex; tight areolation, created with long and narrow leaf cells which do not form regular rows. This combination of features made it possible to distinguish P. angusticellum from similar and closely related species such as P. cavifolium, P. nemorale, and P. longisetum [27]. Ecology. From Poland (from where it was described), this species was recorded mainly in deciduous forests (e.g., eutrophic swamp forests Ribeso nigri-Alnetum Sol.-Górn. (1975) 1987, wet alder riparian forests Fraxino-Alnetum W. Mat. 1952, beech forests Luzulo pilosae-Fagetum W. Mat. et A. Mat. 1973, oaklinden-hornbeam forests Tilio-Carpinetum Tracz. 1962) in epigeic, epiphytic and epilithic habitats. Distribution. Apart from Central Europe, this species is also reported from North America (USA) [191].
- Plagiothecium argentatum (Mitt.) Q. Zuo 2011—described as Hypnum argentatum by Mitten [192], transferred to the genus Struckia by Müller [193], and finally (based on DNA analysis) incorporated into the genus Plagiothecium [36], its integration in this genus was confirmed by other researchers (e.g., [7,23]). A pale green, julaceous plant, with symmetric, concave, plicate, long, non-decurrent leaves, with a serrulate or entire margin, and quadrate alars; it is characterised by quite an unusual (for a member of the genus Plagiothecium) set of features. Most of these features place P. argentatum well in the described genus, but serrulate margins, absence of decurrency and quadrate alars seem to exclude this species from the genus Plagiothecium. This large morphological separateness resulted in the placement of this species (as P. enerve) in a separate section of Struckia [23]. Ecology. The species was recorded in epiphytic and epixylic habitats [7]. Distribution. So far, recorded from East and South Asia.
- Plagiothecium berggrenianum Frisvoll 1981—a medium-sized to large, julaceous, crowded plant; leaves symmetrical, very concave, ovate, long decurrent, and an abruptly narrowed to hooked apex; leaf cells long and narrow, thick-walled; capsules straight and erect. This circumpolar species was described by Frisvoll [171], and is easily distinguishable from other species by leaves with recurved margins and by the shape of its apex, as well as well-developed alar regions. Plagiothecium berggrenianum is similar to P. svalbardense, but the former is longer, with elliptical, plicate leaves, and broadly recurved margins. Wynns [7] states that P. berggrenianum is a possible hybrid. Ecology. This species is recorded in epigeic, epilithic and epixylic habitats [26,171,172]. It is found in swales, tundra, and cliffs; low to moderate elevations [21]. Distribution. Apart from Eurasia, it is also reported from North America (Canada, Greenland, USA) [7,26,172].
- Plagiothecium cavifolium (Brid.) Z. Iwats. 1970—Iwatsuki [29] selected this name for the species previously known as P. roeseanum [1]. A small to medium-sized, pale green to yellowish green, glossy plant in dense mats (Figure 2); ascending to erect, stems julaceous more or less; imbricate, ovate to elliptical, symmetric, concave leaves (Figure 3), often with a curved apex; cells linear-rhomboidal, long and narrow (Figure 4). Ecology. This species is found in shaded locations in low to high elevations [21] and recorded in epigeic, epilithic, epixylic and epiphytic habitats (e.g., [25,28,29,30,34,62,79,194,195,196,197,198,199]). Distribution. Apart from Eurasia, this species is also reported from Africa (Tunisia) [47]; North America (Canada, Falkland Islands, Greenland, USA) [7,21,23,25,79].
- Plagiothecium cavifolium var. orthocladium (Schimp.) Z. Iwats. 1970—Iwatsuki [29] selected this name for the variety previously known as P. orthocladium [1], and also reported on a relationship between this taxon and P. nemorale fo. japonicum (currently P. japonicum) as well as P. succulentum. Wynns [7] mentioned difficulty in distinguishing this taxon, adding that he used this name for olivaceous, boreal specimens, with crispate and spreading leaves. Ecology. This taxon is recorded in epilithic habitat. Distribution. Apart from Europe (Nordic countries), this taxon is also reported from North America (Canada, Greenland) [7].
- Plagiothecium cochleatum Dixon 1938—a dark green plant; leaves loosely imbricated, concave, plicate, with rigid areolation, and quite well-developed alar decurrencies. Wynns [7] indicates that P. cochleatum is similar to P. cavifolium and may be confused with this species. Ecology. It is a rare species, present in disjunct Alpine and Himalayan habitats [23]. Distribution. So far, it is reported from Asia (India).
- Plagiothecium conostegium Herzog 1916—Suzuki [119] recorded this taxon from Japan. Considering the features and pictures published by Suzuki [119] in particular: Asymmetric, in dry condition shrunken leaves, long-hexagonal cells, we believe that the taxon described by Suzuki [119] looks more like P. longisetum. This requires checking herbarium materials, but at this stage, we consider the presence of P. conostegium doubtful in Eurasia. Ecology. In Central and South America, it is recorded as a forest species mostly growing on epigeic, epilithic, epiphytic and epixylic habitats [3,7]. Distribution. Apart from Asia (Japan) [119], the species is reported by Wynns [7] as a mountain species from Central America and Northern South America (Bolivia, Ecuador, Guatelama, Mexico, Peru) and also present at high elevations in North America, the Dominican Republic, Northern Andes and Tierra del Fuego [3].
- Plagiothecium curvifolium Schlieph. ex Limpr. 1897—a small to medium-sized plant, green to yellowish green, glossy (Figure 2); leaves broadly lanceolate to lanceolate, not concave, asymmetric, sometimes downward curving; alar decurrencies wide, hyaline (Figure 3), sometimes even inflated; capsules curved and inclined to horizontal. These features distinguish this species from other closely related species (e.g., P. laetum). Ireland [18] and Iwatsuki [29] (and many after them) did not recognise P. curvifolium and P. laetum as separate species, but DNA analysis clearly proves this [7,23]. Ecology. It is recorded in epigeic, epilithic, epixylic and epiphytic habitats (e.g., [25,28,29,30,34,79,194,200]). Distribution. The species is common in lowland areas [25]. Outside Eurasia, it is reported from Africa [29] but its presence in North Africa considered doubtful by Ros et al. [12]. It is also reported from North America (Canada, USA) [7,25,79].
- Plagiothecium curvifolium fo. julaceum Culm. & E. Bauer 1915—it is a forgotten taxon, which after being described in 1915 it was not later mentioned as a separate or at least as a synonym in any of the major bryological studies [28,30,32,201,202,203,204,205]. Based on molecular analyses, Wynns [7] recognises it as separate, although the description of the gametophyte characteristics (ramis subjulaceis, foliis imbricatis saepe subhomomallis) indicates features similar to P. cavifolium. At this stage, this taxon definitely requires further in-depth research and detailed analysis. Ecology and Distribution. Detailed ecological data and distribution for this taxon are not known exactly and require specific investigation. However, according to Wynns [7], the isotype is found on fir roots near the upper tree line (epiphytic); from Switzerland (Burgfeld in Beatenberg, Canton of Bern).
- Plagiothecium decoratum J.T. Wynns 2015—the species established by Wynns [23] and described as a julaceous to subcomplanate, slender plant, with concave, ovate, more or less symmetrical, plicate leaves, with recurved margins and a curled, denticulate, hyaline and recurved leaf apex. Ecology. It is listed in epiphytic habitat [7]. Distribution. The species is described as endemic of Bhutan and Nepal [7], present in evergreen forests around 3000 m. According to Wynns et al. [23], this taxon should be searched for elsewhere, as it could reasonably be expected to occur in Sikkim (Northeastern India)—which borders Bhutan in the east and Nepal in the west—and in Yunnan (China).
- Plagiothecium denticulatum (Hedw.) Schimp 1851—a fairly large plant, green to yellowish green, often glossy (Figure 2); the stem prostrate to ascending, densely foliate; concave, ovate to lanceolate, asymmetrical, acute to acuminate leaves, with recurved margins (Figure 3), an often denticulate apex, and loose cell areolation (Figure 4); median cells linear-rhomboidal; alar regions well-developed, broadly decurrent, composed of large, inflated, round hyaline cells; sporophytes with inclined capsules. These features allow this species to be distinguished from others. Ecology. It is considered as a circumboreal species, where it is recorded in epigeic, epilithic, epixylic and epiphytic habitats; more abundant in alpine areas (e.g., [28,29,30,34,79,194,198,199,206]). Distribution. Apart from Eurasia, it is cited in the literature from North Africa, but considered doubtful by Ros et al. [12]; also the occurrence of this species in sub-Saharan Africa is considered doubtful by O’Shea [10]. It is also reported from North America (Canada, Greenland, USA) [7,18,25,79,207].
- Plagiothecium denticulatum var. affine Warnst. 1906—a species with small, flat, asymmetric leaves and well-developed alar regions [7]. Warnstorf [208] stated that P. denticulatum var. affine has a delicate form and resembles P. laetum, and that it could be an intermediate form between these species. Intermediate features of this taxon include its delicate structure as well as straight and erect capsules (which is characteristic for all species closely related to P. laetum). Wynns [7] supports this opinion that it may be a hybrid between these two species. Ecology. Warnstorf [208] did not provide details about the ecological preferences of this taxon, however, he indicated that it is a delicate form growing in flat turfs. Distribution. So far, it is reported only from Germany (Bärwalde, between Vietnitz and Nordhausen, Königsberg) [7,208].
- Plagiothecium denticulatum var. obtusifolium (Turner) Moore 1873—a small, subjulaceous, glossy, soft plant; leaves round, ovate or elliptical, with an obtuse apex, recurved margins and well-developed alar regions. These features distinguish this taxon from other species. Plagiothecium denticulatum var. obtusifolium was treated by some scientists as a synonym of P. denticulatum (e.g., [18,159]) or considered to be an ecotypic variety, but DNA sequence analyses indicate that P. denticulatum var. obtusifolium and P. denticulatum are not the same taxon [7,23]. Ecology. Wynns [7] reported on that P. denticulatum var. obtusifolium is restricted to mountains and cliffs, where it is recorded in epigeic, epilithic and epiphytic habitats. Distribution. Outside Eurasia, the species reported from North America (Canada, USA) [7,79].
- Plagiothecium enerve (Broth.) Q. Zuo 2011—due to a rather unusual combination of features: small plant with not tumid branches, narrowly lanceolate leaves bordered by hyaline, elongate, thin-walled cells, with an extremely long, often brownish piliferous apex, this plant was first described as Struckia enervis [209]. However, DNA analysis [36] indicates that it belongs to the genus Plagiothecium. Ecology. This species is recorded in epilithic and epiphytic habitats [7]. Distribution. Until now, it is reported from Asia (China and Russia).
- Plagiothecium euryphyllum (Cardot & Thér.) Z. Iwats. 1970—a medium-sized to robust, glossy plant, with flattened branches; leaves ovate to elliptical, asymmetric, slightly contorted when dry, broadly acute, more or less undulate; median leaf cell linear, narrow, thin-walled, areolation looks tight. Due to this feature combination, P. euryphyllum is similar and confused with P. neckeroideum, but this species does not have intense iridescent and concave leaves as P. euryphyllum. Ecology. The species is recorded in epigeic, epilithic, epixylic and epiphytic habitats [29,64,79]. Distribution. It is widely spread over Asia (China, Formosa, Japan, Korea and Myanmar) and Eastern Europe (Russia) [7,29,32,72,140].
- Plagiothecium fallax Cardot & Thér. 1902—Cardot & Thériot [210] described this species as a robust, light green, shiny plant; leaves broadly ovate-lanceolate, asymmetrical, undulate, with a broad base and wider areolation of thin-walled cells. According to these authors, P. fallax is similar to P. denticulatum sensu lato, but it can be distinguished from this species by very small alar decurrencies. Ireland [18] and Iwatsuki [29] treated this species as a synonym or variety of P. cavifolium. Ecology. This species is recorded in epigeic habitat. Distribution. Apart from Eurasia (Russian Federation and Japan), it is also reported from North America (USA) and considered to be a typical North Pacific element [7].
- Plagiothecium japonicum Sakurai 1949—described by Sakurai [211], but Iwatsuki [29] treated this species as a form of P. nemorale (P. nemorale fo. japonicum), later even as a synonym to this species [33]. Plagiothecium japonicum can be easily recognised by large, broadly ovate, often concave leaves with stiff, extended cells and, as indicated by Wynns [7], it should be treated as a separate species, despite the fact that morphologically and genetically it shows intermediate features between P. nemorale and P. cavifolium. Ecology. This species is recorded in epigeic and epilithic habitats. Distribution. Apart from Asia (Japan), it is also reported from North America (USA) and considered a North Pacific element as P. fallax [7].
- Plagiothecium laetum Schimp. 1851—small plant, pale green to yellowish green, glossy, in loose mats; leaves asymmetrical, narrowly ovate-lanceolate, and narrowly-decurrent, gradually acuminate at apex; median leaf cells linear-rhomboidal; capsules more or less erect. Narrow alar decurrencies and sporophytes with erect capsules easily distinguish this species from closely related ones such as P. curvifolium. A recent taxonomic study of P. laetum complex allowed description of a new species, i.e., P. rossicum [26]. Ecology. The species recorded in epigeic, epilithic, epixylic, and epiphytic habitats (e.g., [25,28,30,66,79,171,172,195,212]). Distribution. Apart from Eurasia, this species is also reported from North America (Canada, Greenland, USA) [7,18,79].
- Plagiothecium laetum var. tenellum (Schimp.) Warnst. 1906—Warnstorf [208] states that this taxon differs from P. laetum by longer, more lanceolate leaves, as well as narrow and long cells. Jedlička [202,203] characterised P. laetum var. tenellum as having small, narrow leaves, very short costae and narrow cells, often with propagules, and small erect capsules. Ecology and Distribution. Warnstorf [208] did not provide data about the ecological preferences of this taxon, therefore detailed ecological data and distribution for this taxon are not known exactly and require specific investigation. However, he indicated that var. tenellum plants are from all locations listed to P. laetum, which include Germany (Crossen, Lübeck, Hamburg and Altmark). The ecological data reported for these locations were: very rare in pine and deciduous forests at the bottom of trees or on forest floor, sometimes also in moors on the edge of old peat holes; often in crevices of lower and higher mountains.
- Plagiothecium latebricola Wilson ex Schimp. 1851—a small, slender, bright green or yellowish green, glossy plant; leaves erect-spreading, symmetrical, narrowly ovate-lanceolate, sometimes complanate, long acuminate, at times shrunken when dry, a margin narrowly recurved, entire or denticulate near the apex; costae very short; median leaf cells linear-rhomboidal; alar regions narrowly decurrent; fusiforme gemmae often present as well as rhizoids at the apex; capsules erect. Because of its small size, colour, short costae, narrow decurrent alar regions and erect capsules, this species can be confused with P. laetum, but even leaf symmetry in the latter helps to distinguish these two species. Ecology. Plagiothecium latebricola is found in swamps, fens, marshes and recorded in epigeic, epilithic, epixylic and epiphytic habitats (e.g., [21,25,28,30,79,101,199,206,213]). Distribution. This species has a circumboreal distribution [7], typically found in lowland, shaded locations [21,25]. Apart from Eurasia, it is also reported from North America (Canada, USA) [18,79].
- Plagiothecium longisetum Lindb. 1872—species described by Lindberg [214], synonymized with P. nemorale by Iwatsuki [29] and treated as such for about 50 years [33], recently resurrected and considered as a separate species [27]. A robust, green to yellow green, plant without metallic lustre (Figure 2); leaves asymmetric to strongly asymmetric, shrunken when dry, ovate to lanceolate; a straight, not denticulate, acute to acuminate apex; leaf cells in regular rows, long and wide, areolation loose; long and burly costae; and very long seta (to 5.5 cm). This feature combination allows us to easily distinguish this species from P. nemorale and other closely related species. Ecology. It is recorded in epigeic, epilithic, epixylic and epiphytic habitats [27]. Distribution. Apart from Eurasia, this species is also reported from North America (Canada, USA) [191].
- Plagiothecium neckeroideum Schimp. 1851—a robust, green to yellowish green, strongly complanate plant; leaves domorphic, triangular, asymmetrical, ovate, undulate, concave, and a serrulate apex; median leaf cells linear-rhomboidal, narrow; alar decurrencies hyaline, thin-walled and well-developed. Apex cells are nematogenous, leaves often with differentiated apical cells, often seen as a longitudinal brown stripe at the leaf apex. Ecology. It is noted in epigeic, epilithic, epixylic and epiphytic habitats (e.g., [29,34,66,79]). Distribution. Wynns [7] reported P. neckeroideum from East and Southeastern Asia and in the Himalayas, from Europe (in the Alps). Deng-ke and Ireland [79] also gives this species from North America.
- Plagiothecium neckeroideum fo. exile J.T. Wynns 2015—a taxon described by Wynns [23] as a small plant, with slender stems and branches, reddish stems; concave, not undulate, acuminate leaves; leaf cell areolation composed of short and narrow cells; decurrencies with enlarged, hyaline alar cells. Ecology. Reported from Quercus semecarpifolia forest, on tree trunk (epiphytic) [23]. Distribution. So far, the taxon is known only from Nepal.
- Plagiothecium neckeroideum var. javense M. Fleisch. 1920—Fleischer [215] describes this taxon as a large, light green plant, with pale, symmetric, concave, undulate, long acuminate leaves, a denticulate apex, short costae, thin-walled leaf cells, enlarged in the basal area, with a vertical stripe of nematogenous cells at the apex. Ecology. This taxon was recorded in epigeic and epilithic habitats. Distribution. Apart from Southeast Asia (Indonesia, Philippines), it is also reported from Papua New Guinea [216] and from East Africa (Ethiopia) [10].
- Plagiothecium neckeroideum var. myurum Molendo 1875—smaller than P. neckeroideum var. javense, other features that make it different from closely related species are that it is a julaceous plant, with strongly concave, not undulate leaves [7,217]. Ecology. It is a montane taxon, recorded in epigeic, epilithic, epixylic and epiphytic habitats [7]. Distribution. Reported from Sino-Himalayan region (Bhutan, China, India, Nepal) [23].
- Plagiothecium neckeroideum var. niitakayamae (Toyama) Z. Iwats. 1970—a big, light green, julaceous plant, with symmetrical, plicate leaves; this variety differs from the species by more julaceous, symmetric, undulate leaves. Ecology. This taxon was recorded in epigeic, epilithic and epiphytic habitats [7,29,79]. Distribution. Recorded from East Asia (China, Japan, Taiwan) and Southeast Asia (Philippines).
- Plagiothecium neckeroideum fo. parvum J.T. Wynns 2015—a form proposed by Wynns [7], who describes it as a small, pale green, crispate when dry, with flat or undulate, very concave, cordate, short, broad and very asymmetrical leaves, with an acuminate, acute and denticulate apex, often with rhizoids; leaf cells narrow; this form is similar to P. subglaucum, but in P. neckeroideum fo. parvum the leaves are broader. Ecology. This taxon was recorded in epigeic habitats [7]. Distribution. So far, it is known only from Taiwan (East Asia).
- Plagiothecium nemorale (Mitt.) A. Jaeger 1878—this species has been too widely described in the last few decades, a taxonomic review of P. nemorale sensu lato indicates that it is actually three separate species: P. nemorale sensu stricto, P. longisetum and P. angusticellum [27]. A medium to large, green to dark green plant, shrunken when dry (Figure 2) and without metallic luster; leaves ovate, symmetric (Figure 3); acute to acuminate, straight; a denticulate apex; leaf cells short and wide, loose areolation, symmetric, in regular rows (Figure 4). This feature combination makes it very easy to distinguish this species from other closely related species. Ecology. The species is recorded in epigeic, epilithic, epixylic, and epiphytic habitats (e.g., [25,27,28,29,34,66,79,141,194,199,200,218]). Distribution. The species is quite common in Eurasia [27]. It is also reported by Ros et al. [12,47] from North Africa (Algeria, Tunisia) and reported from North America (Canada, USA) [27,191].
- Plagiothecium noricum Molendo ex Limpr. 1897—flaccid, not undulate, very concave leaves, with expended cell aerolation, denticulate; rhizoids at the apex. Ecology. Wynns [7] describes P. noricum as a still little-known Alpine species, where are listed from epigeic habitat. Distribution. Reported from the Southern part of Central Europe (Austria) and Southeast Asia (Myanmar).
- Plagiothecium obtusissimum Broth. 1921—a yellowish green to pale green, glossy plant with metallic luster; leaves ovate, rounded-obtuse at the apex, asymmetrical, slightly concave, the margin often erect at one side; leaves with suddenly differentiated alars, composed by hyaline inflated cells; median cells linear-flexuose, very narrow and long, thin-walled. Iwatsuki [29] considered P. obtusissimum to be closely related to P. euryphyllum, due to similar alar cells, leaf cells and setae. Additionally, it is easily distinguished from this species by the plant size and shape of leaf apex. DNA analysis confirms the observations about the close relationship between these species [36]. Ecology. Plagiothecium obtusissimum is recorded in epigeic, epilithic, epixylic and epiphytic habitats (e.g., [29,34]). Distribution. Noguchi [34] considered this species to be endemic to Japan; however, it is later reported from Russia (e.g., [32]).
- Plagiothecium piliferum (Sw.) Schimp. 1851—a small, slender, pale green and glossy plant; leaves ovate, deeply concave, almost symmetrical, abruptly contracted to a piliferous, sometimes flexuose apex, with recurved margins; median leaves linear-rhomboidal, very narrow; alar regions narrowly decurrent. Ecology. Plagiothecium piliferum is recorded in epigeic, epilithic, and epixylic habitats (e.g., [25,28,30,79]). Distribution. Apart from Eurasia, this species is also reported from North America (Canada, Greenland, USA) in low to moderate elevations [7,21,30,79].
- Plagiothecium platyphyllum Mönk. 1927—a medium-sized to robust, green, glossy plant; leaves ovate-lanceolate, asymmetrical, complanate, facid, undulate; median leafcells linear-romboidal, apical cells often bearing rhizoids; alar cells hyaline to pale green. According to Ireland [18], it is an autopolyploid of P. denticulatum, while a DNA study [219] suggested that P. denticulatum var. obtusifolium is a haploid of P. platyphyllum. Ecology. Plagiothecium platyphyllum is recorded in epigeic, epilithic, epixylic, and epiphytic habitats (e.g., [25,28,30,79,128,194]). Distribution. Apart from Eurasia, it is also reported from North America (Canada, USA) [7,23].
- Plagiothecium rhizophyllum Sakuri 1932—described by Sakurai [220] as a small species, with not undulate, loose cell areolation and rhizoids at the apex. Iwatsuki [29,221] and researchers after him (e.g., [33]) consider it as a synonym of P. nemorale, but Wynns [7] treats P. rhizophyllum as a separate species. Ecology. It is recorded in epigeic habitat [7]. Distribution. So far, the species reported only from East Asia (China).
- Plagiothecium rossicum Ignatov & Ignatova 2019—described on the basis of DNA analyses of the P. laetum complex by Ignatova et al. [26]. A small plant with distinctly complanate foliage; leaves asymmetrical, ovate-lanceolate, a narrowly acute to short acuminate apex, margins flat, entire or minutely denticulate at the apex; leaf cells long and very narrow; straight and erect capsules. In terms of many features, this species is similar to P. laetum, but a flat margin and strongly asymmetric leaves are very useful in distinguishing P. rossicum from this species. Many features allow this species to be distinguished also from P. svalbardense, including for example: A flat leaf margin, narrow cells, narrowly acute to short acuminate apex which characterise P. rossicum. Ecology. So far, this species has been recorded in epigeic, epilithic, epixylic and epiphytic habitats [26]. Distribution. The species in common in boreal and hemiboreal forests of Russia, one position is also given from Poland [26].
- Plagiothecium ruthei Limpr. 1897—a medium-sized to large plant; leaves strongly complanate on the stem, transversely undulate when moist, sometimes shrunken when dry, flaccid, acuminate, strongly asymmetrical, on side almost straight, leaves with narrowly recurved margins and well-developed alar regions. These features distinguish this species from other closely related species. It is recognised as a separate species throughout Eurasia, despite the fact that DNA data places this plant closer to P. denticulatum, even closer than P. denticulatum var. obtusifolium [7,23]. Ecology. Plagiothecium ruthei is typical of wetlands species, recorded in epigeic, epixylic, and epiphytic habitats (e.g., [25,30,194]). Distribution. Apart from Eurasia, this species is also reported from North Africa but considered doubtful by Ros et al. [12]; and from North America (Canada, USA) [7].
- Plagiothecium ruthei var. rupincola Limp. 1897—Limpricht [222] described this taxon as similar to P. ruthei due to the size and cell areolation, but different due to closer foliage; symmetrical leaves, lacking recurved margin. Ecology. Limpricht [222] described it as an epilithic, alpine taxon. Distribution. According to the protologue and Wynns [7], this taxon is reported from Central, Northern and Western Europe (Asutria, Czech Republic, France, Germany, Norway and Sweden).
- Plagiothecium subglaucum Thwaites & Mitt. 1873—Mitten [223] described this species as a plant with ovate, flat leaves, with an acute to acuminate apex. Plagiothecium subglaucum is similar to and can be confused with P. neckeroideum. Both species require further in-depth research [7]. Ecology. It is recorded in epigeic and epiphytic habitats. Distribution. So far, known only from Sri Lanka (South Asia) and Myanmar (Southeast Asia).
- Plagiothecium succulentum (Wilson) Lindb. 1865—a robust, yellowish green to golden green, very glossy plant, leaves symmetric, lanceolate, not shrunken when dry, with an entire apex; median leaf cells very long. Plagiothecium succulentum differs from P. nemorale by lanceolate leaves, longer cells and a smooth apex; and from P. longisetum by lanceolate, symmetrical leaves; from other closely related species (e.g., P. angusticellum), it is very easy to distinguish, for example, by leaf symmetries and loose cells areolation [27]. Wynns [7] considered P. succulentum as problematic and described this species as polyphyletic or intermediate between P. nemorale and P. cavifolium. Plagiothecium succulentum in some countries is indicated as doubtful (Table 2, Figure S4). In our opinion, the relationship between these above-mentioned species requires a detailed analysis. Ecology. Plagiothecium succulentum is recorded in epigeic, epilithic, and epiphytic habitats (e.g., [25,28,30,62,79,194]). Distribution. Apart from Eurasia, this species is also reported from North Africa (a single record from Tunisia) [47]; and from North America (Canada, USA) [191].
- Plagiothecium succulentum fo. propaguliferum E. Bauer 1902—a very dark, small plant, with shrunken leaves when dry. These are the features that distinguish this form from P. succulentum. Wynns [7] commented that this taxon can be frequently found in herbaria under the name P. succulentum. Ecology. This taxon is recorded in epilithic and epiphytic habitats [7]. Distribution. Currently, P. succulentum fo. propaguliferum is recorded in Western, Northern and Western Europe [7], and from North America (Canada, USA) [7,191].
- Plagiothecium svalbardense Frisvoll 1996—a small, growing erect plant, crispy when dry; leaves small, weakly undulate, concave, symmetrical to slightly asymmetrical, short, ovate, gradually tapered to the apex; margins narrowly recurved, entire or minutely denticulate at the apex; a subpiliferous apex; capsules straight and erect. Plagiothecium svalbardense is different from P. laetum by leaf shape and apex shape, the described species is also similar to P. piliferum due to its apex, but the latter has narrower leaf cells and a longer apex. The shape and arrangement of capsules is similar to P. laetum and P. berggrenianum but different from P. curvifolium. Ecology. Wynns [7] described P. svalbardense as an arctic species, where it is recorded in epilithic and epixylic habitats [26]. Distribution. Apart from Eurasia (Russia, Svalbard, Sweden), this species is also reported from North America (Greenland) [7].
- Plagiothecium undulatum (Hedw.) Schimp. 1851—a large white to pale green, dull plant; leaves large, imbricate, crispate, slightly asymmetric, acute and serrulate at the apex; rhizoids occur at the leaf insertion; leaf cells with papillae on abixal surfaces. These mentioned features led Ireland [224] to create for this species (as well as P. draytonii (Sull.) E.B. Bartram) a separate genus—Buckiella Ireland. Plagiothecium undulatum is similar to P. neckeroideum, but it differs by size and colour of the plant as well as longer and broader median leaf cells. Ecology. Plagiothecium undulatum was recorded in epigeic, epilithic, and epiphytic habitats (e.g., [25,28,30,79,197]). Distribution. Apart from Eurasia, this species is also reported from North America (Canada, USA) [7,18,79].
3. Materials and Methods
3.1. The Area of the Checklist
- Europe (Northern, Middle, Southwestern, Southeastern, and Eastern Europe);
- Asia-Temperate (Siberia, Russian Far East, Middle Asia, Caucasus (excl. partially recognised countries such as Abkhazia), Western Asia, the Arabian Peninsula, China, Mongolia, and Eastern Asia); and
- Asia-Tropical (Indian Subcontinent, Indo-China, Malaysia, but excl. Papuasia).
3.2. Data Collection and Presentation
3.3. Nomenclature and Taxonomy
3.4. Conspectus of Classification of Plagiothecium
- Plagiotheciaceae M.Fleisch., Nova Guinea 8: 748. 1912;
- Hypnaceae subfam. Plagiothecioideae Brotherus in Engler and Prantl, Nat. Pflan-
- zenfam. 1(3): 1021, 1078. 1908, “Plagiothecieae”. Type. Plagiothecium Schimper.
- Plagiothecium Schimper in Bruch, Schimper and Gümbel, Bryol. Eur. 5:179. 1851 ≡
- Stereodon (Bridel) Mitten sect. Plagiothecium (Schimper) Mitten, J. Linn. Soc., Bot. 4:
- 88. 1859. Type. Plagiothecium denticulatum (Hedwig) Schimper in Bruch, Schimper and Gümbel, Bryol. Eur.
- Plagiothecium Schimp. sect. Plagiothecium ≡ Plagiothecium sect. Falciphyllum Jedl., nom. illeg. Spisy Vydá. Přír. Fak. Masarykovy Univ. 308: 23. 1948. = Plagiothecium sect. Rostriphyllum Jedl. Spisy Vydá. Přír. Fak. Masarykovy Univ. 308: 32. 1948.This section consists of 12 species: P. brasiliense (Hampe) A. Jaeger, P. cochleatum Dixon, P. conostegium Herzog, P. denticulatum (Hedw.) Schimp, P. lamprostachys (Hampe) A. Jaeger, P. berggrenianum Frisvoll, P. membranosulum Müll. Hal., P. nitens Dixon, P. ovalifolium Cardot, P. platyphyllum Mönk., P. ruthei Limpr., P. selaginelloides Müll. Hal.
- Plagiothecium sect. Orthophyllum Jedl. Spisy Vydá. Přír. Fak. Masarykovy Univ. 308: 35. 1948.This section consists of 7 species: P. angusticellum G.J. Wolski & P. Nowicka-Krawczyk, P. cavifolium (Brid.) Z. Iwats., P. japonicum Sakurai, P. longisetum Lindb., P. nemorale (Mitt.) A. Jaeger, P. rhizophyllum Sakurai, P. succulentum (Wilson) Lindb.
- Plagiothecium sect. Leptophyllum Jedl. Spisy Vydá. Přír. Fak. Masarykovy Univ. 308: 23. 1948. = Plagiothecium sect. Philoscia (Berk.) Ochyra. Biodiversity of Poland 3: 177. 2003. ≡ Philoscia Berk. Handbook of British Mosses 49, 146. 1863.This section consists of 12 species: P. andinum (Hampe) A. Jaeger, P. curvifolium Schlieph. ex Limpr., P. funale J.T. Wynns, P. laetum Schimp., P. latebricola Wilson ex Schimp., P. lucidum (Hook. f. and Wilson) Paris, P. mollicaule R.S. Williams, P. pacificum J.T. Wynns, P. rhizolucidum J.T. Wynns, P. rossicum Ignatov & Ignatova, P. svalbardense Frisvoll.
- Plagiothecium sect. Pseudo-Neckera (Kindb.) J.T. Wynns. Cladistics 34: 469–501. 2018. ≡ Plagiothecium subgen. Pseudo-Neckera Kindb. European and North American Bryineae (Mosses) 1: 69. 1897.This section consists of 4 species: P. decoratum J.T. Wynns, P. neckeroideum Schimp., P. noricum Molendo ex Limpr., P. subglaucum Thwaites & Mitt.
- Plagiothecium sect. Lycambium Jedl. 1948. Spisy Vydá. Přír. Fak. Masarykovy Univ. 308: 10. 1948. ≡ Buckiella Ireland. Novon 11(1): 55. 2001.This section consists of 3 species: P. draytonii (Sull.) E.B. Bartram, P. fallax Cardot & Thér., P. undulatum (Hedw.) Schimp.
- Plagiothecium sect. Saviczia (Abramova & I.I. Abramov) Z. Iwats. J. Hattori Bot. Lab. 33: 341. 1970. ≡ Saviczia Abramova & I.I. Abramov. Novosti Sist. Nizsh.Rast. 1966: 298. 1966.This section consists of 2 species: P. euryphyllum (Cardot & Thér.) Z. Iwats., P. obtusissimum Broth.
- Plagiothecium sect. Struckia (Müll. Hal.) J.T. Wynns. Cladistics 34: 469–501. 2018. ≡ Struckia Müll. Hal. Arch. Vereins Freunde Naturgesch. Mecklenburg 47: 129. 1893.This section consists of 2 species: P. argentatum (Mitt.) Q. Zuo, P. enerve (Broth.) Q. Zuo.
- Plagiothecium sect. Rectithecium (Hedenäs & Huttunen) J.T. Wynns. Cladistics 34: 469–501. 2018. ≡ Rectithecium Hedenäs & Huttunen. Bot. J. Linn. Soc. 171(2): 344. 2013.This section consists of one species: P. piliferum (Sw.) Schimp.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Buckiella undulata (Hedw.) Ireland = Plagiothecium undulatum |
| Cephalocladium enerve (Broth.) Abramova & I. I. Abramov = Plagiothecium enerve |
| Fissidens denticulatus Baumg. = Plagiothecium denticulatum |
| Fobronia enervis Broth. = Plagiothecium enerve |
| Hypnum argentatum Mitt. = Plagiothecium argentatum |
| Hypnum cavifolius Brid. = Plagiothecium cavifolium |
| Hypnum denticulatum auct. non Hedw. = Plagiothecium cavifolium |
| Hypnum denticulatum L. ex Hedw. = Plagiothecium denticulatum |
| Hypnum denticulatum var. densum (Schimp.) Lesq. & James = Plagiothecium denticulatum |
| Hypnum denticulatum var. donnianum (Sm.) Hook. = Plagiothecium denticulatum |
| Hypnum denticulatum var. laetum (Schimp.) Lindb. = Plagiothecium laetum |
| Hypnum denticulatum var. majus Boulay = Plagiothecium denticulatum |
| Hypnum denticulatum var. obtusifolium Turner = Plagiothecium denticulatum var. obtusifolium |
| Hypnum denticulatum var. piliferum (Hartm.) Wahlenb. = Plagiothecium piliferum |
| Hypnum denticulatum var. sublaetum Lind. = Plagiothecium laetum |
| Hypnum denticulatum var. succulentum Wilson = Plagiothecium succulentum |
| Hypnum denticulatum var. tenellum (Schimp.) Husn. = Plagiothecium laetum |
| Hypnum lamprostachys Hamp. = Plagiothecium denticulatum |
| Hypnum letabricola (Schimp.) Hobk. = Plagiothecium latebricola |
| Hypnum obtusifolium (Turner) Brid. = Plagiothecium denticulatum var. obtusifolium |
| Hypnum orthocarpum Aongstr. = Plagiothecium piliferum |
| Hypnum roeseanum Hampe = Plagiothecium curvifolium |
| Hypnum silvaticum Hedw. ex. Lindb. nom. inval. in synon. err. orthogr. pro. Hypnum |
| sylvaticum Brid. = Plagiothecium denticulatum |
| Hypnum succulentum Wilson = Plagiothecium succulentum |
| Hypnum sullivantiae Sull. & Lesq. = Plagiothecium cavifolium |
| Hypnum sylvaticum auct. non Brid. = Plagiothecium nemorale |
| Hypnum sylvaticum Brid. = Plagiothecium denticulatum |
| Hypnum trichophorum Spruce = Plagiothecium piliferum |
| Hypnum trichophorum var. brevipile (Schimp.) Lindb. = Plagiothecium piliferum |
| Hypnum undulatum Hedw. = Plagiothecium undulatum |
| Isopterygiopsis piliferum (Hartm.) Loeske = Plagiothecium piliferum |
| Isopterygium euryphyllum Cardot & Thér. = Plagiothecium eurphyllum |
| Isopterygium latebricola (Schimp.) Delogne = Plagiothecium latebricola |
| Isopterygium piliferum (Sw.) Loeske = Plagiothecium piliferum |
| Leskea laeta (Schimp.) Bergger. = Plagiothecium laetum |
| Leskea latebricola (Schimp.) Wilson = Plagiothecium latebricola |
| Leskea pilifera Sw. ex. Hartm. = Plagiothecium piliferum |
| Plagiothcium nemorale fo. japonicum (Sakurai) Z. Iwats. ≡ Plagiothecium japonicum |
| Plagiotheciella latebricola (Schimp.) M. Fleisch. = Plagiothecium latebricola |
| Plagiotheciella pilifera (Sw.) M. Fleisch. = Plagiothecium piliferum |
| Plagiothecium annotinum Stirt. ex Dixon nom. inval. = Plagiothecium denticulatum var. obtusifolium |
| Plagiothecium apiculatum Sakurai = Plagiothecium cavifolium |
| Plagiothecium auritum (Kern.) Jedl. = Plagiothecium ruthei |
| Plagiothecium cavifolium fo. otii (Sakurai) Z. Iwats. = Plagiothecium cavifolium |
| Plagiothecium cavifolium var. fallax (Cardot & Thér.) Z. Iwats. = Plagiothecium fallax |
| Plagiothecium chuzensii Iisiba ex Sakurai = Plagiothecium platyphyllum |
| Plagiothecium cochlearifolimus Dixon = Plagiothcium cavifolium |
| Plagiothecium crudum Sakurai = Plagiothcium cavifolium |
| Plagiothecium denticulatum fo. laticuspis F. Koppe = Plagiothecium denticulatum var. obtusifolium |
| Plagiothecium denticulatum subsp. aptychus Spruce = Plagiothecium curvifolium |
| Plagiothecium denticulatum subsp. donnianum (Sm.) Giacom. = Plagiothecium denticulatum |
| Plagiothecium denticulatum subsp. laetum (Schimp.) Kindb. = Plagiothecium laetum |
| Plagiothecium denticulatum subsp. ruthei (Limpr.) Kindb. = Plagiothecium ruthei |
| Plagiothecium denticulatum subsp. sulcatum Spruc. = Plagiothecium denticulatum |
| Plagiothecium denticulatum var. aptychus Lees in Dix. = Plagiothecium curvifolium |
| Plagiothecium denticulatum var. cryptarum Renauld & Hérib. = Plagiothecium cavifo- lium |
| Plagiothecium denticulatum var. curvifolium (Limpr.) Meylan = Plagiothecium curvi- folium |
| Plagiothecium denticulatum var. donnianum (Sm.) Lindb. ex Weim. = Plagiothecium denticulatum |
| Plagiothecium denticulatum var. donnii Lindb. = Plagiothecium denticulatum var. obtusifolium |
| Plagiothecium denticulatum var. eciliatum Pfeff. = Plagiothecium laetum |
| Plagiothecium denticulatum var. gravetii (Piré) Husn. = Plagiothecium laetum |
| Plagiothecium denticulatum var. imbricatum (Boulay) Meyl. = Plagiothecium denticu- latum |
| Plagiothecium denticulatum var. laetum (Schimp.) Lindb. = Plagiothecium laetum |
| Plagiothecium denticulatum var. laxum Schimp. = Plagiothecium denticulatum |
| Plagiothecium denticulatum var. majus (Boulay) Delogne = Plagiothecium denticulatum |
| Plagiothecium denticulatum var. microcarpum Renauld & Cardot = Plagiothecium curvifolium |
| Plagiothecium denticulatum var. myurum Schimp. = Plagiothecium cavifolium |
| Plagiothecium denticulatum var. nervosum (Renauld) Mém. = Plagiothecium platyphyllum |
| Plagiothecium denticulatum var. orthocladium (Schimp.) Hérib. = Plagiothecium cavifolium var. orthocladium |
| Plagiothecium denticulatum var. orthocladum Warnst. = Plagiothecium denticulatum |
| Plagiothecium denticulatum var. phyllorhizans Schiffn. = Plagiothecium denticulatum |
| Plagiothecium denticulatum var. podperae Jedl. = Plagiothecium cavifolium |
| Plagiothecium denticulatum var. propaguliferum (R. Ruthe ex Limpr.) Warnst. = Plagi- othecium denticulatum |
| Plagiothecium denticulatum var. recurvum Warnst. = Plagiothecium curvifolium |
| Plagiothecium denticulatum var. roeseanum (Schimp.) Hérib. = Plagio- thecium cavifolium |
| Plagiothecium denticulatum var. ruthei (Limpr.) Riehm. = Plagiothecium ruthei |
| Plagiothecium denticulatum var. secundum Lindb. = Plagiothecium laetum |
| Plagiothecium denticulatum var. sublaetum (Lindb.) Breidl. = Plagiothecium laetum |
| Plagiothecium denticulatum var. succulentum (Wilson) Dixon = Plagiothecium succu- lentum |
| Plagiothecium denticulatum var. sullivantiae (Sull.) Dix. = Plagiothecium cavifolium |
| Plagiothecium denticulatum var. tenellum Schimp. = Plagiothecium laetum var. tenellum |
| Plagiothecium denticulatum var. undulatum R. Ruthe ≡ Plagiothecium ruthei |
| Plagiothecium dimorphophyllum Sakurai = Plagiothecium euryphyllum |
| Plagiothecium doii Sakurai = Plagiothecium euryphllum |
| Plagiothecium donnianum (Sm.) Mitt. = Plagiothecium denticulatum var. obtusifolium |
| Plagiothecium erectum Broth. = Plagiothecium latebricola |
| Plagiothecium euryphyllum var. brevirameum (Cardot.) Z. Iwats. = Plagiothecium euryphyllum |
| Plagiothecium fujiyamae Sakurai = Plagiothecium cavifolium |
| Plagiothecium gravetii Piré = Plagiothecium laetum |
| Plagiothecium hakusanense Sakurai = Plagiothecium euryphyllum |
| Plagiothecium hattorii Sakurai = Plagiothecium euryphyllum |
| Plagiothecium ikedamii Sakurai = Plagiothecium cavifolium |
| Plagiothecium insigne Cardot = Plagiothecium fallax |
| Plagiothecium kanedae Sakurai = Plagiothecium euryphyllum |
| Plagiothecium laetum fo. julaceum Jedl. = Plagiothecium curvifolium |
| Plagiothecium laetum fo. tenellum (Schimp.) Mönk. = Plagiothecium laetum var. tenellum |
| Plagiothecium laetum subsp. curvifolium (Schlieph. ex Limpr.) Szafran = Plagiothecium curvifolium |
| Plagiothecium laetum subsp. succulentum fo. longifolium (Mönk.) Jedl. = Plagi- othecium succulentum |
| Plagiothecium laetum var. curvifolium (Limpr.) Mastracci & M. Sauer = Plagiothecium curvifolium |
| Plagiothecium laetum var. densum (Schimp.) Warnst. = Plagiothecium laetum |
| Plagiothecium laetum var. secundum (Lindb.) Frisvoll et al. = Plagiothecium curvifo- lium |
| Plagiothecium laetum var. sublaetum (Breidl.) Warnst. = Plagiothecium laetum |
| Plagiothecium laetum var. tenellum (Schimp.) Warnst. = Plagiothecium laetum |
| Plagiothecium latifolium Cardot = Plagiothecium neckeroideum var. myurum |
| Plagiothecium laxum Sakurai = Plagiothecium cavifolium |
| Plagiothecium longicaule Sakurai = Plagiothecium neckeroideum |
| Plagiothecium longisetum var. brevinerve Iisiba = Plagiothecium longisetum |
| Plagiothecium lucens Sauter ex Rabenhorst = Plagiothecium cavifolium |
| Plagiothecium luridum (Molendo) Molendo & Lorentz = Plagiothecium laetum |
| Plagiothecium maedae Sakurai = Plagiothecium neckeroideum |
| Plagiothecium magufuki Sakurai = Plagiothecium japonicum |
| Plagiothecium matsumarae S. Okamura = Plagiothecium euryphyllum |
| Plagiothecium mauiense Broth. = Plagiothecium longisetum |
| Plagiothecium neckeroideum var. angustatum Cardot = Plagiothecium neckeroideum |
| Plagiothecium neckeroideum var. argenteus Dixon = Plagiothecium euryphyllum |
| Plagiothecium neckeroideum var. sikkimense Renauld & Cardot = Plagiothecium neckeroideum var. myurum |
| Plagiothecium neglectum Mönkm. = Plagiothecium nemorale |
| Plagiothecium neglectum subsp. platyphyllum (Mönk.) Szafran = Plagiothecium platyphyllum |
| Plagiothecium neglectum subsp. platyphyllum fo. fontana (Mönk.) Jedl. = Plagiothecium platyphyllum |
| Plagiothecium niitakayamae Toyama = Plagiothecium neckeroideum var. niitakayamae |
| Plagiothecium nipponense Sakurai = Plagiothecium neckeroideum |
| Plagiothecium nitellum Wilson ex Braithw. = Plagiothecium curvifolium |
| Plagiothecium obtusifolium (Turner) J. J. Amann = Plagiothecium denticulatum var. obtusifolium |
| Plagiothecium orthocladium Schimp. = Plagiothecium cavifolium var. orthocladium |
| Plagiothecium orthothecioides (Meyl.) Jedl. = Plagiothecium ruthei |
| Plagiothecium otii Sakurai = Plagiothecium cavifolium |
| Plagiothecium pallidum S. Okamura = Plagiothecium euryphyllum |
| Plagiothecium podperae Jedl. (Jedl.) = Plagiothecium cavifolium |
| Plagiothecium pseudolaetum var. japonicum Cardot = Plagiothecium curvifolium |
| Plagiothecium pseudosylvaticum Warnst. = Plagiothecium ruthei |
| Plagiothecium ptychocarpum Thér. & Dixon = Plagiothecium cavifolium |
| Plagiothecium rigens Broth. = Plagiothecium cavifolium |
| Plagiothecium roeseanum (Hampe) = Plagiothecium cavifolium |
| Plagiothecium roeseanum fo. angustirete (Warnst.) Jedl. = Plagiothecium cavifolium |
| Plagiothecium roeseanum fo. flagellaceum (Warnst.) Mönk. = Plagiothecium cavifolium |
| Plagiothecium roeseanum fo. gracile (Breidl.) Jedl. = Plagiothecium cavifolium |
| Plagiothecium roeseanum fo. heterophyllum (Warnst.) Jedl. = Plagiothecium cavifolium |
| Plagiothecium roeseanum fo. propaguliferum R. Rute = Plagiothecium cavifolium |
| Plagiothecium roeseanum var. gracile Breidl. = Plagiothecium cavifolium |
| Plagiothecium roeseanum var. gracilescens E. Bauer = Plagiothecium cavifolium |
| Plagiothecium roeseanum var. julaceum Cardot = Plagiothecium cavifolium |
| Plagiothecium roeseanum var. flagellaceum Warnst. = Plagiothecium cavifolium |
| Plagiothecium roeseanum var. orthocladium (Schimp.) Limpr. = Plagiothecium cavifo- lium var. orthocladium |
| Plagiothecium roeseanum var. propaguliferum R. Ruthe = Plagiothecium cavifolium |
| Plagiothecium roesei Milde = Plagiothecium cavifolium |
| Plagiothecium rosei Schimp. = Plagiothecium cavifolium |
| Plagiothecium rufovirescens Stirt. = Plagiothecium denticulatum |
| Plagiothecium ruthei var. gracile Meyl. = Plagiothecium ruthei |
| Plagiothecium ruthei var. pseudosylvaticum (Warnst.) Warnst. = Plagiothecium ruthei |
| Plagiothecium ruthei var. rupestris R. Ruthe = Plagiothecium ruthei |
| Plagiothecium ruthei var. subundulatum R. Ruthe ex Warnst. = Plagiothecium ruthei |
| Plagiothecium sakuraii Reimers = Plagiothecium cavifolium |
| Plagiothecium sandbergii Renauld & Cardot = Plagiothecium denticulatum var. obtusifolium |
| Plagiothecium saxicola Sakurai = Plagiothecium nemorale |
| Plagiothecium silvaticum Bruch & Schimp. in Lindb. = Plagiothecium sylvaticum |
| Plagiothecium silvaticum var. latifolium Cardot = Plagiothecium nemorale |
| Plagiothecium silvaticum var. nemorale (Mitt.) Par. = Plagiothecium nemorale |
| Plagiothecium silvaticum var. rhynchostegioides Cardot = Plagiothecium nemorale |
| Plagiothecium solutans Mol. ex Warnst. = Plagiothecium curvifolium |
| Plagiothecium splendens Schimp. ex Cardot = Plagiothecium euryphyllum |
| Plagiothecium splendens var. brevirameum Cardot = Plagiothecium euryphyllum |
| Plagiothecium splendens var. minus Cardot = Plagiothecium euryphyllum |
| Plagiothecium splendens var. paraphylliferum Sakurai = Plagiothecium euryphyllum |
| Plagiothecium splendens var. punctatum Sakurai = Plagiothecium euryphyllum |
| Plagiothecium stoloniferum Velen. = Plagiothecium ruthei |
| Plagiothecium subdenticulatum Correns = Plagiothecium ruthei |
| Plagiothecium sublaetum (Lindb.) Lindb. = Plagiothecium laetum |
| Plagiothecium succulentum fo. lignicolum Jedl. = Plagiothecium succulentum fo. propaguliferum |
| Plagiothecium succulentum var. fontanum (Schiffn.) Riehm. = Plagiothecium platyphyllum |
| Plagiothecium succulentum var. longifolium Mönk = Plagiothecium succulentum fo. propaguliferum |
| Plagiothecium sullivantiae (Schimp. ex Sull.) A.Jaeger = Plagio thecium cavifolium |
| Plagiothecium sylvaticum (Brid.) Schimp. = Plagiothecium denticulatum |
| Plagiothecium sylvaticum auct. non. (Brid.) Bruch & Schimp. = Plagiothecium nemorale |
| Plagiothecium sylvaticum auct. non. Hypnum sylvaticum Brid. = Plagiothecium nemo- rale |
| Plagiothecium sylvaticum subsp. roesei Lindb. (Kindb.) = Plagiothecium cavifolium |
| Plagiothecium sylvaticum subsp. succulentum (Wils.) J.J. Amann & Meyl. = Plagiothecium succulentum |
| Plagiothecium sylvaticum var. auritum Kern = Plagiothecium ruthei |
| Plagiothecium sylvaticum var. cavifolium Jur. = Plagiothecium cavifolium |
| Plagiothecium sylvaticum var. cryptarum (Renauld & Hérib.) P.Syd. = Plagiothecium cavifolium |
| Plagiothecium sylvaticum var. flavescens Warnst. = Plagiothecium platyphyllum |
| Plagiothecium sylvaticum var. fluitans Podp. = Plagiothecium platyphyllum |
| Plagiothecium sylvaticum var. fontanum Schiffn. = Plagiothecium platyphyllum |
| Plagiothecium sylvaticum var. latifolium Cardot = Plagiothecium nemorale |
| Plagiothecium sylvaticum var. laxum Molendo = Plagiothecium cavifolium |
| Plagiothecium sylvaticum var. monoricum Breidl. in Limpr. = Plagiothecium ruthei var. rupincola |
| Plagiothecium sylvaticum var. myurum Molendo = Plagiothecium cavifolium |
| Plagiothecium sylvaticum var. neglectum (Mönk.) F. Koppe = Plagiothecium nemorale |
| Plagiothecium sylvaticum var. nemorale (Mitt.) Paris ≡ Plagiothecium nemorale |
| Plagiothecium sylvaticum var. nervosum Renauld = Plagiothecium platyphyllum |
| Plagiothecium sylvaticum var. orthocladium (Schimp.) Schimp. = Plagiothecium cavi- folium var. orthocladium |
| Plagiothecium sylvaticum var. phyllorhizans Spruce = Plagiothecium platyphyllum |
| Plagiothecium sylvaticum var. platyphyllum (Mönk) F. Koppe = Plagiothecium |
| platyphyllum |
| Plagiothecium sylvaticum var. pseudoneckeroideum Schiffn. = Plagiothecium ruthei |
| Plagiothecium sylvaticum var. pseudo-roeseanum Cardot = Plagiothecium cavifolium |
| Plagiothecium sylvaticum var. rhynchostegioides Cardot = Plagiothecium nemorale |
| Plagiothecium sylvaticum var. rivulare Debat = Plagiothecium nemorale |
| Plagiothecium sylvaticum var. robustum Roell. = Plagiothecium ruthei |
| Plagiothecium sylvaticum var. roeseanum (Schimp.) A. W. H. Walther & Molendo = Plagiothecium cavifolium |
| Plagiothecium sylvaticum var. squarrosum Kind. = Plagiothecium platyphyllum |
| Plagiothecium sylvaticum var. succulentum (Wilson) Spruce = Plagiothecium succu- lentum |
| Plagiothecium sylvaticum var. sullivantiae (Sull.) Ren. & Card. = Plagiothecium cavi- folium |
| Plagiothecium takahashii Sakurai = Plagiothecium cavifolium |
| Plagiothecium trichodeum Stirt. = Plagiothecium denticulatum |
| Plagiothecum ruthei var. subjulaceum Warnst. = Plagiothecium cavifolium |
| Plagiothecium ruthei var. orthothecioides Meyl. = Plagiothecium platyphyllum |
| Rectithecium piliferum (Sw.) Hadenäs & Huttunen = Plagiothecium piliferum |
| Saviczia obtusissima (Broth.) Abramova & I. I. Abramov = Plagiothecium obtusissi- mum |
| Plagiothecium watanabei Dixon = Plagiothecium euryphyllum |
| Stereodon denticulatus (Hedw.) Mitt. = Plagiothecium denticulatum |
| Stereodon nemoralis Mitt. ≡ Plagiothecium nemorale |
| Stereodon sylvaticus (Brid.) Brid. = Plagiothecium denticulatum |
| Struckia argentata (Mitt.) Müll. Hal. = Plagiothecium argentatum |
| Struckia argentata var. enervis (Broth.) B. C. Tan, W. R. Buck & Ignatov = Plagiothe- cium enerve |
| Struckia enervis (Broth.) Ignatov, T. J. Kop. & D. G. Long = Plagiothecium enerve |
Appendix B
- Plagiothecium Schimp. sect. Plagiothecium Type: Hypnum denticulatum Hedw. Species Muscorum Frondosorum 237. 1801.
- 1.
- Plagiothecium conostegium Herzog 1916. Bibiotheca Botanica 87: 154. f. 73: a–d.JAP [119].
- 2.
- Plagiothecium denticulatum (Hedw.) Schimp. in BSG 1851. Bryologia Europea 5: 190, 501 (Table VIII).ALB [9,48]; AND [9,47,48,51,52]; ARM [32,53]; AUT [7,9,48,54]; AZE [32]; AZO [47]; BGM [7,9,48,62]; BAN [59,60]; BUL [9,48,49,68]; BIH [9,47,48,49]; BLR [9,32,48,61,228]; CHI [9,48]; SWI [9,48,176,177]; CHN [77,78,79,80]; COR [9,47,48,62]; CZE [7,9,48,84]; GER [7,9,48,95,96,98]; DEN [7,9,30,48,87]; SPA [9,47,48,51,167]; EST [9,32,48,88]; FIN [7,9,28,48,91,92]; FRA [9,47,48,94]; FRO [7,9,48,89,90]; GRB [7,9,25,48,109]; GEO [32]; GRC [9,47,48,49]; CRO [9,47,48,49]; HUN [9,48,99]; IND [103]; IRE [9,25,48,109]; IRN [45,110]; IRQ [106,107]; ICE [9,48,102]; ITA [9,48,114,115]; JAP [33,34,78,118]; KAZ; KGZ [32]; KOS [9]; SKO [78,86]; LAV [9,32,48,124]; LIE [9,48,126]; LTU [9,32,48,127]; LUX [9,48,129,130]; MDR [9,47,131]; MKD [9,47,48,49,145]; MNE [9,47,48,49]; MON [139]; NET [9,48,143,144]; NOR [7,9,48]; NEP [142]; PAK [149,150,151]; POL [7,9,48,154]; NKO [78,86]; POR [9,47,48,51,155]; ROM [9,48,49,158]; RUS [32,48,159]; SAR [9,48]; SIC [9,47,48]; SVA [9,48,173]; SRB [9,47,48,49]; SVK [9,48,164]; SVN [9,47,48,49]; SWE [7,9,48,174,175]; TZK [32]; TUR [9,45,47,48,49,180,181]; UKR [9,32,48,183,185].
- 3.
- Plagiothecium denticulatum var. affine Warnst. 1906. Kryptogamenflora der Mark Brandenburg, Laubmoose 822, 838: f. 1.GER [7].
- 4.
- Plagiothecium denticulatum var. obtusifolium (Turner) Moore 1873. Proceedings of the Royal Irish Academy 1: 424.BUL [9,47,48]; SWI [7]; CHN [7,79,80]; CZE [7,9,48,84]; GER [7]; SPA [47,48,167]; FIN [7,9,48]; FRA [7,9,47,48,94]; GRB [9,25,48,109]; HUN [7]; IRE [9,25,48,109]; IRN [43,45,110]; ICE [7]; ITA [9,47,48,115,116,117]; JAP [33,118]; KOS [9,120]; LUX [9,48,129,130]; NEP [7]; POL [9,48,154]; RUS [7,32,48,162]; SVN [9,48]; SWE [7,9,48,174,175]; TUR [9,45,47,48,181]; UKR [9,32,48].
- 5.
- Plagiothecium platyphyllum Mönk. 1927. Die Laubmoose Europas 866. f. 207b.AND [9,52]; AUT [7,9,48,54]; BUL [9,47,48,49,68]; SWI [9,48,176,177]; CHN [77,79,80]; COR [9,47,48,62]; CZE [7,9,48,84]; GER [7,9,48,95,97,98]; DEN [30]; SPA [9,47,48,51,167]; FIN [7,9,28,48,91,92,93]; FRA [9,47,48,78,94]; GRB [7,9,25,48]; GEO [32]; GRC [9,48]; CRO [9,83]; HUN [7,9,48,99]; IRE [9,25,48,109]; IRN [43,45,110]; ITA [9,47,48,114,117]; JAP [78]; KOS [9,120]; SKO [78]; LTU [9,128]; LUX [9,48,129,130]; MKD [9,47,48,49]; MNE [9,47,48,49]; NOR [7,9,48]; POL [9,48,154]; NKO [78]; POR [9,156]; ROM [9,48,49,158]; RUS [32,48,159]; SRB [9,47,48,49]; SVK [9,48,164]; SVN [9,47,48,49]; SWE [7,9,48,174,175]; TUR [9,43,45,47,48,49,180,181]; UKR [9,32,48,184].
- 6.
- Plagiothecium ruthei Limpr. 1877. Die Laubmoose Deutschlands, Oesterreichs und der Schweiz 3: 271.AUT [9,48,54]; BUL [47,68]; BLR [9,48]; SWI [7,9,48,176,177]; CHN [77]; CZE [9,48,84]; GER [7,9,48,95,96,98]; DEN [7,9,30,48,87]; SPA [9,47,48,167]; EST [9,32,48,88]; FIN [7,9,48,91,92]; FRA [9,47,48,94]; GRB [9,25,48,109]; HUN [9,48,99]; IRE [25,109]; ITA [47,48,114]; JAP [33,118]; LAV [9,32,48,124]; LIE [9,48,126]; LTU [9,32,48,127]; LUX [9,48,117,129,130]; NET [7,9,48,143,144]; POL [7,9,48,154]; ROM [9,48]; RUS [32,48,159]; SVK [9,48,164]; SWE [9,48,174,175]; UKR [9,32,48,185].
- 7.
- Plagiothecium ruthei var. rupincola Limpr. 1897 Die Laubmoose Deutschland Oesterreich und der Schweiz 3: 273.AUT; CZE; GER; FRA; NOR; SWE [7].
- Plagiothecium sect. Orthophyllum Jedl. Spisy Prír. Fak. Masarykovy Univ. 308: 35. 1948.
- 8.
- Plagiothecium angusticellum G. J. Wolski & P. Nowicka-Krawczyk 2020. PLOS ONE 15(3): e0230237.CZE; EST; HUN; LAV; LTU; POL [27].
- 9.
- Plagiothecium cavifolium (Brid.) Z. Iwats. 1970. Jounral of the Hattori Botanical Labolatory 33: 360.ALB [9,48]; AND [9,52]; AUT [9,48,54]; AZE [32]; BGM [9,48,62]; BUL [9,47,48,49,68]; BIH [9,47,48,49]; BHU [63,64]; BLR [9,32,48,61,228]; SWI [7,9,48,176,177]; CHN [7,64,77,78,79,80]; COR [9,47,48,62]; CZE [7,9,48,84]; GER [7,9,48,95,96,98]; DEN [7,9,30,48,87]; SPA [9,47,48,51,167]; EST [9,32,48,88]; FIN [9,28,48,91,92]; FRA [7,9,47,48,94]; FRO [9,48,89,90]; GRB [9,25,48,109]; GEO [32]; GRC [9,47,48,49]; CRO [9,47,48,49]; HUN [7,9,48,99]; IND [64,103]; IRE [9,25,48,109]; IRN [111]; ICE [7,9,48,102]; ITA [9,47,48,114,115,116,117]; JAP [7,33,34,64,78,118]; KAZ [32]; SKO [64,78]; LAO [78]; LAV [7,9,32,48,124]; LIE [9,48]; LTU [9,32,48,127]; LUX [9,48,129,130]; MOL [9,32,48]; MKD [9,47,48,49,145]; MNE [9,47,48,49]; MON [139]; NET [9,48,143,144]; NOR [9,48]; NEP [64,142]; PAK [148,149,151]; POL [7,9,48,154]; NKO [64,78]; POR [9,47,48,51,155]; ROM [9,48,49,158]; RUS [7,48,64,159]; SIC [9,47,48]; SRB [9,47,48,49]; SVK [9,48,164]; SVN [9,47,48,49]; SWE [9,48,174,175]; TUR [9,45,47,48,49,181]; TAI [178]; UKR [9,32,48,183,185].
- 10.
- Plagiothecium cavifolium var. orthocladium (Schimp.) Z. Iwats. 1970 Jounral of the Hattori Botanical Labolatory 33: 371.FIN; FRO; SWE [7].
- 11.
- Plagiothecium cochleatum Dixon 1938. Notes on the moss collections of the Royal Botanic Garden, Edinburgh. Notes from the Royal Botanic Garden, Edinburgh 19: 299. f. 12.
- 12.
- Plagiothecium japonicum Salurai 1949. Botanical Magazine (Tokyo) 62: 112. f. 1.JAP [7].
- 13.
- Plagiothecium longisetum Lindb. 1872. Contributio ad Floram Cryptogamam Asiae Boreali-Orientalis 232.AUT; AZO; BGM; SWI; CHN; GER; DEN; SPA; EST; FIN; FRA; GRB; GEO; IND; IRN; JAP; MDR; NEP; NOR; POL; RUS; SWE; TUR [27].
- 14.
- Plagiothecium nemorale (Mitt.) A. Jaeger 1878. Bericht über die Thätigkeit der St. Gallischen Naturwissenschaftlichen Gesellschaft 1876-77: 451 (Gen. Sp, Musc. 2: 1269).ALB [9,47,48,49]; AND [9,47,48,51,52]; ARM [32,53]; AUT [9,27,48,54]; AZE (Ignatov et al. 2006) [32]; AZO [9,47,48,55,56,57]; BGM [9,27,48,62]; BUL [9,47,48,49,68]; BIH [9,47,48,49]; BHU [7,63]; BLR [9,32,48,61,228]; CNY [9,47,48]; CHI [9,48]; SWI [9,48,176,177]; CHN [7,27,66,77,78,79,80]; COR [9,47,48,62]; CZE [7,9,27,48,84]; GER [7,9,27,48,95,96,98]; DEN [7,9,27,30,48,87]; SPA [9,47,48,51,167]; EST [9,27,32,48,88]; FIN [9,28,48,91,92,93]; FRA [7,9,27,47,48,94]; FRO [90]; GRB [7,9,25,27,48,109]; GEO [27,32]; GRC [9,47,48,49]; CRO [47,48,82]; HUN [7,9,27,48,99]; IND [7,27,103]; IRE [9,25,48,109]; IRN [7,27,43,45,111]; ITA [7,9,27,47,48,114,115,116,117]; JAP [7,27,33,34,66,78,118]; KOS [9,120]; SKO [7,66,78,86]; LAV [9,27,32,48,124]; LIE [9,48,126]; LTU [9,27,32,48,127]; LUX [9,48,129,130]; MDR [9,47,48,51,131]; MKD [9,47,48,49,145]; MYA [72]; MNE [9,47,48,49]; NET [9,48,143,144]; NOR [9,27,48]; NEP [7,141,142]; PAK [150,151]; PHI [152,153]; POL [9,27,48,154]; NKO [7,66,78,85,86]; POR [9,27,47,48,51,155]; ROM [9,48,49,158]; RUS [7,32,48,66,78,159]; SAR [9,47,48]; SIC [9,47,48]; SRB [9,47,48,49]; SVK [9,27,48,164]; SVN [9,47,48,49,165]; SWE [7,9,27,48,174,175]; TUR [7,9,27,43,45,47,48,49,180,181]; TAI [66,78,178]; UKR [9,32,48,183,185]; VIE [189].
- 15.
- Plagiothecium rhizophyllum Sakurai 1932. Botanical Magazine (Tokyo) 46: 501.CHN [7].
- 16.
- Plagiothecium succulentum (Wilson) Lindb. 1865. Botaniska Notiser 1865: 143.ALB [9,48]; AND [52]; AUT [9,48,54]; AZO [9,47,48,55,56,57]; BGM [9,48,62]; BUL; BIH [9,47,48,49]; BLR [9,48]; CNY [9,47,48,74]; CHI [9,48]; SWI [9,48,176,177]; CHN [77,79,80]; COR [9,47,48,62]; CZE [7,9,48,84]; GER [7,9,48,95,96,98]; DEN [7,9,30,48,87]; SPA [9,47,48,51,167]; EST [9,32,48,88]; FIN [9,28,48,91]; FRA [9,47,48,78,94]; FRO [7,9,48,89,90]; GRB [9,25,48,109]; GEO (Ignatov et al. 2006) [32]; GRC [9,47,48,49]; HUN [9,48,99]; IRE [9,25,48,109]; IRN [45,110]; ICE [9,48,102]; ITA [9,47,48,114,116,117]; SKO [78]; KOS [9,120]; LAV [9,32,48,124]; LTU [9,32,48,127]; LUX [9,48,129,130]; MDR [9,47,48,51,131,132]; MKD [9,146]; MNE; NOR [9,48]; NET [9]; POL [9,48,154]; NKO [78]; POR [9,47,48,51,155]; ROM [9,48,49,158]; RUS [32,48,162]; SRB [9,47,48,49]; SVK [9,48,164]; SVN [9,47,48,49]; SWE [7,9,48,174,175]; TUR [9,45,47,48,49,181]; UKR [9,32,48,184,185].
- 17.
- Plagiothecium succulentum fo. propaguliferum E. Bauer 1902. Deutsche Botanische Monatsschrift 20: 2.AUT; CZE; GER; DEN; LAV [7].
- Plagiothecium sect. Leptophyllum Jedl. Spisy Prír. Fak. Masarykovy Univ. 308: 23. 1948.
- 18.
- Plagiothecium berggrenianum Frisvoll 1981. Lindbergia 7: 96. f. 2: a–i.
- 19.
- Plagiothecium curvifolium Schlieph. ex Limpr. 1897. Die Laubmoose Deutschlands, Oesterreichs und der Schweiz 3: 269.ALB; AUT [9,48]; BGM [9,48,62]; BUL [9,47,48]; BIH [9,47,48,49]; CHI [9,48]; SWI [9,48,177]; CHN [77,79,80]; COR [9,47,48,62]; CZE [7,9,48,84]; GER [7,9,26,48,95,98]; DEN [7,9,30,48,87]; SPA [9,47,48,51,167]; EST [9,32,48,88]; FIN [9,28,48,91,92]; FRA [9,47,48,94]; GRB [9,25,48,109]; GRC [9,47,48,49]; CRO [9,83]; HUN [9,48,100]; IRE [9,25,48,109]; ITA [9,47,48,116,117]; JAP [34]; KOS [9,120]; LAV [7,9,32,48,124]; LIE [9,48,126]; LTU [9,32,48,127]; LUX [9,48,129,130]; MKD [9,47,48]; MNE [9,47,48,49]; NET [9,143]; NOR [9,48]; POL [7,9,26,48,154]; ROM [9,48,49,158]; RUS [26,32,48,161,162]; SRB [9,47,48,49]; SVK [7,9,48,164]; SVN [9,47,48,49]; SWE [9,48,174,175]; TUR [9,45,47,48,49,181]; UKR [9,26,32,48,185].
- 20.
- Plagiothecium curvifolium fo. julaceum Clum. & E. Bauer 1915. Musci Europaei Exsiccati 27: 1307.SWI [7].
- 21.
- Plagiothecium laetum Schimp. in BSG 1851. Bryologia Europaea 5: 185, 495 (Table II).ALB [9,50]; AND [9,47,48,51,52]; AUT [9,26,48]; AZE [32]; BGM [7,9,48,62]; BUL [9,47,48,49,68]; BIH [9,26,47,48,49]; BLR [9,32,48,61,228]; SWI [9,48,176,177]; CHN [77,79,80]; CZE [7,9,26,48,84]; GER [7,9,26,48,95,96,98]; DEN [7,9,30,48,87]; SPA [9,47,48,51,167]; EST [9,32,48,88]; FIN [7,9,26,28,48,91,92]; FRA [9,47,48,94]; GRB [9,25,48,109]; GEO [32]; GRC [9,47,48,49]; HUN [9,26,48,99]; IRE [9,25,48,109]; IRN [43,45,110]; ITA [9,47,48,114,115,116,117]; JAP [33,78,118]; KAZ; KGZ [32]; KOS [9,120]; SKO [78]; LAV [9,32,48,124]; LIE [9,48,126]; LTU [9,32,48,127]; LUX [9,48,129,130]; MDR [47,51,132]; MNE [9,47,48,49]; MON [139]; NET [9,48,143,144]; NOR [9,26,48]; POL [7,9,26,48,154]; NKO [78]; [47,48,51]; ROM [9,48,49,158]; RUS [7,26,32,48,159]; SRB [9,47,48,49]; SVK [9,48,164]; SVN [9,47,48,49,165]; SWE [7,9,26,48,174,175]; TUR [7,45,47,181]; TAI [66]; UKR [9,32,48,183,185].
- 22.
- Plagiothecium laetum var. tenellum (Schimp.) Warnst. 1906. Kryptogamenflora der Mark Brandenburg, Laubmoose 835.BGM [7].
- 23.
- Plagiothecium latebricola Wilson ex Schimp. in BSG 1851. Bryologia Europaea 5: 184, 494 (Table I).AUT [9,48,54]; BGM [7,9,48,62]; BLR [9,32,48,61,228]; SWI [48]; CHN [76,77,79,80]; CZE [9,48,84]; GER [9,48,95,96,98,222]; DEN [7,9,30,48,87]; EST [9,32,48,88]; FIN [7,9,28,48,91,92,93]; FRA [9,47,48]; GRB [7,9,25,48,109]; GEO [32]; HUN [9,101]; IRE [9,25,48,109]; ITA [9,47,48,114,117]; JAP [33,34,118]; KGZ [32]; LAV [9,32,48,124]; LTU [9,32,48,127]; SRL [170]; LUX [9,48,129,130]; NET [7,9,143,144]; NOR [9,48]; PAK [150,151]; POL [9,48,154]; POR [9,47,48,155]; ROM [9,48,49,158]; RUS [32,48,159]; SRB [9,47,48,49]; SVK [9,48,164]; SWE [9,48,174,175]; TUR [43,45,47,180,181]; UKR [9,32,48,185].
- 24.
- Plagiothecium rossicum Ignatov & Ignatova 2019. Arctoa 28: 28-45.
- 25.
- Plagiothecium svalbardense Frisvoll 1996. Norsk Polarinstitutt Skrifter 198: 103.
- Plagiothecium sect. Pseudo-Neckera (Kindb.) J.T. Wynns 2015
- 26.
- Plagiothecium decoratum J. T. Wynns 2015. Molecular phylogeny and systematic revision of the pleurocarpous moss genus Plagiothecium. PhD Thesis, University of Copenhagen: 126.BHU; NEP [7].
- 27.
- Plagiothecium neckeroideum Schimp. in BSG 1851. Bryologia Europea 5: 194, 505 (Table XII).AUT [7,9,48,54]; BOR [65,66]; BHU [63]; SWI [7,9,48,176,177]; CHN [7,66,77,78,79,80]; CZE [9,48,84]; GER [7,9,48,95,97,98]; IDN [105]; IND [78,103]; JAP [7,33,34,66,78,118]; SKO [78]; MLY [133]; NEP [7,142]; PHI [7,66,152,153]; NKO [78]; ROM [9,48,49,158]; RUS [32,66,78,159]; SIN [133]; SUM [66,105]; SVN [9,47,48,49,166]; THA [66,72,179]; TAI [66,178]; UKR [9,32,48,184].
- 28.
- Plagiothecium neckeroideum fo. exile J. T. Wynns 2015. Molecular phylogeny and systematic revision of the pleurocarpous moss genus Plagiothecium. PhD Thesis, University of Copenhagen: 210.NEP [7].
- 29.
- Plagiothecium neckeroideum var. javense M. Fleisch. 1920. Die Musci der Flora von Buitenzorg 4: 1168. f. 194.
- 30.
- Plagiothecium neckeroideum var. myurum Molendo 1875. Jahres-Bericht de Naturhistirischen Vereins in Passau 10: 234.BHU; CHN, IND; NEP [7].
- 31.
- Plagiothecium neckeroideum var. niitakayamae (Toyama) Z. Iwats. 1970. Journal of the Hattori Botanical Laboratory 33: 354.
- 32.
- Plagiothecium neckeroideum fo. parvum J. T. Wynns 2015. Molecular phylogeny and systematic revision of the pleurocarpous moss genus Plagiothecium. PhD Thesis, University of Copenhagen: 213.TAI [7].
- 33.
- Plagiothecium noricum Molendo ex Limpr. 1897. Die Laubmoose Deutschlands, Oesterreichs und der Schweiz 3: 257.
- 34.
- Plagiothecium subglaucum Thwaites & Mitt. 1873. Journal of the Linnean Society, Botany 13: 321.
- Plagiothecium sect. Lycambium Jedl. 1948. Spisy Prír. Fak. Masarykovy Univ. 308: 10. 1948.
- 35.
- Plagiothecium fallax Cardot & Thér. 1902. Proceedings of the Washington Academy of Sciences 4: 336. pl. 22: f. 4.JAP; RUS [7].
- 36.
- Plagiothecium undulatum (Hedw.) Schimp. in BSG 1851. Bryologia Europea 5: 195, 506 (Table XIII).AUT [9,48,54]; AZE [32]; BGM [9,48,62]; BUL [9,47,48,49,68]; BIH [47,48,49]; BLR [9,32,48,228]; CHI [9,48]; SWI [9,48,176,177]; CHN [77,79]; CZE [9,48,84]; GER [7,9,48,95,96,98]; DEN [7,9,30,48,87]; SPA [9,47,48,51,167]; EST [9,32,48,88]; FIN [9,28,48,91,92]; FRA [7,9,47,48,94]; FRO [7,9,48,89,90]; GRB [7,9,25,48,109]; CRO [9,47,48,49]; HUN [9,48,99,100]; IRE [9,25,48,109]; IRN [43,45,110]; ITA [9,47,48,114,115,116,117]; LAV [9,32,48,124]; LIE [9,48,126]; LTU [9,32,48,127]; LUX [9,48,129,130]; NET [9,48,143,144]; NOR [9,48]; POL [7,9,48,154]; POR [9,47,48,51]; ROM [9,48,49,158]; RUS [32,48,159]; SIC [9,47,48]; SRB [9,47,48,49]; SVK [9,48,164]; SVN [9,47,48,49]; SWE [7,9,48,174,175]; TUR [43,45,47,180,181]; UKR [9,32,48,184].
- Plagiothecium sect. Saviczia (Abramova & I. I. Abramov) Z. Iwats. J. Hattori Bot. Lab. 33: 341. 1970.
- 37.
- Plagiothecium euryphyllum (Cardot & Thér.) Z. Iwats. 1970. Jounral of the Hattori Botanical Labolatory 33: 348.
- 38.
- Plagiothecium obtusissimum Broth. 1921. Oefversigt at Förhandlingar, Finska Vetenskaps-Societeten 62A(9): 45.
- Plagiothecium sect. Struckia (Müll. Hal.) J.T. Wynns 2015
- 39.
- Plagiothecium argentatum (Mitt.) Q. Zuo 2011. Journal of Bryology 33(3): 227.
- 40.
- Plagiothecium enerve (Broth.) Q. Zuo 2011. Journal of Bryology 33(3): 227.
- Plagiothecium sect. Rectithecium (Hedenäs & Huttunen) J. T. Wynns 2015
- 41.
- Plagiothecium piliferum (Sw.) Schimp. in BSG 1851. Bryologia Europea 5: 186, 496 (Table III).AND [9,52]; SWI [9,48,176,177]; CHN [77,78,79,80]; COR [9,47,48,62]; DEN [30]; SPA [9,47,48,51,167]; FIN [7,9,28,48,91,92]; FRA [9,47,48,78,94]; GRB [7,9,25,48,109]; IRE [25,109]; ITA [9,47,48,114,115,117]; JAP [33,78]; SKO [78]; LAV [9,125]; NOR [7,9,48]; NKO [78]; POR [9,47,48,51,155]; ROM [9,48,49,158]; RUS [7,32,48,78,159]; SAR [9,47,48]; SVN [9,47,48,49,165]; SWE [7,9,48,174,175]; TUR [47,182]; UKR [9,32,48,184,185].
References
- Bruch, P.; Schimper, W.P.; Gümbel, W.T. Bryologia Europaea Seu Genera Muscorum Europaeorum Monographice Illustrata; Schimper, W.P., Ed.; Sumptibus Librariae E. Schweizerbart: Stuttgart, Germany, 1851; Volume 5. [Google Scholar]
- Buck, W.R.; Ireland, R.R. A Reclassification of the Plagiotheciaceae. Nova Hedwig. 1985, 41, 89–125. [Google Scholar]
- Buck, W.R.; Ireland, R.R. Plagiotheciaceae. Flora Neotropica, Monograph 50; The New York Botanical Garden: New York, NY, USA, 1989. [Google Scholar]
- Buck, W.R. Plagiotheciaceae. In Pleurocarpous Mosses of the West Indies; The New York Botanical Garden: New York, NY, USA, 1998; pp. 295–299. [Google Scholar]
- Pedersen, N.; Hedenäs, L. Phylogenetic Relationships within the Plagiotheciaceae. Lindbergia 2001, 26, 62–76. [Google Scholar]
- Pedersen, N.; Hedenäs, L. Phylogeny of the Plagiotheciaceae Based on Molecular and Morphological Evidence. Bryologist 2002, 105, 310–324. [Google Scholar] [CrossRef]
- Wynns, J.T. Molecular Phylogeny and Systematic Revision of the Pleurocarpous Moss Genus Plagiothecium. Ph.D. Thesis, University of Copenhagen, Copenhagen, Denmark, 2015. [Google Scholar]
- Hodgetts, N.G.; Söderström, L.; Blockeel, T.L.; Caspari, S.; Ignatov, M.S.; Konstantinova, N.A.; Lockhart, N.; Papp, B.; Schröck, C.; Sim-Sim, M.; et al. An Annotated Checklist of Bryophytes of Europe, Macaronesia and Cyprus. J. Bryol. 2020, 42, 1–116. [Google Scholar] [CrossRef]
- Hodgetts, N.; Lockhart, N. Checklist and Country Status of European Bryophytes—Update 2020; Irish Wildlife Manuals, No. 123.; National Parks and Wildlife Service. Department of Culture, Heritage and the Gaeltacht: Dublin, Ireland, 2020.
- O’Shea, B.J. Checklist of the Mosses of Sub-Saharan Africa (Version 5, 12/06). Trop. Bryol. Res. Rep. 2006, 6, 1–255. [Google Scholar]
- Ochyra, R. Plagiothecium lamprostachys (Hampe) A.Jaeger (Plagiotheciaceae), a Forgotten Name for an Australasian Moss. J. Bryol. 2002, 24, 85–86. [Google Scholar] [CrossRef]
- Ros, R.M.; Cano, M.J.; Guerra, J. Bryophyte Checklist of Northern Africa. J. Bryol. 1999, 21, 207–244. [Google Scholar] [CrossRef]
- Schultze-Motel, W. Katalog Der Laubmoose von West-Afrika. [Catalogue of Mosses of West-Africa]. Willdenowia 1975, 7, 473–535. [Google Scholar]
- Ochyra, R.; Lewis-Smith, R.I.; Bednarek-Ochyra, H. The Illustrated Moss Flora of Antarctica; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Ochyra, R.; Bednarek-Ochyra, H.; Lewis Smith, R.I. New and Rare Moss Species from Subantarctic South Georgia. Nova Hedwig. 2002, 74, 121–147. [Google Scholar] [CrossRef]
- Ireland, R.R. Studies of the Genus Plagiothecium in Australasia. Bryologist 1992, 95, 221–224. [Google Scholar] [CrossRef]
- Klazenga, N. Australian Mosses Online 18. Plagiotheciaceae. Australian Biological Resources Study, Canberra. 2012. Available online: http://www.anbg.gov.au/abrs/Mosses_online/18_Plagiotheciaceae.html (accessed on 29 January 2020).
- Ireland, R.R. A Taxonomic Revision of the Genus Plagiothecium for North America, North of Mexico; National Museum of Natural Sciences Publications in Botany, The National Museums of Canada: Ottawa, ON, Canada, 1969. [Google Scholar]
- Ireland, R.R. Synopsis of the Genus Plagiothecium in North America. Lindbergia 1986, 12, 49–56. [Google Scholar]
- Anderson, L.E.; Crum, H.A.; Buck, W.R. List of the Mosses of North America North of Mexico. Bryologist 1990, 93, 448–499. [Google Scholar] [CrossRef]
- Ireland, R.R. Family Plagiotheciaceae. In Flora of North America North of Mexico; Flora of North America Editorial Committee, Ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2014; Volume 28, pp. 483–488. ISBN 978-0-19-020275-0. [Google Scholar]
- Churchill, S.P.; Griffin, D.G.; Muñoz, F.J. A Checklist of the Mosses of the Tropical Andean Countries; Monografías del Real Jardín Botánico; Consejo Superior de Investigaciones Científicas: Madrid, Spain, 2000; Volume 17, ISBN 978-84-00-07993-2. [Google Scholar]
- Wynns, J.T.; Munk, K.R.; Lange, C.B.A. Molecular Phylogeny of Plagiothecium and Similar Hypnalean Mosses, with a Revised Sectional Classification of Plagiothecium. Cladistics 2018, 34, 469–501. [Google Scholar] [CrossRef]
- Wolski, G.J. Are Plagiothecium cavifolium, P. nemorale and P. succulentum Indeed Variable Species? Pak. J. Bot. 2018, 50, 1579–1589. [Google Scholar]
- Smith, A.J.E. The Moss Flora of Britain and Ireland, 2nd ed.; Cambridge University Press: Cambridge, UK, 2004; ISBN 978-0-521-54672-0. [Google Scholar]
- Ignatova, E.A.; Fedorova, A.V.; Kuznetsova, O.I.; Ignatov, M.S. Taxonomy of the Plagiothecium laetum Complex (Plagiotheciaceae, Bryophyta) in Russia. Arctoa 2019, 28, 28–45. [Google Scholar] [CrossRef]
- Wolski, G.J.; Nowicka-Krawczyk, P. Resurrection of the Plagiothecium longisetum Lindb. and Proposal of the New Species—P. angusticellum. PLoS ONE 2020, 15, e0230237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyholm, E. Illustrated Moss Flora of Fennoscandia; CWK Gleerup: Lund, Sweden, 1965; Volume 2. [Google Scholar]
- Iwatsuki, Z. A Revision of Plagiothecium and Its Related Genera from Japan and Her Adjacent Areas. J. Hattori Bot. Lab. 1970, 33, 332–385. [Google Scholar]
- Lewinsky, J. The Family Plagiotheciaceae in Denmark. Lindbergia 1974, 2, 185–217. [Google Scholar]
- Hill, M.O.; Bell, N.; Bruggeman-Nannenga, M.A.; Brugues, M.; Cano, M.J.; Enroth, J.; Flatberg, K.I.; Frahm, J.-P.; Gallego, M.T.; Garilleti, R.; et al. An Annotated Checklist of the Mosses of Europe and Macaronesia. J. Bryol. 2006, 28, 198–267. [Google Scholar] [CrossRef]
- Ignatov, M.S.; Afonina, O.M.; Ignatova, E.A.; Āboliņa, A.; Akatova, T.V.; Baisheva, E.Z.; Bardunov, L.V.; Baryakina, E.A.; Belkina, O.A.; Bezgodov, A.G.; et al. Check-List of Mosses of East Europe and North Asia. Arctoa 2006, 15, 1–130. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T. A Revised New Catalog of the Mosses of Japan. Hattoria 2016, 7, 9–223. [Google Scholar] [CrossRef]
- Noguchi, A. Illustrated Moss Flora of Japan; Hattori Botanical Laboratory: Nichinan, Japan, 1994; Volume 5, ISBN 978-4-938163-09-9. [Google Scholar]
- Huttunen, S.; Ignatov, M.S.; Quandt, D.; Hedenӓs, L. Phylogenetic Position and Delimitation of the Moss Family Plagiotheciaceae in the Order Hypnales. Bot. J. Linn. Soc. 2013, 171, 330–353. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Q.; Higuchi, M.; Wang, Y.F.; Arikawa, T.; Hirayama, Y. The Status of Struckia Müll.Hal. (Plagiotheciaceae, Bryopsida) Inferred from Multiple Nuclear and Chloroplast Loci. J. Bryol. 2011, 33, 221–228. [Google Scholar] [CrossRef]
- Wynns, J.T.; Schröck, C. Range Extensions for the Rare Moss Plagiothecium handelii, and Its Transfer to the Resurrected Genus Ortholimnobium. Lindbergia 2018, 41, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, J. Étude Comparative Des Plagiotheciacea de Belgique a l’égard de Certains Facteurs Influencant Leur Relation Hydrique Avec Le Milieu [Comparative Study of Plagiotheciacea from Belgium with Regard to Certain Factors Influencing Their Water Relationship with the Environment]. Bull. Soc. Roy. Bot. Belg. 1970, 103, 63–70. [Google Scholar]
- Lefebvre, J. Étude autécologique des Plagiotheciaceae de Belgique. [Autecological study of Plagiotheciaceae from Belgium]. Bull. Soc. Roy. Bot. Belg. 1970, 103, 51–62. [Google Scholar]
- Szweykowski, J.; Zieliński, R. Isoenzymatic Variation in Polish Population of the Moss Plagiothecium undulatum (Hedw.) B.S.G.—A Preliminary Report. J. Hattori Bot. Lab. 1983, 54, 119–123. [Google Scholar]
- Inoue, S.; Iwatsuki, Z. Cytotaxonomic Studies of Plagiothecium B.S.G. and its Related Genera. J. Hattori Bot. Lab. 1987, 63, 453–471. [Google Scholar]
- Hofman, A. A Preliminary Survey of Allozyme Variation in the Genus Plagiothecium (Plagiotheciaceae, Bryopsida). J. Hattori Bot. Lab. 1988, 64, 143–150. [Google Scholar]
- Frey, W.; Kürschner, H. Conspectus Bryophytorum Orientalum et Arabicorum. An Annotated Catalogue of the Bryophytes of Southwest Asia; J. Cramer in der Gebrüder Borntraeger Verlagsbuchhandlung: Berlin-Stuttgart, Germany, 1991. [Google Scholar]
- Frey, W.; Kürschner, H. New Records of Bryophytes from Afghanistan—With a Note on the Bryological Exploration of the Country. Nova Hedwig. 2009, 88, 503–511. [Google Scholar] [CrossRef]
- Kürschner, H.; Frey, W. Liverworts, Mosses and Hornworts of Southwest Asia (Marchantiophyta, Bryophyta, Anthoceroptophyta). Nova Hedwig. 2011, 139, 179–180. [Google Scholar]
- Kürschner, H.; Frey, W. Liverworts, Mosses and Hornworts of Afghanistan—Our Present Knowledge. Acta Mus. Siles. Sci. Nat. 2019, 68, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Ros, R.M.; Mazimpaka, V.; Abou-Salama, U.; Aleffi, M.; Blockeel, T.L.; Brugués, M.; Cros, R.M.; Dia, M.G.; Dirkse, G.M.; Draper, I.; et al. Mosses of the Mediterranean, an Annotated Checklist. Cryptogam. Bryol. 2013, 34, 99–283. [Google Scholar] [CrossRef]
- Hodgetts, N.G. Checklist and Country Status of European Bryophytes—Towards a New Red List for Europe. Irish Wildlife Manuals, No. 84; National Parks and Wildlife Service, Department of Arts, Heritage and the Gaeltacht: Dublin, Ireland, 2015.
- Sabovljević, M.; Natcheva, R.; Dihoru, G.; Tsakiri, E.; Dragićević, S.; Erdağ, A.; Papp, B. Check-List of the Mosses of SE Europe. Phytol. Balcan. 2008, 14, 207–244. [Google Scholar]
- Marka, J.; Blockeel, T.L.; Long, D.G.; Papp, B. Bryophytes New to Albania from the British Bryological Society Field Meeting in 2014. J. Bryol. 2018, 40, 163–172. [Google Scholar] [CrossRef]
- Sérgio, C.; Brugués, M.; Cros, R.M.; Casas, C.; Garcia, C. The Red List and an Updated Checklist of Bryophytes of the Iberian Peninsula (Portugal, Spain and Andorra). Lindbergia 2006, 31, 108–124. [Google Scholar]
- Sotiaux, A.; Vanderpoorten, A. A Checklist of the Bryophytes of Andorra. J. Bryol. 2017, 39, 353–367. [Google Scholar] [CrossRef]
- Manakyan, V.A. Results of Bryological Studies in Armenia. Arctoa 1995, 5, 15–33. [Google Scholar] [CrossRef] [Green Version]
- Köckinger, H.; Schröck, C.; Krisai, R.; Zechmeister, H.G. Checklist of Austrian Bryophytes (a Continuously Updated Checklist). [Online Source]. Available online: https://cvl.univie.ac.at/projekte/moose/ (accessed on 19 March 2021).
- Frahm, J.-P. An Evaluation of the Bryophyte Flora of the Azores. Bryophyt. Divers. EVolume 2005, 26, 57–79. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, R.; Sjögren, E.; Schumacker, R.; Sérgio, C.; Aranda, S.C.; Claro, D.; Homem, N.; Martins, B. List of Bryophytes (Anthocerotophyta, Marchantiophyta, Bryophyta). In A List of the Terrestrial and Marine Biota from the Azores; Borges, P.A.V., Costa, A., Cunha, R., Gabriel, R., Gonçalves, V., Martins, A.F., Melo, I., Parente, M., Raposeiro, P., Rodrigues, P., et al., Eds.; Princípia: Cascais, Portugal, 2010; pp. 99–115. [Google Scholar]
- Gabriel, R.; Homem, N.; Couto, A.; Aranda, S.C.; Borges, P.A.V. Azorean Bryophytes: A Preliminary Review of Rarity Patterns. Açoreana 2011, 7, 149–206. [Google Scholar]
- Cros, R.M.; Sáez, L.; Brugués, M. The Bryophytes of the Balearic Islands: An Annotated Checklist. J. Bryol. 2008, 30, 74–95. [Google Scholar] [CrossRef]
- O’Shea, B.J. An Overview of the Mosses of Bangladesh, with a Revised Checklist. J. Hattori Bot. Lab. 2003, 93, 259–272. [Google Scholar]
- Banu-Fattah, K.; Sarker, S.K. Bryophyte Flora of Greater Mymensingh District of Bangladesh—Class: Bryopsida. Bangladesh J. Plant Taxon. 2007, 14, 47–66. [Google Scholar] [CrossRef] [Green Version]
- Shabeta, M.S.; Rykovsky, G.F. Bryophyte Diversity in The Belarus Coniferous Forests. Arctoa 2015, 24, 541–546. [Google Scholar] [CrossRef] [Green Version]
- Sotiaux, A.; Pioli, A.; Royaud, A.; Schumacker, R.; Vanderpoorten, A. A Checklist of the Bryophytes of Corsica (France): New Records and a Review of the Literature. J. Bryol. 2007, 29, 41–53. [Google Scholar] [CrossRef]
- Long, D.G. Mosses of Bhutan II *. A Checklist of the Mosses of Bhutan. J. Bryol. 1994, 18, 339–364. [Google Scholar] [CrossRef]
- Asthana, A.K.; Sahu, V. Two New Records of Plagiothecium from India. Acta Bot. Hung. 2015, 57, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Suleiman, M.; Akiyama, H.; Tan, B.C. A Revised Catalogue of Mosses Reported from Borneo. J. Hattori Bot. Lab. 2006, 99, 107–183. [Google Scholar] [CrossRef]
- Higuchi, M.; Yao, K.-Y.; Lin, S.-H. Mosses of Mt. Chilai, Taiwan. Mem. Natl. Sci. Mus. Tokyo 2012, 48, 159–175. [Google Scholar]
- Tan, B.C.; Mohamed, H. A New Moss Checklist of Negara Brunei Darussalam. Pol. Bot. J. 2013, 58, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Natcheva, R.; Ganeva, A. Check-List of the Bryophytes of Bulgaria. II. Musci. Cryptogam. Bryol. 2005, 26, 209–232. [Google Scholar]
- Tixier, P. Bryophytae Indosinicae. A Preliminary Contribution to the Bryoflora of Angkhor (Cambodia). Nat. Hist. Bull. Siam Soc. 1966, 21, 129–133. [Google Scholar]
- Tixier, P. A Preliminary Contribution to the Knowledge of the Coastal Southern Bryoflora of Cambodia. Nat. Hist. Bull. Siam Soc. 1975, 26, 11–24. [Google Scholar]
- Tixier, P. Cambodian Bryoflora. Collections from Phnom Kulen. Nova Hedwig. 1980, 32, 369–376. [Google Scholar]
- Tan, B.C.; Iwatsuki, Z. A Checklist of Indochinese Mosses. J. Hattori Bot. Lab. 1993, 74, 325–405. [Google Scholar]
- Higuchi, M. Moss Flora of Angkor, Cambodia. Bull. Natn. Sci. Mus. Tokyo Ser. B 2009, 35, 141–150. [Google Scholar]
- Dirkse, G.; Bouman, A.; Losada-Lima, A. Bryophytes of the Canary Islands: An Annoted Checklist. Cryptogam. Bryol. 1993, 14, 1–47. [Google Scholar]
- Dirkse, G.M.; Losada-Lima, A. Additions and Amendments to the Moss Flora of the Canary Island. Cryptogam. Bryol. 2011, 32, 37–41. [Google Scholar] [CrossRef]
- He, S.; Redfearn, P.L. Three Mosses New to Mainland China. Bryologist 1995, 98, 383–384. [Google Scholar] [CrossRef]
- Redfearn, P.L.; Tan, B.C.; He, S. A Newly Updated and Annotated Checklist of Chines Mosses. J. Hattori Bot. Lab. 1996, 79, 163–357. [Google Scholar]
- Park, K.W.; Choe, K. New List of Bryophytes in Korea; Korea National Arboretum: Pocheon, Korea, 2007. [Google Scholar]
- Deng-ke, L.; Ireland, R.R. Plagiotheciaceae. In Moss Flora of China; Amblystegiaceae to, Plagiotheciacea; Ren-liang, H., You-fang, W., Crosby, M.R., Eds.; Science Press and Missouri Botanical Garden Press: Beijing, China; St. Louis, MO, USA, 2008; Volume 7, pp. 219–245. [Google Scholar]
- A Checklist of Chinese Mosses. (Date Unknown). Available online: http://www.mobot.org/MOBOT/moss/China/china-p.html (accessed on 3 January 2020).
- Blockeel, T.L. Notes on Some Rare and Newly Recorded Bryophytes from Crete, Greece. J. Bryol. 2007, 29, 197–198. [Google Scholar] [CrossRef]
- Papp, B.; Sabovljević, M. Notes on Some New and Interesting Bryophyte Records from Croatia. J. Bryol. 2009, 31, 272–275. [Google Scholar] [CrossRef]
- Papp, B.; Alegro, A.; Šegota, V.; Šapić, I.; Vukelić, J. Additions to the Bryophyte Flora of Croatia. J. Bryol. 2013, 35, 140–143. [Google Scholar] [CrossRef]
- Kučera, J.; Váňa, J. Check- and Red List of Bryophytes of the Czech Republic. Preslia 2003, 75, 193–222. [Google Scholar]
- Tong, C.; Yuhuan, W. An Enumeration of Bryophytes Collected from North Korea. J. Res. 1998, 9, 226–228. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, S.Y.; Lee, B.Y.; Yoon, Y.J.; Choi, S.S.; Sun, B.Y. A Field Guide to Bryophytes in Korea; National Institute of Biological Resources: Incheon, Korea, 2013.
- Mogensen, G.S.; Goldberg, I. Danske Navne for Tørvemosser, Sortmosser, og Bladmosser der Forekommer i Danmark. Ver. 4. [Danish and Latin Names for Sphagna, Andreaeas and Mosses that Occur in Denmark. Vers. 4.]; Botanisk Have & Museum, Københavns Universitet: Copenhagen, Denmark, 2005. [Google Scholar]
- Vellak, K.; Ingerpuu, N.; Leis, M.; Ehrlich, L. Annotated Checklist of Estonian Bryophytes. Folia Cryptogam. Est. 2015, 52, 109–127. [Google Scholar] [CrossRef] [Green Version]
- Boesen, D.F.; Lewinsky, J.; Rasmussen, L. A Check-List of the Bryophytes of the Faroes. Lindbergia 1975, 3, 69–78. [Google Scholar]
- Lewinsky, J.; Jóhansen, J. The Vegetation and Bryophyte Flora of the Faroe Island (Denmark), Excursion Guide; XIV International Botanical Congress: Berlin, Germany, 1987. [Google Scholar]
- Koponen, T.; Isoviita, P.; Lammes, T. The Bryophytes of Finland: An Annotated Checklist. Flora Fenn. 1977, 6, 1–77. [Google Scholar]
- Plagiothecium B.S.G. in Finland. 1998. Available online: http://www.nic.funet.fi/pub/sci/bio/life/plants/bryophyta/bryopsida/bryales/plagiotheciaceae/plagiothecium/ (accessed on 2 December 2019).
- Juutinen, R.; Syrjänen, K.; Korvenpää, T.; Laitinen, P.; Ahonen, I.; Huttunen, S.; Korvenpää, T.; Kypärä, T.; Parnela, A.; Ryömä, R.; et al. Bryophytes. In Suomen Lajien Uhanalaisuus—Punainen Kirja 2019 [The 2019 Red List of Finnish Species]; Hyvärinen, E., Juslén, A., Kemppainen, E., Uddström, A., Liukko, U.-M., Eds.; Finnish Environment Institute: Helsinki, Finland, 2019; ISBN 978-952-11-4974-0. [Google Scholar]
- Hugonnot, V.; Celle, J. Première liste rouge des bryophytes d’Auvergne [First red list of Auvergne bryophytes]. Evaxiana 2015, 1, 5–29. [Google Scholar]
- Ludwig, G.; Schnittler, M. Rote Liste gefährdeter Pflanzen Deutschlands [Red List of Endangered Plants in Germany]. Schr. Für Veg. Ser. Publ. Veg. Sci. 1996, 28, 1–744. [Google Scholar]
- Klawitter, J.; Köstler, H. Rote Liste und Gesamtartenliste der Moose (Bryophyta) von Berlin [Red list and total species list of mosses (Bryophyta) from Berlin]. In Rote Listen der Gefährdeten Pflanzen, Pilze und Tiere von Berlin; Der Landesbeauftragte für Naturschutz und Landschaftspflege/Senatsverwaltung für Umwelt, Verkehr und Klimaschutz, Ed.; Universitätsverlag der Technische Universität (TU): Berlin, Germany, 2017; pp. 1–31. [Google Scholar]
- Moose Deutschland (Mosses of Germany). 2017. Available online: http://www.moose-deutschland.de/organismen/?qstring=Plagiothecium (accessed on 7 January 2020).
- Caspari, S.; Dürhammer, O.; Sauer, M.; Schmidt, C. Rote Liste und Gesamtartenliste der Moose (Anthocerotophyta, Marchantiophyta und Bryophyta) Deutschlands [Red list and total species list of mosses (Anthocerotophyta, Marchantiophyta and Bryophyta) in Germany]. In Rote Liste Gefährdeter Tiere, Pflanzen und Pilze Deutschlands, Band 7: PflanzenPilze [Red List of Endangered Animals, Plants and Fungi of Germany, Volume 7: Plants]; Metzing, D., Hofbauer, N., Ludwig, G., Matzke-Hajek, G., Eds.; Federal Agency for Nature Conservation: Bonn, Germany, 2018; Naturschutz und Biologische Viefalt [Nature conservation and biological diversity]; Volume 70, pp. 361–489. ISBN 978-3-7843-5612-9. [Google Scholar]
- Erzberger, P.; Papp, B. Annotated Checklist of Hungarian Mosses. Studia Bot. Hung. 2004, 35, 91–149. [Google Scholar]
- Papp, B.; Erzberger, P.; Ódor, P.; Hock, Z.S.; Szövényi, P.; Szurdoki, E.; Tóth, Z. Updated Checklist and Red List of Hungarian Bryophytes. Studia Bot. Hung. 2010, 41, 31–59. [Google Scholar]
- Erzberger, P.; Baráth, K. Plagiothecium latebricola a New Member of the Hungarian Bryoflora. Studia Bot. Hung. 2017, 48, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Checklist of Icelandic Bryophytes. 2003–2019. Available online: http://www.floraislands.is/Annad/moslist.html (accessed on 10 December 2019).
- Gangulee, H.C. Mosses of Eastern India and Adjacent Regions; Published by the author: Calcutta, India, 1980; Volume 3. [Google Scholar]
- Dandotiya, D.; Govindapyari, H.; Suman, S.; Uniyal, P.L. Checklist of the Bryophytes of India. Arch. Bryol. 2011, 88, 71–72. [Google Scholar]
- Ho, B.-C.; Tan, B.C.; Hernawati, N.S. A Checklist of Mosses of Sumatra, Indonesia. J. Hattori Bot. Lab. 2006, 100, 143–190. [Google Scholar]
- Aziz, F. Seventeen Spp. New Records for the Moss Flora of Iraq. Φhyton 2011, 80, 36–46. [Google Scholar]
- Aziz, F.H.; Al-Niama, B. Species Chick [Sic] List of Bryophyte, Pteridophyta, Lichens and Mashroom [Sic] of Iraq and Kurdistan Region. Zanco J. Pure Appl. Sci. 2017, 29, 53–73. [Google Scholar]
- Agnew, S.; Vondráček, M. A Moss Flora of Iraq. Feddes Reper. 1975, 86, 341–489. [Google Scholar] [CrossRef]
- Hill, M.O.; Preston, C.D.; Bosanquet, S.D.S.; Roy, D.B. BRYOATT—Attributes of British and Irish Mosses, Liverworts and Hornworts With Information on Native Status, Size, Life Form, Life History, Geography and Habitat; Centre for Ecology and Hydrology: Huntingdon, UK, 2007; ISBN 978-1-85531-236-4. [Google Scholar]
- Akhani, H.; Kürschner, H. An Annotated and Updated Checklist of the Iranian Bryoflora. Cryptogam. Bryol. 2004, 25, 315–347. [Google Scholar]
- Zare, H.; Akbarinia, M.; Hedenäs, L.; Maassumi, A.A. Eighteen Mosses from the Hyrcanian Forest Region New to Iran. J. Bryol. 2011, 33, 62–65. [Google Scholar] [CrossRef]
- Herrnstadt, I.; Heyn, C.C.; Crosby, M.R. A Checklist of the Mosses of Israel. Bryologist 1991, 94, 168–178. [Google Scholar] [CrossRef]
- Herrnstadt, I.; Heyn, C.C. Part I. Bryopsida (Mosses). In The Bryophyte Flora of Israel and Adjacent Regions; Flora Palaestina; Heyn, C.C., Herrnstadt, I., Eds.; Israel Academy of Sciences and Humanities: Jerusalem, Israel, 2004; pp. 17–18. ISBN 978-965-208-152-0. [Google Scholar]
- Pedrotti, C.C. Flora dei Muschi d’Italia, Parte 2: Bryopsida (II Parte) [Flora of the Mosses of Italy, Part 2: Bryopsida (Second Part)]; Flora dei Muschi d’Italia; Antonio Delfino Editore, Medicina-Scienze: Roma, Italy, 1992; Volume 2, ISBN 978-88-7287-370-0. [Google Scholar]
- Aleffi, M.; Tacchi, R.; Pedrotti, C.C. Checklist of the Hornworts, Liverworts and Mosses of Italy. Bocconea 2008, 22, 5–254. [Google Scholar]
- Sguazzin, Francesco Check-List Delle Briofite Del Friuli Venezia Giulia (NE Italia) [Check-List of the Bryophytes of Friuli Venezia Giulia (NE Italy). Gortania Bot. Zool 2010, 32, 17–114.
- Tacchi, R.; Aleffi, M. Mosses and Liverworts of Italy/Muschi Ed Epatiche d’Italia (2011). Available online: http://dryades.units.it/briofite/ (accessed on 26 March 2020).
- Iwatsuki, Z. New Catalog of the Mosses of Japan. J. Hattori Bot. Lab. 2004, 96, 1–182. [Google Scholar]
- Suzuki, T. Many Interesting Mosses Newly Found in Japan. Hattoria 2017, 8, 1–88. [Google Scholar] [CrossRef]
- Pantović, J.P.; Sabovljević, M.S. Bryophytes of Kosovo. Phytotaxa 2017, 306, 101–123. [Google Scholar] [CrossRef]
- El-Saadawi, W. Some Mosses from Kuwait. Bryologist 1976, 79, 515–518. [Google Scholar] [CrossRef]
- Kürschner, H. Bryophyte Flora of the Arabian Peninsula and Socotra; Bryophytorum Bibliotheca; Gebrüder Borntraeger Verlag: Berlin, Germany, 2000; Volume 55, ISBN 978-3-443-62027-1. [Google Scholar]
- Ho, B.-C.; Luong, T.-T.; Tan, B.C.; Dinh, N.-L. Additional New and Noteworthy Moss (Bryophyta) Records from Vietnam and Laos. Bryophyt. Divers. EVolume 2015, 37, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Āboliņa, A. Latvijas sūnu saraksts. [List of bryophytes of Latvia]. Latv. Veģetācija 2001, 3, 47–86. [Google Scholar]
- Ellis, L.T.; Aleffi, M.; Bączkiewicz, A.; Buczkowska, K.; Bambe, B.; Boiko, M.; Zagorodniuk, N.; Brusa, G.; Burghardt, M.; Calleja, J.A.; et al. New National and Regional Bryophyte Records, 60. J. Bryol. 2019, 41, 285–299. [Google Scholar] [CrossRef]
- Senn, H. Die Moose Des Fürstentums Liechtenstein [The Mosses of the Principality of Liechtenstein]; Naturkundliche Forschungim Fürstentum Liechtenstein, Regierung des Fürstentums Liechtenstein: Vaduz, Liechtenstein, 2000; Volume 17, ISBN 3-9521855-1-5. [Google Scholar]
- Jukonienė, I. Checklist of Lithuanian Mosses [Lietuvos Lapsamanių Sąvadas]. Bot. Lith. 2002, 8, 304–321. [Google Scholar]
- Ellis, L.T.; Afonina, O.M.; Doroshina, G.Y.; Agudelo, C.; Andriamiarisoa, R.L.; Asthana, A.K.; Gupta, D.; Gupta, R.; Rawat, K.K.; Sahu, V.; et al. New National and Regional Bryophyte Records, 58. J. Bryol. 2019, 41, 63–84. [Google Scholar] [CrossRef]
- Werner, J. Liste rouge des bryophytes du Luxembourg. Mesures de conservation et perspectives. [Red List of the Bryophytes of Luxembourg. Conservation measures and perspectives]. Ferrantia 2003, 35, 9–71. [Google Scholar]
- Werner, J. Les bryophytes du Luxembourg—Liste annotée et atlas. [The bryophytes of Luxembourg-annotated list and atlas]. Ferrantia 2011, 65, 12–136. [Google Scholar]
- Borges, P.A.V.; Abreu, C.; Aguiar, A.M.F.; Carvalho, P.; Jardim, R.; Melo, I.; Oliveira, P.; Sérgio, C.; Serrano, A.R.M.; Vieira, P. A List of the Terrestrial Fungi, Flora and Fauna of Madeira and Selvagens Archipelagos; Direcção Regional do Ambiente da Madeira and Universidade dos Açores, Funchal and Angra do Heroísmo: Madeira, Portugal, 2008; ISBN 978-989-95790-0-2. [Google Scholar]
- Sérgio, C.; Sim-Sim, M.; Carvalho, M. Annotated Catalogue of Madeiran Bryophytes. Bol. Mus. Munic. Funchal 2006, 10, 5–164. [Google Scholar]
- Yong, K.T.; Tan, B.C.; Ho, B.C.; Ho, Q.Y.; Mohamed, H. A Revised Moss Checklist of Peninsular Malaysia and Singapore; FRIM Research Pamphlet Series; Forest Research Institute Malaysia (FRIM): Kuala Lumpur, Malaysia, 2013; Volume 133, ISBN 978-967-5221-99-6.
- Ellis, L.T. Some Mosses New to the Maldives. J. Bryol. 1988, 15, 493–498. [Google Scholar] [CrossRef]
- Menzel, M.; Passow-Schindhelm, R. The Mosses of the Maldive Islands. Cryptogam. Bryol. 1990, 11, 363–367. [Google Scholar]
- Frahm, J.-P.; Lüth, M. The Bryophyte Flora of the Maltese Islands. Arch. Bryol. 2008, 29, 1–10. [Google Scholar]
- Mifsud, S.D. An Update on the Moss Flora of the Maltese Islands. Cryptogam. Bryol. 2012, 33, 405–418. [Google Scholar] [CrossRef]
- Karczmarz, K. New and Rare Bryophytes in the Flora of Mongolia. Bryologist 1981, 84, 339–343. [Google Scholar] [CrossRef]
- Tsegmed, T. Checklist and Distribution of Mosses in Mongolia. Arctoa 2001, 10, 1–18. [Google Scholar] [CrossRef]
- Müller, F.; Wynns, J.T. A Revised Classification of Plagiothecium in Myanmar. J. Bryol. 2020, 42, 169–178. [Google Scholar] [CrossRef]
- Karczmarz, K. Bryophytes from Nepal. Lindbergia 1981, 7, 126–130. [Google Scholar]
- Kattel, L.P.; Adhikari, M.K. Mosses of Nepal; Natural History Society of Nepal: Kathmandu, Nepal, 1992. [Google Scholar]
- Dirkse, G.; van Melick, H.; Touw, A. Checklist of Dutch Bryophytes. Lindbergia 1989, 14, 167–175. [Google Scholar]
- Siebel, H.N.; During, H.J.; van Melick, H.M.H. Veranderingen in de Standaardlijst van de Netherlandse Blad-, Lever- En Hauwmossen [Checklist of Dutch Bryophytes and Liverworts]. Buxbaumiella 2005, 73, 26–54. [Google Scholar]
- Martinčič, A. Contributions to the Bryophyte Flora of Republic of Macedonia. Hacquetia 2009, 8, 97–114. [Google Scholar] [CrossRef] [Green Version]
- Papp, B.; Pantović, J.; Szurdoki, E.; Sabovljević, M.S. New Bryophyte Records for the Republic of Macedonia. J. Bryol. 2016, 38, 168–171. [Google Scholar] [CrossRef]
- Noguchi, A. Mosses from Pakistan. J. Hattori Bot. Lab. 1956, 16, 76–82. [Google Scholar]
- Karczmarz, K. Bryophytes from Western Jammu and Kashmir. Ann. Univ. Mariae Curie-SkłodowskaC Biol. 1980, 35, 34–41. [Google Scholar]
- Higuchi, M. Mosses from Pakistan collected by Botanical Expedition of National Science Museum, Tokyo. In Cryptogamic Flora of Pakistan; Nakaike, T., Malik, S., Eds.; National Sciences Museum: Islamabad, Pakistan, 1992; Volume 1, pp. 245–259. [Google Scholar]
- Nishimura, N.; Watanabe, R.; Kanda, H.; Takaki, N.; Mizushima, U. Pleurocarpous mosses from Pakistan. In Crypotogamic Flora of Pakistan; Nakaike, T., Malik, S., Eds.; National Sciences Museum: Islamabad, Pakistan, 1993; Volume 2, pp. 255–269. [Google Scholar]
- Higuchi, M.; Nishimura, N. Mosses of Pakistan. J. Hattori Bot. Lab. 2003, 93, 273–291. [Google Scholar]
- Iwatsuki, Z.; Tan, B.C. A Tentative Checklist of Philippine Moss Species; Hattori Bot. Lab.: Nichinan, Japan; Univ. of British Columbia: Vancouver, BC, Canada, 1977. [Google Scholar]
- Tan, B.C.; Iwatsuki, Z. A New Annotated Philippine Moss Checklist. Harv. Pap. Bot. 1991, 1, 1–64. [Google Scholar]
- Ochyra, R.; Żarnowiec, J.; Ochyra-Bednarek, H. Census Catalogue of Polish Mosses. Biodiversity of Poland; Polish Academy of Sciences, Institute of Botany: Kraków, Poland, 2003; Volume 3. [Google Scholar]
- Sérgio, C.; Carvalho, S. Annotated Catalogue of Portuguese Bryophytes. Port. Acta Biol. 2003, 21, 5–230. [Google Scholar]
- Ellis, L.T.; Afonina, O.M.; Atwood, J.J.; Bednarek-Ochyra, H.; Burghardt, M.; Dragićević, S.; Vuksanović, S.; Espinoza-Prieto, B.; Opisso, J.; Goga, M.; et al. New National and Regional Bryophyte Records, 62. J. Bryol. 2020, 42, 195–208. [Google Scholar] [CrossRef]
- Kürschner, H.; Alatalo, J.M.; Al-Mesaifrib, M.A.S.L.; Alsafran, S.A. Closing a Gap—First Records of Bryophytes from the Qatar Peninsula. Cryptogam. Bryol. 2018, 39, 77–82. [Google Scholar] [CrossRef]
- Ştefănuţ, S.; Goia, I. Checklist and Red List of Bryophytes of Romania. Nova Hedwig. 2012, 95, 59–104. [Google Scholar] [CrossRef]
- Ignatov, M.S.; Afonina, O.M. Check-List of Mosses of the Former USSR. Arctoa 1992, 1, 1–85. [Google Scholar] [CrossRef] [Green Version]
- Ellis, L.T.; Aleffi, M.; Asthana, A.K.; Srivastava, A.; Bakalin, V.A.; Batan, N.; Özdemir, T.; Bednarek-Ochyra, H.; Borovichev, E.A.; Brugués, M.; et al. New National and Regional Bryophyte Records, 40. J. Bryol. 2014, 36, 223–244. [Google Scholar] [CrossRef]
- Cherdantseva, V.; Pisarenko, O.; Ignatov, M.; Ignatova, E.; Fedosov, V.; Dudov, S.; Bakalin, V. Mosses of the Southern Russian Far East, an Annotated Check-List. Bot. Pac. 2018, 7, 1–29. [Google Scholar] [CrossRef]
- Moss Flora of Russia. (Date Unknown). Available online: http://www.arctoa.ru/Flora/spisok-vidov.htm (accessed on 10 December 2019).
- Flora of Slovakia. (Date Unknown). [Online Source] Slovakia: Institute of Botany SAS (The Institute of Botany of the Slovak Academy of Sciences). Available online: www.ibot.sav.sk/checklist/index.php?lang=en&doc=list (accessed on 25 December 2019).
- Mišíková, K.; Godovičová, K.; Širka, P.; Šoltés, R. Checklist and Red List of Mosses (Bryophyta) of Slovakia. Biologia 2020, 75, 21–37. [Google Scholar] [CrossRef]
- Skudnik, M.; Sabovljević, A.; Batič, F.; Sabovljević, M. The Bryophyte Diversity of Ljubljana (Slovenia). Pol. Bot. J. 2013, 58, 319–324. [Google Scholar] [CrossRef] [Green Version]
- Martinčič, A. Updated Red List of Bryophytes of Slovenia. Hacquetia 2016, 15, 107–126. [Google Scholar] [CrossRef] [Green Version]
- Casas, R. New Checklist of Spanish Mosses. Orsis 1991, 6, 3–26. [Google Scholar]
- Jansen, M.A.B.; Abeywickrama, B.A. A Checklist of the Mosses of Sri Lanka; National Science Council of Sri Lanka: Colombo, Sri Lanka, 1978.
- O’Shea, B.J. Checklist of the Mosses of Sri Lanka. J. Hattori Bot. Lab. 2002, 92, 125–164. [Google Scholar]
- Ruklani, N.C.S.; Rubasinghe, S.C.K. A Preliminary Survey of Bryophytes in the Central Province of Sri Lanka. Ceylon J. Sci. 2013, 42, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Frisvoll, A.A. Fifteen Bryophytes New to Svalbard, Including Notes on Some Rare or Interesting Species. Lindbergia 1981, 7, 91–102. [Google Scholar]
- Frisvoll, A.A. The Distribution of Plagiothecium berggrenianum Frisv. Bryologist 1984, 87, 167. [Google Scholar] [CrossRef]
- Frisvoll, A.A.; Elvebakk, A. Part 2. Bryophytes. In A Catalogue of Svalbard Plants, Fungi, Algae and Cyanobacteria; Elvebakk, A., Prestrud, P., Eds.; Norwegian Polar Institute: Oslo, Norway, 1996; pp. 57–172. [Google Scholar]
- Söderström, L.; Hedenäs, L. Checklista över Sveriges mossor. [Checklist of Swedish Bryophytes]. Myrinia 1998, 8, 58–90. [Google Scholar]
- Hallingbäck, T.; Hedenӓs, L.; Weibull, H. Ny checklista för Sveriges mossor [New checklist of Swedish bryophytes]. Sven. Bot. Tidskr. 2006, 100, 96–148. [Google Scholar]
- Meier, M.K.; Urmi, E.; Schnyder, N.; Bergamini, A.; Hofmann, H. Checkliste der Schweizer Moose. [Checklist of Swiss mosses]; Nationales Inventar der Schweizer Moosflora, Institut für Systematische Botanik der Universität Zürich: Zürich, Switzerland, 2013. [Google Scholar]
- Swissbryophytes Working Group 2020. Checkliste. Concept “Swissbryophytes 2017”. Available online: https://www.swissbryophytes.ch/index.php/de/datenzentrum/checkliste (accessed on 30 December 2019).
- Kuo, C.-M.; Chiang, T.Y. Index of Taiwan Mosses. Taiwania 1987, 32, 119–207. [Google Scholar]
- He, S. An Annotated Checklist and Atlas of the Mosses of Thailand. 1995–2014. Available online: http://www.mobot.org/MOBOT/moss/Thailand/thai-p.shtml (accessed on 3 January 2020).
- Cetin, B. Checklist of the Mosses of Turkey. Lindbergia 1988, 14, 15–23. [Google Scholar]
- Uyar, G.; Çetin, B. A New Check-List of the Mosses of Turkey. J. Bryol. 2004, 26, 203–220. [Google Scholar] [CrossRef]
- Ellis, L.; Asthana, A.; Sahu, V.; Bednarek-Ochyra, H.; Ochyra, R.; Cano, M.; Costa, D.; Cykowska-Marzencka, B.; Philippov, D.; Dulin, M.; et al. New National and Regional Bryophyte Records, 25. J. Bryol. 2010, 32, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Bolyukh, V. A Comparison of Moss Flora of Central Podolia (Ukraine) and Adjacent Regions. Arctoa 1995, 4, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Virchenko, V. A List of Pleurocarpous Mosses of Ukraine; National Academy of Sciences of Ukraine, MH Kholodny Institute of Botany: Kiev, Ukraine, 2000. [Google Scholar]
- Virchenko, V. Мoхoпoдібні Прирoднo-Запoвідних Теритoрій Українськoгo Пoлісся. [Bryophytes of protected areas of the Ukrainian Polissya]; TOV NVP “Interservis”: Kiev, Ukraine, 2014. [Google Scholar]
- Aleffi, M. The Bryophyte Flora of the Vatican City State. Cryptogam. Bryol. 2015, 36, 155–169. [Google Scholar] [CrossRef]
- Aleffi, M. Contribution to the Knowledge of the Bryophyte Flora of the Vatican City State: The Pontifical Villas of Castel Gandolfo (Rome, Italy). Flora Medit. 2017, 27, 137–150. [Google Scholar] [CrossRef]
- Ninh, T. Mosses of the Tam Dao Mountains, Vietnam. Bryologist 1993, 96, 573–581. [Google Scholar] [CrossRef]
- He, S.; Khang, N.S. New Records and an Updated Checklist of the Mosses of Vietnam. Bryophyt. Divers. EVolume 2012, 34, 32–88. [Google Scholar] [CrossRef]
- Kürschner, H. A Bryophyte Flora of Socotra Island, Yemen. In Biodiversity of Socotra Forests, Woodlands and Bryophyte; Kilian, N., Hubaishan, M.A., Eds.; Englera. No. 28.; Botanischer Garten und Botanisches Museum (BGBM): Berlin-Dahlem, Germany, 2006; pp. 97–162. ISBN 0170-4818. [Google Scholar]
- Wolski, G.J. Reassessing the Taxonomic Diversity of Plagiothecium Section Orthophyllum in the North American Bryoflora. Brittonia 2020, 72, 337–350. [Google Scholar] [CrossRef]
- Mitten, W. Musci Indiae Orientalis; an Enumeration of the Mosses of the East Indies; Journal of the Proceedings of the Linnean Society, Supplement to Botany. No. 1; Longman, Brown, Green, Longmans & Roberts, and Williams and Norgate: London, UK, 1859. [Google Scholar]
- Müller, C. Struckia, Eine Neue Laubmoos-Gattung [Struckia, a New Genus of Deciduous Moss]. Arch. Des Ver. Der Freunde Der Nat. Mecklenbg. 1894, 47, 127–130. [Google Scholar]
- Greene, S.W. The British Species of the Plagiothecium denticulatum—P. silvaticum Group. Trans. Brit. Bryol. Soc. 1957, 3, 181–190. [Google Scholar] [CrossRef]
- Sofronova, E.N.; Abakarova, A.S.; Afonina, O.M.; Badmaeva, N.K.; Borovichev, E.A.; Boychuk, M.A.; Czernyadjeva, I.V.; Doroshina, G.Y.; Dulin, M.V.; Dyachenko, A.P.; et al. New Bryophyte Records. 1—Нoвые Бриoлoгические Нахoдки. 1. Arctoa 2012, 21, 275–300. [Google Scholar] [CrossRef] [Green Version]
- Sofronova, E.V.; Afonina, O.M.; Andrejeva, E.N.; Antipin, V.K.; Baisheva, E.Z.; Bakalin, V.A.; Borovichev, E.A.; Boychuk, M.A.; Czernyadjeva, I.V.; Dulin, M.V.; et al. New Bryophyte Records. 3—Нoвые Бриoлoгические Нахoдки. 3. Arctoa 2014, 23, 219–238. [Google Scholar] [CrossRef] [Green Version]
- Sofronova, E.V.; Afonina, O.M.; Andrejeva, E.N.; Beldiman, L.N.; Bezgodov, A.G.; Borovichev, E.A.; Boychuk, M.A.; Chepinoga, V.V.; Czernyadjeva, I.V.; Doroshina, G.Y.; et al. New Bryophyte Records. 6—Нoвые Бриoлoгические Нахoдки. 6. Arctoa 2016, 25, 183–228. [Google Scholar] [CrossRef] [Green Version]
- Ellis, L.T.; Ah-Peng, C.; Aranda, S.C.; Bednarek-Ochyra, H.; Borovichev, E.A.; Cykowska-Marzencka, B.; Duarte, M.C.; Enroth, J.; Erzberger, P.; Fedosov, V.; et al. New National and Regional Bryophyte Records, 45. J. Bryol. 2015, 37, 308–329. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.; Alam, J.; Fiaz, M. A Checklist of Mosses of District Mansehra. Sci. Int. 2016, 28, 2569–2575. [Google Scholar]
- Sofronova, E.V.; Andrejeva, E.N.; Bakalin, V.A.; Beldiman, L.N.; Belyakov, E.A.; Blagovetshenskiy, I.V.; Borovichev, E.A.; Boychuk, M.A.; Doroshina, G.Y.; Dulin, M.V.; et al. New Bryophyte Records. 8—Нoвые Бриoлoгические Нахoдки. 8. Arctoa 2017, 26, 105–125. [Google Scholar] [CrossRef] [Green Version]
- Mönkemeyer, W. Die Laubmoose Europas. IV Band Ergänzungsband Andreales—Bryales. In Kryptogamen-Flora von Deutschland, Österreich und der Schweiz; Rabenhorst, L., Ed.; Akademische Verlagsgesellschaft m. b. H. (Druckt von E. Haberland, Leipzig): Leipzig, Germany, 1927. [Google Scholar]
- Jedlička, J. Monographia Specierum Europaearum Gen. Plagiothecium s.s.; Pȓirodovĕdeckou Fakultou Masarykovy University: Brno, Czech, 1948; Volume 308. [Google Scholar]
- Jedlička, J. Monographia Specierum Europaearum Gen. Plagiothecium s.s. Icones; Pȓirodovĕdeckou Fakultou Masarykovy University: Brno, Czech, 1950; Volume 318. [Google Scholar]
- Podpéra, J. Conspectus Muscorum Europaeorum; Nakladatelství Československé Akademie Věd: Prague, Czech, 1954. [Google Scholar]
- Van der Wijk, R.; Margadant, W.D.; Florschütz, P.A. Index Muscorum; International Bureau for Plant Taxonomy and Nomenclature: Utrecht, The Netherlands, 1967; Volume 4. [Google Scholar]
- Sofronova, E.V.; Afonina, O.M.; Akatova, T.V.; Andrejeva, E.N.; Baisheva, E.Z.; Bezgodov, A.G.; Blagovetshenskiy, I.V.; Borovichev, E.A.; Chemeris, E.V.; Chernova, A.M.; et al. New Bryophyte Records. 4—Нoвые Бриoлoгические Нахoдки. 4. Arctoa 2015, 24, 224–264. [Google Scholar] [CrossRef] [Green Version]
- Lewinsky, J.; Mogensen, G. Distribution Maps of Bryophytes in Greenland 4. Lindbergia 1977, 4, 135–142. [Google Scholar]
- Warnstorf, C. Kryptogamenflora der Mark Brandenburg und angrenzender Gebiete. Zweiter Band: Laubmoose [Cryptogamic flora of Mark Brandenburg and adjacent areas. Volume 2: Mosses]; Gebrüder Borntraeger: Leipzig, Germany, 1906. [Google Scholar]
- Tan, B.C.; Buck, W.R.; Ignatov, M.S. On the Himalayan Struckia C. Muell. and Russian Cephalocladium Lazar. (Musci, Hypnaceae). Lindbergia 1990, 16, 100–104. [Google Scholar]
- Cardot, J.; Thétiot, I. Papers from the Harriman Alaska Expedition XXIX. The Mosses of Alaska. In Proceedings of the Washington Academy of Sciences; Washington Academy of Sciences: Washington, DC, USA, 1902; Volume 4, pp. 293–372. [Google Scholar]
- Sakurai, K. Classification of the Genus Plagiothecium in East Asia. Bot. Mag. 1949, 62, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Özdemir, T.; Batan, N. The Bryophyte Checklist of Trabzon Province of Turkey. Arctoa 2017, 26, 58–67. [Google Scholar] [CrossRef]
- Āboliņa, A.A.; Afonina, O.M.; Badmaeva, N.K.; Bakalin, V.A.; Belkina, O.A.; Borovichev, E.A.; Chemeris, E.V.; Cherdantseva, V.Y.; Cherednichenko, O.V.; Czernyadjeva, I.V.; et al. New Bryophyte Records. Arctoa 2011, 20, 247–268. [Google Scholar] [CrossRef]
- Lindberg, S.O. Contributio Ad Flora Cryptogamam Asiae Boreali-Orientalis. Acta Soc. Sci. Fenn. 1872, 10, 221–280. [Google Scholar]
- Fleischer, M. Die Musci der Flora von Buitenzorg: Zugleich Laubmoosflora von Java; E.J. Brill: Leiden, The Netherlands, 1915. [Google Scholar]
- Enroth, J. Bryophyte Flora of the Huon Penisula, Papua Nwe Guinea. XLVIII. Plagiotheciaceae (Musci). Ann. Bot. Fenn. 1991, 28, 111–115. [Google Scholar]
- Molendo, L. Bayerns Laubmoose: Vorläufige Übersicht Mit Besonderer Rücksicht Auf Niederbayern; Bericht des Naturhistorischen Vereins in Passau: Passau, Germany, 1875; Volume 10. [Google Scholar]
- Kara, R.; Ezer, T.; Düzenlí, A. The Bryophyte Flora of Northern Amanos (Nur) Mountain (Hatay-Turkey). Evansia 2013, 30, 1–14. [Google Scholar] [CrossRef]
- Wynns, J.T.; Lange, C.B.A. A Comparison of 16 DNA Regions for Use as Phylogenetic Markers in the Pleurocarpous Moss Genus Plagiothecium (Hypnales). Am. J. Bot. 2014, 101, 652–669. [Google Scholar] [CrossRef]
- Sakurai, K. Beobachtungen Über Japanische Moosflora (II). [Observations on Japanese Moss Flora (II)]. Bot. Mag. 1932, 46, 496–509. [Google Scholar] [CrossRef] [Green Version]
- Iwatsuki, Z. Catalog of the Mosses of Japan; Hattori Botanical Laboratory: Nichnan, Japan, 1991. [Google Scholar]
- Limpricht, K.G. Die Laubmoose Deutschlands, Oesterreichs und der Schweiz [The Mosses of Germany, Austria and Switzerland]; E. Kummer: Leipzig, Germany, 1895; Volume 3. [Google Scholar]
- Mitten, W. New Species of Musci Collected in Ceylon by Dr. Thwaites. J. Linn. Soc. Bot. 1873, 13, 293–326. [Google Scholar] [CrossRef]
- Ireland, R.R. Buckiella, a New Genus in the Hypnaceae (Musci). Novon 2001, 11, 55–62. [Google Scholar] [CrossRef]
- El-Saadawi, W.; Shabbara, H.; Khalil, M.; Taha, M. An Annotated Checklist of Egyptian Mosses. Taeckholmia 2015, 35, 1–23. [Google Scholar] [CrossRef]
- Brummitt, R.K. World Geographical Scheme for Recording Plant Distributions, Biodiversity Information Standards (TDWG), 2nd ed.; Hunt Institute for Botanical Documentation Carnegie Mellon University: Pittsburgh, PA, USA, 2001; Available online: http://www.tdwg.org/standards/109 (accessed on 12 November 2019).
- Brummitt, R.K.; Powell, C.E. (Eds.) Authors of Plant Names: A List of Authors of Scientific Names of Plants, with Recommended Standard Form of Their Names Including Abbreviations; Royal Botanic Gardens: Kew, UK, 1992; ISBN 0-947643-44-3. [Google Scholar]
- Rykovsky, G.F.; Maslovsky, O.M. Flora of Belarus. Bryophytes. Andreaeopsida—Bryopsida; Минск, Тэхналoгія [Minsk, Technalogia]: Minsk, Belarus, 2004; Volume 1. [Google Scholar]





| No. | Country/Region | Abbreviation | Main Source(s) of Information |
|---|---|---|---|
| 1. | Afghanistan | AFG | [43,44,45,46] |
| 2. | Albania | ALB | [9,47,48,49,50] |
| 3. | Andorra | AND | [9,47,48,51,52] |
| 4. | Armenia | ARM | [32,53] |
| 5. | Austria | AUT | [7,9,26,27,48,54] |
| 6. | Azerbaijan | AZE | [32] |
| 7. | Azores (Portugal) | AZO | [9,27,47,48,55,56,57] |
| 8. | Bahrain | BAH | [43,45] |
| 9. | Balearic Islands (Spain) | BAL | [47,48,58] |
| 10. | Bangladesh | BAN | [59,60] |
| 11. | Belarus | BLR | [9,32,48,61] |
| 12. | Belgium | BGM | [7,9,27,48,62] |
| 13. | Bhutan | BHU | [7,63,64] |
| 14 | Borneo (Indonesia, Malaysia, Brunei) | BOR | [65,66] |
| 15. | Bosnia and Herzegovina | BIH | [9,26,47,48,49] |
| 16. | Brunei Darussalam | BRN | [67] |
| 17. | Bulgaria | BUL | [9,47,48,49,68] |
| 18. | Cambodia | CBD | [69,70,71,72,73] |
| 19. | Canary Islands (Spain) | CNY | [9,47,48,74,75] |
| 20. | Channel Islands (United Kingdom) | CHI | [9,48] |
| 21. | China | CHN | [7,27,36,64,66,76,77,78,79,80] |
| 22. | Corsica (France) | COR | [9,47,48,62] |
| 23. | Crete (Greece) | CRE | [47,48,81] |
| 24. | Croatia | CRO | [9,47,48,49,82,83] |
| 25. | Cyprus | CYP | [43,47,48] |
| 26. | Czech Republic | CZE | [7,9,26,27,48,84] |
| 27. | Democratic People’s Republic of Korea (North Korea) | NKO | [7,64,66,78,85,86] |
| 28. | Denmark | DEN | [7,9,27,30,48,87] |
| 29. | Estonia | EST | [9,27,32,48,88] |
| 30. | Faroe Islands (Denmark) | FRO | [7,9,48,89,90] |
| 31. | Finland | FIN | [7,9,26,27,28,48,91,92,93] |
| 32. | France | FRA | [7,9,27,47,48,78,94] |
| 33. | Germany | GER | [7,9,26,27,48,95,96,97,98] |
| 34. | Greece | GRC | [9,47,48,49] |
| 35. | Hungary | HUN | [7,9,26,27,48,99,100,101] |
| 36. | Iceland | ICE | [7,9,48,102] |
| 37. | India | IND | [7,27,64,78,103,104] |
| 38. | Indonesia | IDN | [7,105] |
| 39. | Iraq | IRQ | [43,45,106,107,108] |
| 40. | Ireland | IRE | [9,25,48,109] |
| 41. | Islamic Republic of Iran | IRN | [7,27,43,45,110,111] |
| 42. | Israel | ISR | [43,45,47,112,113] |
| 43. | Italy | ITA | [7,9,27,47,48,114,115,116,117] |
| 44. | Japan | JAP | [7,27,29,33,34,64,66,78,118,119] |
| 45. | Jordan | JOR | [43,45,47] |
| 46. | Kazakhstan | KAZ | [32,48] |
| 47. | Kosovo | KOS | [9,48,120] |
| 48. | Kuwait | KUW | [43,45,121,122] |
| 49. | Kyrgyzstan | KGZ | [32] |
| 50. | Lao People’s Democratic Republic | LAO | [72,78,123] |
| 51. | Latvia | LAV | [7,9,27,32,48,124,125] |
| 52. | Lebanon | LBN | [43,45,47] |
| 53. | Liechtenstein | LIE | [9,48,126] |
| 54. | Lithuania | LTU | [9,27,32,48,127,128] |
| 55. | Luxembourg | LUX | [9,48,129,130] |
| 56. | Madeira (Portugal) | MDR | [9,27,47,48,51,131,132] |
| 57. | Malaysia | MLY | [133] |
| 58. | Maldives | MDV | [134,135] |
| 59. | Malta | MAL | [47,48,136,137] |
| 60. | Monaco | MCO | [48] |
| 61. | Mongolia | MON | [138,139] |
| 62. | Montenegro | MNE | [9,47,48,49] |
| 63. | Myanmar (Burma) | MYA | [72,140] |
| 64. | Nepal | NEP | [7,27,64,141,142] |
| 65. | Netherlands | NET | [7,9,48,143,144] |
| 66. | Norway | NOR | [7,9,26,27,48] |
| 67. | North Macedonia (formerly Macedonia) | MKD | [9,47,48,49,145,146] |
| 68. | Oman | OMA | [43,45,122] |
| 69. | Pakistan | PAK | [147,148,149,150,151] |
| 70. | Palestine | PAL | No information available |
| 71. | Philippines | PHI | [7,66,152,153] |
| 72. | Poland | POL | [7,9,26,27,48,154] |
| 73. | Portugal | POR | [9,27,47,48,51,155,156] |
| 74. | Qatar | QAT | [43,45,157] |
| 75. | Republic of Korea (South Korea) | SKO | [7,64,66,78,86] |
| 76. | Republic of Moldova | MOL | [9,32,48] |
| 77. | Romania | ROM | [9,48,49,158] |
| 78. | Russia Federation | RUS | [7,9,26,27,32,48,64,78,159,160,161,162] |
| 79. | San Marino (Italy) | SMR | [9,48] |
| 80. | Sardinia (Italy) | SAR | [9,47,48] |
| 81. | Saudi Arabia | SAU | [43,45,122] |
| 82. | Serbia | SRB | [9,47,48,49] |
| 83. | Sicily (Italy) | SIC | [9,47,48] |
| 84. | Singapore | SIN | [133] |
| 85. | Slovakia | SVK | [7,9,27,48,163,164] |
| 86. | Slovenia | SVN | [9,47,48,49,165,166] |
| 87. | South Georgia | GEO | [27,32] |
| 88. | Spain | SPA | [9,27,47,48,51,167] |
| 89. | Sri Lanka | SRL | [7,168,169,170] |
| 90. | Sumatra (Indonesia) | SUM | [66,105] |
| 91. | Svalbard (Norway) | SVA | [7,9,26,48,171,172,173] |
| 92. | Sweden | SWE | [7,9,26,27,48,174,175] |
| 93. | Switzerland | SWI | [7,9,27,48,176,177] |
| 94. | Syrian Arab Republic | SYR | [43,45,47] |
| 95. | Taiwan | TAI | [7,66,77,78,178] |
| 96. | Tajikistan | TZK | [32] |
| 97. | Thailand | THA | [66,72,179] |
| 98. | Timor-Leste | TLS | No information available |
| 99. | Turkey | TUR | [7,9,27,43,45,47,48,49,180,181,182] |
| 100 | Turkmenistan | TKM | [32] |
| 101 | Ukraine | UKR | [9,26,32,48,183,184,185] |
| 102 | United Arab Emirates | UAE | [43,45,122] |
| 103. | United Kingdom | GRB | [7,9,25,27,48,109] |
| 104. | Uzbekistan | UZB | [32] |
| 105. | Vatican City | VAT | [48,186,187] |
| 106. | Vietnam | VIE | [72,123,188,189] |
| 107. | Yemen | YEM | [43,45,122,190] |
| Countries/Islands | Plagiothecium Taxa Recorded in Eurasia | Σ1 | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | ||
| Afghanistan | – | |||||||||||||||||||||||||||||||||||||||||
| Albania | • | • | • | • | • | • | 6 | |||||||||||||||||||||||||||||||||||
| Andorra | • | • | • | • | • | • | • | 7 | ||||||||||||||||||||||||||||||||||
| Armenia | • | • | 2 | |||||||||||||||||||||||||||||||||||||||
| Austria | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | 16 | |||||||||||||||||||||||||
| Azerbaijan | • | • | • | • | • | 5 | ||||||||||||||||||||||||||||||||||||
| Azores | • | • | ? | 3 | ||||||||||||||||||||||||||||||||||||||
| Bahrain | – | |||||||||||||||||||||||||||||||||||||||||
| Balearic Islands | – | |||||||||||||||||||||||||||||||||||||||||
| Bangladesh | • | 1 | ||||||||||||||||||||||||||||||||||||||||
| Belarus | • | • | • | • | • | • | ? | • | 8 | |||||||||||||||||||||||||||||||||
| Belgium | • | • | • | • | • | • | • | • | • | • | 10 | |||||||||||||||||||||||||||||||
| Bhutan | • | • | • | • | • | • | 6 | |||||||||||||||||||||||||||||||||||
| Bosnia and Herzegovina | • | • | • | • | • | • | • | 7 | ||||||||||||||||||||||||||||||||||
| Brunei Darussalam | – | |||||||||||||||||||||||||||||||||||||||||
| Bulgaria | • | • | • | • | • | • | • | • | • | • | 10 | |||||||||||||||||||||||||||||||
| Cambodia | – | |||||||||||||||||||||||||||||||||||||||||
| Canary Islands | • | • | 2 | |||||||||||||||||||||||||||||||||||||||
| Channel Islands | • | • | • | • | • | 5 | ||||||||||||||||||||||||||||||||||||
| China | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | 20 | |||||||||||||||||||||
| Corsica | • | • | • | • | • | • | • | 7 | ||||||||||||||||||||||||||||||||||
| Crete | – | |||||||||||||||||||||||||||||||||||||||||
| Croatia | • | • | • | • | • | • | • | 7 | ||||||||||||||||||||||||||||||||||
| Cyprus | – | |||||||||||||||||||||||||||||||||||||||||
| Czech Republic | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | 15 | ||||||||||||||||||||||||||
| Democratic People’s Republic of Korea | • | • | • | • | • | • | • | • | • | 9 | ||||||||||||||||||||||||||||||||
| Denmark | • | • | • | • | • | • | • | • | • | • | • | • | • | 13 | ||||||||||||||||||||||||||||
| Estonia | • | • | • | • | • | • | • | • | • | • | • | 11 | ||||||||||||||||||||||||||||||
| Faroe Islands | • | • | • | • | • | • | 6 | |||||||||||||||||||||||||||||||||||
| Finland | • | • | • | • | • | • | • | • | • | • | • | • | • | • | 14 | |||||||||||||||||||||||||||
| France | • | • | • | • | • | • | • | • | • | • | • | • | • | • | 14 | |||||||||||||||||||||||||||
| Germany | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | 16 | |||||||||||||||||||||||||
| Greece | • | • | • | • | • | • | • | 7 | ||||||||||||||||||||||||||||||||||
| Hungary | • | • | • | • | • | • | • | • | • | • | • | • | 12 | |||||||||||||||||||||||||||||
| Iceland | • | • | • | • | 4 | |||||||||||||||||||||||||||||||||||||
| India | • | • | • | • | • | • | • | • | • | 9 | ||||||||||||||||||||||||||||||||
| Indonesia | • | • | 2 | |||||||||||||||||||||||||||||||||||||||
| Iraq | • | 1 | ||||||||||||||||||||||||||||||||||||||||
| Ireland | • | • | • | • | • | • | • | • | • | • | • | • | 12 | |||||||||||||||||||||||||||||
| Islamic Republic of Iran | • | • | • | • | • | • | • | • | • | 9 | ||||||||||||||||||||||||||||||||
| Israel | – | |||||||||||||||||||||||||||||||||||||||||
| Italy | • | • | • | • | • | • | • | • | • | • | • | • | 12 | |||||||||||||||||||||||||||||
| Japan | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | 18 | |||||||||||||||||||||||
| Jordan | – | |||||||||||||||||||||||||||||||||||||||||
| Kazakhstan | • | • | • | 3 | ||||||||||||||||||||||||||||||||||||||
| Kosovo | • | • | • | • | • | • | • | 7 | ||||||||||||||||||||||||||||||||||
| Kuwait | – | |||||||||||||||||||||||||||||||||||||||||
| Kyrgyzstan | • | • | • | 3 | ||||||||||||||||||||||||||||||||||||||
| Lao People’s Democratic Republic | • | 1 | ||||||||||||||||||||||||||||||||||||||||
| Latvia | • | • | • | • | • | • | • | • | • | • | • | • | 12 | |||||||||||||||||||||||||||||
| Lebanon | – | |||||||||||||||||||||||||||||||||||||||||
| Liechtenstein | • | • | • | • | • | • | • | 7 | ||||||||||||||||||||||||||||||||||
| Lithuania | • | • | • | • | • | • | • | • | • | • | • | 11 | ||||||||||||||||||||||||||||||
| Luxembourg | • | • | • | • | • | • | • | • | • | • | • | 11 | ||||||||||||||||||||||||||||||
| Madeira | • | • | • | • | • | 5 | ||||||||||||||||||||||||||||||||||||
| Malaysia | • | 1 | ||||||||||||||||||||||||||||||||||||||||
| Maldives | – | |||||||||||||||||||||||||||||||||||||||||
| Malta | – | |||||||||||||||||||||||||||||||||||||||||
| Monaco | – | |||||||||||||||||||||||||||||||||||||||||
| Mongolia | • | • | • | 3 | ||||||||||||||||||||||||||||||||||||||
| Montenegro | • | • | • | • | • | • | • | • | • | • | 10 | |||||||||||||||||||||||||||||||
| Myanmar | • | • | • | • | 4 | |||||||||||||||||||||||||||||||||||||
| Nepal | • | • | • | • | • | • | • | • | • | • | 10 | |||||||||||||||||||||||||||||||
| Netherlands | • | • | • | • | • | • | • | • | • | • | 10 | |||||||||||||||||||||||||||||||
| North Macedonia | • | • | • | • | • | • | 6 | |||||||||||||||||||||||||||||||||||
| Norway | • | • | • | • | • | • | • | • | • | • | • | • | • | 13 | ||||||||||||||||||||||||||||
| Oman | – | |||||||||||||||||||||||||||||||||||||||||
| Pakistan | • | • | • | • | 4 | |||||||||||||||||||||||||||||||||||||
| Palestine | – | |||||||||||||||||||||||||||||||||||||||||
| Philippines | • | • | • | • | 4 | |||||||||||||||||||||||||||||||||||||
| Poland | • | • | • | • | • | • | • | • | • | • | • | • | • | • | 14 | |||||||||||||||||||||||||||
| Portugal | • | • | • | • | • | • | • | • | • | 9 | ||||||||||||||||||||||||||||||||
| Qatar | – | |||||||||||||||||||||||||||||||||||||||||
| Republic of Korea | • | • | • | • | • | • | • | • | • | 9 | ||||||||||||||||||||||||||||||||
| Republic of Moldova | • | 1 | ||||||||||||||||||||||||||||||||||||||||
| Romania | • | • | • | • | • | • | • | • | • | • | • | • | 12 | |||||||||||||||||||||||||||||
| Russian Federation | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | 20 | |||||||||||||||||||||
| San Marino | – | |||||||||||||||||||||||||||||||||||||||||
| Sardinia | • | • | • | 3 | ||||||||||||||||||||||||||||||||||||||
| Saudi Arabia | – | |||||||||||||||||||||||||||||||||||||||||
| Serbia | • | • | • | • | • | • | • | • | • | 9 | ||||||||||||||||||||||||||||||||
| Sicily | • | • | • | • | 4 | |||||||||||||||||||||||||||||||||||||
| Singapore | ? | 1 | ||||||||||||||||||||||||||||||||||||||||
| Slovakia | • | • | • | • | • | • | • | • | • | • | 10 | |||||||||||||||||||||||||||||||
| Slovenia | • | • | • | • | • | • | • | • | • | • | • | 11 | ||||||||||||||||||||||||||||||
| South Georgia | • | • | • | • | • | • | • | • | 8 | |||||||||||||||||||||||||||||||||
| Spain | • | • | • | • | • | • | • | • | • | • | • | • | 12 | |||||||||||||||||||||||||||||
| Sri Lanka | • | • | 2 | |||||||||||||||||||||||||||||||||||||||
| Sumatra | • | • | 2 | |||||||||||||||||||||||||||||||||||||||
| Svalbard | • | • | • | 3 | ||||||||||||||||||||||||||||||||||||||
| Sweden | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | 16 | |||||||||||||||||||||||||
| Switzerland | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | 15 | ||||||||||||||||||||||||||
| Syrian Arab Republic | – | |||||||||||||||||||||||||||||||||||||||||
| Taiwan | • | • | • | • | • | • | • | 7 | ||||||||||||||||||||||||||||||||||
| Tajikistan | • | 1 | ||||||||||||||||||||||||||||||||||||||||
| Thailand | • | 1 | ||||||||||||||||||||||||||||||||||||||||
| Timor-Leste | – | |||||||||||||||||||||||||||||||||||||||||
| Turkey | • | • | • | • | • | • | • | • | • | • | • | • | 12 | |||||||||||||||||||||||||||||
| Turkmenistan | – | |||||||||||||||||||||||||||||||||||||||||
| Ukraine | • | • | • | • | • | • | • | • | • | • | • | • | • | 13 | ||||||||||||||||||||||||||||
| United Arab Emirates | – | |||||||||||||||||||||||||||||||||||||||||
| United Kingdom | • | • | • | • | • | • | • | • | • | • | • | • | • | 13 | ||||||||||||||||||||||||||||
| Uzbekistan | - | – | ||||||||||||||||||||||||||||||||||||||||
| Vatican City State | – | |||||||||||||||||||||||||||||||||||||||||
| Vietnam | • | • | 2 | |||||||||||||||||||||||||||||||||||||||
| Yemen | – | |||||||||||||||||||||||||||||||||||||||||
| Σ2 | 6 | 3 | 2 | 59 | 3 | 1 | 1 | 41 | 1 | 2 | 65 | 1 | 26 | 2 | 10 | 2 | 1 | 51 | 1 | 34 | 22 | 22 | 1 | 3 | 4 | 4 | 1 | 64 | 2 | 2 | 24 | 39 | 1 | 2 | 29 | 6 | 2 | 50 | 5 | 4 | 41 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolski, G.J.; Nour-El-Deen, S.; Cienkowska, A.; Bożyk, D.; El-Saadawi, W. The Genus Plagiothecium Schimp. (Plagiotheciaceae, Bryophyta) in Eurasia: An Annotated Checklist with Distribution and Ecological Data. Plants 2021, 10, 868. https://doi.org/10.3390/plants10050868
Wolski GJ, Nour-El-Deen S, Cienkowska A, Bożyk D, El-Saadawi W. The Genus Plagiothecium Schimp. (Plagiotheciaceae, Bryophyta) in Eurasia: An Annotated Checklist with Distribution and Ecological Data. Plants. 2021; 10(5):868. https://doi.org/10.3390/plants10050868
Chicago/Turabian StyleWolski, Grzegorz J., Samar Nour-El-Deen, Alicja Cienkowska, Daniel Bożyk, and Wagieh El-Saadawi. 2021. "The Genus Plagiothecium Schimp. (Plagiotheciaceae, Bryophyta) in Eurasia: An Annotated Checklist with Distribution and Ecological Data" Plants 10, no. 5: 868. https://doi.org/10.3390/plants10050868