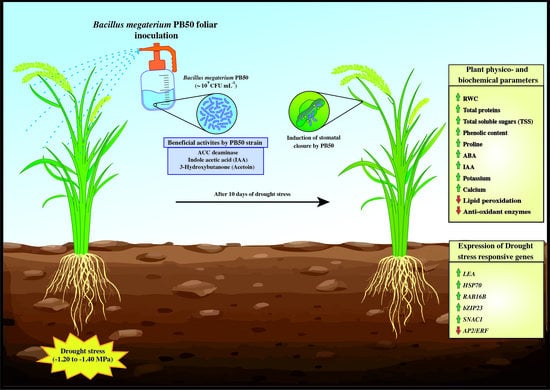
The Foliar Application of Rice Phyllosphere Bacteria induces Drought-Stress Tolerance in Oryza sativa (L.)
Abstract
:1. Introduction
2. Results
2.1. Plant Stress-Related Attributes of Rice Leaves under Different Treatments
2.2. Expression of Stress-Related Genes
2.3. Yield Parameters
2.4. Association of PB50 Strain on Rice Leaf Surface and Its Impact on Stomatal Closure
3. Discussion
3.1. Effect of Bacterial Application on Physicochemical and Biochemical Properties Related to Stress Tolerance in Rice Leaves
3.2. Effect of Bacterial Application on the Expression of Stress-Related Genes in Rice Leaves
3.3. Effect of Bacterial Application on Stomatal Closure
4. Materials and Methods
4.1. Experimental Design and Preparation of Bacterial Inocula
4.2. Sampling and Physiochemical Analysis of Leaves
4.3. Biochemical Analysis
4.3.1. Determination of Total Protein, Total Soluble Sugar, and Proline Contents
4.3.2. Total Phenolic Content
4.3.3. Determination of Lipid Peroxidation
4.3.4. Estimation of IAA and ABA Hormones
4.3.5. Assay of Antioxidant Enzymes
4.4. RNA Extraction from Rice Leaves and Reverse Transcriptase Quantitative PCR (qPCR) Analysis
4.5. Yield Parameters
4.6. The Gnotobiotic Experiment for the Study of Bacteria Colonization on the Rice Leaf and Its Role of Stomatal Modulation
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- De Oliveira, A.C.; Pegoraro, C.; Viana, V.E. (Eds.) The Future of Rice Demand: Quality Beyond Productivity; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Bouman, B.A.M.; Tuong, T.P. Field water management to save water and increase its productivity in irrigated lowland rice. Agric. Water Manag. 2001, 49, 11–30. [Google Scholar] [CrossRef]
- O’Neill, B.C.; MacKellar, F.L.; Lutz, W. Population and Climate Change; Cambridge University Press: Cambridge, UK, 2005; ISBN 9780521662420. [Google Scholar]
- Fitton, N.; Alexander, P.; Arnell, N.; Bajzelj, B.; Calvin, K.; Doelman, J.; Gerber, J.S.; Havlik, P.; Hasegawa, T.; Herrero, M.; et al. The vulnerabilities of agricultural land and food production to future water scarcity. Global Environ. Chang. 2019, 58, 101944. [Google Scholar] [CrossRef]
- Lipiec, J.; Doussan, C.; Nosalewicz, A.; Kondracka, K. Effect of drought and heat stresses on plant growth and yield: A review. Int. Agrophys. 2013, 27, 463–477. [Google Scholar] [CrossRef]
- Kamoshita, A.; Rodriguez, R.; Yamauchi, A.; Wade, L. Genotypic variation in response of rainfed lowland rice to prolonged drought and rewatering. Plant Prod. Sci. 2004, 7, 406–420. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, T.; Mogami, J.; Yamaguchi-Shinozaki, K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant. Biol. 2014, 21, 133–139. [Google Scholar] [CrossRef]
- Lata, C.; Yadav, A.; Prasad, M. Role of plant transcription factors in abiotic stress tolerance. In Abiotic Stress Response in Plants—Physiological, Biochemical and Genetic Perspectives; Shanker, A., Venkateswarlu, B., Eds.; INTECH Open Access Publishers: London, UK, 2011; Volume 10, pp. 269–296. [Google Scholar] [CrossRef] [Green Version]
- Joshi, R.; Wani, S.H.; Singh, B.; Bohra, A.; Dar, Z.A.; Lone, A.A.; Pareek, A.; Singla-Pareek, S.L. Transcription factors and plants response to drought stress: Current understanding and future directions. Front. Plant. Sci. 2016, 7, 1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef]
- Manavalan, L.P.; Guttikonda, S.K.; Phan Tran, L.S.; Nguyen, H.T. Physiological and molecular approaches to improve drought resistance in soybean. Plant Cell Physiol. 2009, 50, 1260–1276. [Google Scholar] [CrossRef] [Green Version]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [Green Version]
- Goyal, D.; Prakash, O.; Pandey, J. Rhizospheric Microbial Diversity: An Important Component for Abiotic Stress Management in Crop Plants Toward Sustainable Agriculture. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, J.S., Singh, D.P., Eds.; Elsevier: Duivendrecht, The Netherlands, 2019; pp. 115–134. [Google Scholar] [CrossRef]
- Jogawat, A.; Bisht, D.; Johri, A.K. Root Endosymbiont-mediated Priming of Host Plants for Abiotic Stress Tolerance. In Molecular Plant Abiotic Stress; Roychoudhury, A., Tripathi, D., Eds.; John Wiley & Sons Ltd.: West Sussex, UK, 2020; pp. 283–300. [Google Scholar] [CrossRef]
- Priyanka, J.P.; Goral, R.T.; Rupal, K.S.; Saraf, M. Rhizospheric Microflora: A Natural Alleviator of Drought Stress in Agricultural Crops. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management. Microorganisms for Sustainability; Sayyed, R., Arora, N., Reddy, M., Eds.; Springer: Singapore, 2019; Volume 12, pp. 103–115. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, L.; Hao, R.; Bai, X.; Wang, Y.; Yu, X. Drought-tolerant plant growth-promoting rhizobacteria isolated from jujube (Ziziphus jujuba) and their potential to enhance drought tolerance. Plant Soil. 2020, 452, 423–440. [Google Scholar] [CrossRef]
- Kembel, S.W.; O’Connor, T.K.; Arnold, H.K.; Hubbell, S.P.; Wright, S.J.; Green, J.L. Relationships between phyllosphere bacterial communities and plant functional traits in a neotropical forest. Proc. Natl. Acad. Sci. USA 2014, 111, 13715–13720. [Google Scholar] [CrossRef] [Green Version]
- Ji, S.H.; Gururani, M.A.; Chun, S.C. Isolation and characterization of plant growth promoting endophytic diazotrophic bacteria from Korean rice cultivars. Microbiol. Res. 2014, 169, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Carvalhais, L.C.; Crawford, M.; Singh, E.; Dennis, P.G.; Pieterse, C.M.; Schenk, P.M. Inner plant values: Diversity, colonization and benefits from endophytic bacteria. Front. Microbiol. 2017, 8, 2552. [Google Scholar] [CrossRef]
- Parasuraman, P.; Pattnaik, S.; Busi, S. Phyllosphere Microbiome: Functional Importance in Sustainable Agriculture. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, J.S., Singh, D.P., Eds.; Elsevier: Duivendrecht, The Netherlands, 2019; pp. 135–148. [Google Scholar] [CrossRef]
- Yang, X.; Wang, B.; Chen, L.; Li, P.; Cao, C. The different influences of drought stress at the flowering stage on rice physiological traits, grain yield, and quality. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arun, K.D.; Sabarinathan, K.G.; Gomathy, M.; Kannan, R.; Balachandar, D. Mitigation of drought stress in rice crop with plant growth-promoting abiotic stress-tolerant rice phyllosphere bacteria. J. Basic Microbiol. 2020, 60, 768–786. [Google Scholar] [CrossRef] [PubMed]
- Kannaiyan, S.; Govindarajan, K.; Lewin, H.D. Effect of foliar spray of Azotobacter chroococcum on rice crop. Plant Soil. 1980, 56, 487–490. [Google Scholar] [CrossRef]
- Pati, B.R.; Chandra, A.K. Effect of spraying nitrogen-fixing phyllospheric bacterial isolates on wheat plants. Plant Soil. 1981, 61, 419–427. [Google Scholar] [CrossRef]
- Sudhakar, P.; Chattopadhyay, G.N.; Gangwar, S.K.; Ghosh, J.K. Effect of foliar application of Azotobacter, Azospirillum and Beijerinckia on leaf yield and quality of mulberry (Morus alba). J. Agric. Sci. 2000, 134, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.S.; Sridar, R.; Uthandi, S. Mitigation of drought in rice by a phyllosphere bacterium Bacillus altitudinis FD48. Afr. J. Microbiol. Res. 2017, 11, 1614–1625. [Google Scholar] [CrossRef] [Green Version]
- Armada, E.; Probanza, A.; Roldán, A.; Azcón, R. Native plant growth promoting bacteria Bacillus thuringiensis and mixed or individual mycorrhizal species improved drought tolerance and oxidative metabolism in Lavandula dentata plants. J. Plant Physiol. 2016, 192, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Obst, M.; Dynes, J.J.; Lawrence, J.R.; Swerhone, G.D.; Benzerara, K.; Karunakaran, C.; Kaznatcheev, K.; Tyliszczak, T.; Hitchcock, A.P. Precipitation of amorphous CaCO3 (aragonite-like) by cyanobacteria: A STXM study of the influence of EPS on the nucleation process. Geochim. Cosmochim. Acta 2009, 73, 4180–4198. [Google Scholar] [CrossRef]
- Tyerman, S.D.; Niemietz, C.M.; Bramley, H. Plant aquaporins: Multifunctional water and solute channels with expanding roles. Plant Cell Environ. 2002, 25, 173–194. [Google Scholar] [CrossRef] [Green Version]
- Goyal, K.; Walton, L.J.; Tunnacliffe, A. LEA proteins prevent protein aggregation due to water stress. Biochem. J. 2005, 388, 151–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.M.; Babar, A. Impacts of plant growth promoters and plant growth regulators on rainfed agriculture. PLoS ONE 2020, 15, e0231426. [Google Scholar] [CrossRef] [Green Version]
- Sandhya, V.S.K.Z.; Ali, S.Z.; Grover, M.; Reddy, G.; Venkateswarlu, B. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 2010, 62, 21–30. [Google Scholar] [CrossRef]
- Naseem, H.; Bano, A. Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J. Plant Interact. 2014, 9, 689–701. [Google Scholar] [CrossRef] [Green Version]
- Bahadur, A.; Singh, U.P.; Sarnia, B.K.; Singh, D.P.; Singh, K.P.; Singh, A. Foliar application of plant growth-promoting rhizobacteria increases antifungal compounds in pea (Pisum sativum) against Erysiphe pisi. Mycobiology 2007, 35, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Chiappero, J.; del Rosario Cappellari, L.; Alderete, L.G.S.; Palermo, T.B.; Banchio, E. Plant growth promoting rhizobacteria improve the antioxidant status in Mentha piperita grown under drought stress leading to an enhancement of plant growth and total phenolic content. Ind. Crops Prod. 2019, 139, 111553. [Google Scholar] [CrossRef]
- Jha, Y.; Subramanian, R.B. PGPR regulate caspase-like activity, programmed cell death, and antioxidant enzyme activity in paddy under salinity. Physiol. Mol. Biol. Plants 2014, 20, 201–207. [Google Scholar] [CrossRef]
- Saikia, J.; Sarma, RK.; Dhandia, R.; Yadav, A.; Bharali, R.; Gupta, V.K.; Saikia, R. Alleviation of drought stress in pulse crops with ACC deaminase producing rhizobacteria isolated from acidic soil of Northeast India. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Porcel, R.; Zamarreño, Á.M.; García-Mina, J.M.; Aroca, R. Involvement of plant endogenous ABA in Bacillus megaterium PGPR activity in tomato plants. BMC Plant Biol. 2014, 14, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Cohen, A.C.; Bottini, R.; Piccoli, P.N. Azospirillum brasilense Sp 245 produces ABA in chemically defined culture medium and increases ABA content in Arabidopsis plants. Plant Growth Regul. 2008, 54, 97–103. [Google Scholar] [CrossRef]
- Sangiorgio, D.; Cellini, A.; Donati, I.; Pastore, C.; Onofrietti, C.; Spinelli, F. Facing Climate Change: Application of Microbial Biostimulants to Mitigate Stress in Horticultural Crops. Agronomy 2020, 10, 794. [Google Scholar] [CrossRef]
- Xiang, Y.; Tang, N.; Du, H.; Ye, H.; Xiong, L. Characterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice. Plant Physiol. 2008, 148, 1938–1952. [Google Scholar] [CrossRef] [Green Version]
- Iuchi, S.; Kobayashi, M.; Taji, T.; Naramoto, M.; Seki, M.; Kato, T.; Tabata, S.; Kakubari, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 2001, 27, 325–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakashima, K.; Yamaguchi-Shinozaki, K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013, 32, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Saakre, M.; Baburao, T.M.; Salim, A.P.; Ffancies, R.M.; Achuthan, V.P.; Thomas, G.; Sivarajan, S.R. Identification and characterization of genes responsible for drought tolerance in rice mediated by Pseudomonas fluorescens. Rice Sci. 2007, 24, 291–298. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Rossi, M.J.; Glick, B.R. Ethylene and 1-Aminocyclopropane-1-carboxylate (ACC) in plant–bacterial interactions. Front. Plant Sci. 2018, 9, 114. [Google Scholar] [CrossRef]
- Vaishnav, A.; Choudhary, D.K. Regulation of drought-responsive gene expression in Glycine max l. Merrill is mediated through Pseudomonas simiae strain AU. J. Plant Growth Regul. 2019, 38, 333–342. [Google Scholar] [CrossRef]
- Shen, J.; Lv, B.; Luo, L.; He, J.; Mao, C.; Xi, D.; Ming, F. The NAC-type transcription factor OsNAC2 regulates ABA-dependent genes and abiotic stress tolerance in rice. Sci. Rep. 2017, 7, 40641. [Google Scholar] [CrossRef]
- Javidnia, K.; Faghih-Mirzaei, E.; Miri, R.; Attarroshan, M.; Zomorodian, K. Biotransformation of acetoin to 2, 3-butanediol: Assessment of plant and microbial biocatalysts. Res. Pharm. Sci. 2016, 11, 349. [Google Scholar] [CrossRef] [Green Version]
- Nuruzzaman, M.; Sharoni, A.M.; Kikuchi, S. Roles of NAC transcription factors in the regulation of biotic and abiotic stress responses in plants. Front. Microbiol. 2013, 4, 248. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Niu, K.; Huang, B.; Liu, W.; Ma, H. Transcriptional responses of creeping bentgrass to 2, 3-butanediol, a bacterial volatile compound (BVC) analogue. Molecules 2017, 22, 1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Li, C.; Zhang, B.; Yi, J.; Yang, Y.; Kong, C.; Lei, C.; Gong, M. The role of the late embryogenesis-abundant (LEA) protein family in development and the abiotic stress response: A comprehensive expression analysis of potato (Solanum Tuberosum). Genes 2019, 10, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, S.; Prasad, V.; Chauhan, P.S.; Lata, C. Bacillus amyloliquefaciens confers tolerance to various abiotic stresses and modulates plant response to phytohormones through osmoprotection and gene expression regulation in rice. Front. Plant Sci. 2017, 8, 1510. [Google Scholar] [CrossRef] [Green Version]
- Yu, A.; Li, P.; Tang, T.; Wang, J.; Chen, Y.; Liu, L. Roles of Hsp70s in stress responses of microorganisms, plants, and animals. Biomed. Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Gamez, R.M.; Rodríguez, F.; Vidal, N.M.; Ramirez, S.; Alvarez, R.V.; Landsman, D.; Mariño-Ramírez, L. Banana (Musa acuminata) transcriptome profiling in response to rhizobacteria: Bacillus amyloliquefaciens Bs006 and Pseudomonas fluorescens Ps006. BMC Genom. 2019, 20, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Chen, T.; Li, G.; Islam, M.R.; Fu, W.; Feng, B.; Tao, L.; Fu, G. Abscisic acid synergizes with sucrose to enhance grain yield and quality of rice by improving the source-sink relationship. BMC Plant Biol. 2019, 19, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Aung, K.; Jiang, Y.; He, S.Y. The role of water in plant–microbe interactions. Plant J. 2018, 93, 771–780. [Google Scholar] [CrossRef] [Green Version]
- Stone, B.W.; Weingarten, E.A.; Jackson, C.R. The role of the phyllosphere microbiome in plant health and function. Ann. Plant Rev. Online 2018, 1, 533–556. [Google Scholar] [CrossRef]
- Wu, L.; Li, X.; Ma, L.; Borriss, R.; Wu, Z.; Gao, X. Acetoin and 2, 3-butanediol from Bacillus amyloliquefaciens induce stomatal closure in Arabidopsis thaliana and Nicotiana benthamiana. J. Exp. Bot. 2018, 69, 5625–5635. [Google Scholar] [CrossRef]
- Brewer, C.A.; Smith, W.K.; Vogelmann, T.C. Functional interaction between leaf trichomes, leaf wettability and the optical properties of water droplets. Plant Cell Environ. 1991, 14, 955–962. [Google Scholar] [CrossRef]
- Hirsch, A.M.; Lum, M.R.; Fujishige, N.A. Microbial Encounters of a Symbiotic Kind: Attaching to Roots and Other Surfaces. In Plant Cell Monographs; Emons, A.M.C., Ketelaar, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 12. [Google Scholar] [CrossRef]
- Van der Wal, A.; Leveau, J.H. Modelling sugar diffusion across plant leaf cuticles: The effect of free water on substrate availability to phyllosphere bacteria. Environ. Microbiol. 2011, 13, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Karl, T.; Harren, F.; Warneke, C.; De Gouw, J.; Grayless, C.; Fall, R. Senescing grass crops as regional sources of reactive volatile organic compounds. J. Geophys. Res. Atmos. 2015, 110, D15. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.A.; Sabarinathan, K.G.; Kannan, R.; Balachandar, D.; Gomathy, M. Isolation and Characterization of Drought Tolerant Bacteria from Rice Phyllosphere. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2655–2664. [Google Scholar] [CrossRef]
- Abid, M.; Ali, S.; Qi, LK.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Sethuraman, P.; Balasubramanian, N. Removal of Cr (VI) from aqueous solution using Bacillus subtilis, Pseudomonas aeruginosa and Enterobacter cloacae. Int. J. Eng. Sci. Technol. 2010, 2, 1811–1825. [Google Scholar]
- Fukami, J.; Ollero, F.J.; Megías, M.; Hungria, M. Phytohormones and induction of plant-stress tolerance and defense genes by seed and foliar inoculation with Azospirillum brasilense cells and metabolites promote maize growth. AMB Express. 2017, 7, 153. [Google Scholar] [CrossRef]
- Preininger, C.; Sauer, U.; Bejarano, A.; Berninger, T. Concepts and applications of foliar spray for microbial inoculants. Appl. Microbiol. Biot. 2018, 102, 7265–7282. [Google Scholar] [CrossRef]
- Turner, N.C. Techniques and experimental approaches for the measurement of plant water status. Plant Soil 1981, 58, 339–366. [Google Scholar] [CrossRef]
- Sahrawat, K.L.; Ravi Kumar, G.; Rao, J.K. Evaluation of triacid and dry ashing procedures for determining potassium, calcium, magnesium, iron, zinc, manganese, and copper in plant materials. Commun. Soil. Sci. Plan. 2002, 33, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Havre, G.N. The flame photometric determination of sodium, potassium and calcium in plant extracts with special reference to interference effects. Anal. Chim. Acta 1961, 25, 557–566. [Google Scholar] [CrossRef]
- Tucker, B.B.; Kurtz, L.T. Calcium and magnesium determinations by EDTA titrations. Soil Sci. Soc. Am. J. 1961, 25, 27–29. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F.J. Phenol sulphuric acid method for total carbohydrate. Anal. Chem. 1956, 26, 350. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Barka, E.A.; Nowak, J.; Clément, C. Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth-promoting rhizobacterium, Burkholderia phytofirmans strain PsJN. Appl. Environ. Microbiol. 2006, 72, 7246–7252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Pan, X.; Welti, R.; Wang, X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography–mass spectrometry. Nat. Protoc. 2010, 5, 986–992. [Google Scholar] [CrossRef]
- Knörzer, O.C.; Burner, J.; Boger, P. Alterations in the antioxidative system of suspension-cultured soybean cells (Glycine max) induced by oxidative stress. Physiol. Plant 1996, 97, 388–396. [Google Scholar] [CrossRef]
- Bradford, N.A. A rapid and sensitive method for the quantitation microgram quantities of a protein isolated from red cell membranes. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Method Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Zaharieva, T.; Yamashita, K.; Matsumoto, H. Iron deficiency induced changes in ascorbate content and enzyme activities related to ascorbate metabolism in cucumber roots. Plant Cell Physiol. 1999, 40, 273–280. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gao, T.; Wu, Y.; Zhang, Y.; Liu, L.; Ning, Y.; Wang, D.; Tong, H.; Chen, S.; Chu, C.; Xie, Q. OsSDIR1 overexpression greatly improves drought tolerance in transgenic rice. Plant Mol. Biol. 2011, 76, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Pathan, A.K.; Bond, J.; Gaskin, R.E. Sample preparation for scanning electron microscopy of plant surfaces—Horses for courses. Micron 2008, 39, 1049–1061. [Google Scholar] [CrossRef]
- Thioulouse, J.; Dray, S. Interactive multivariate data analysis in R with the ade4 and ade4TkGUI packages. J. Stat. Softw. 2007, 22, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, S.; Konietschke, F.; Pauly, M. Resampling-Based Analysis of Multivariate Data and Repeated Measures Designs with the R Package MANOVA.RM. R J. 2019, 11, 380–400. [Google Scholar] [CrossRef]










| Treatments | Physico-Chemical Parameters | Contents of Biochemical Compounds | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RWC (%) | Potassium (ppm g–1 DW) | Calcium (%) | Total Proteins (µg g–1 FW) | TSS (µg g–1 W) | Prolines (µg g–1 FW) | Total Phenolics (mg GAE g–1) | MDA (nmol g–1 FW) | IAA (ng g–1) | ABA (ng g–1) | |
| PB3 | 72.8 ± 0.6 c | 28.1 ± 0.1 c | 0.78 ± 0.01 bc | 277 ± 3 b | 2.99 ± 0.03 b | 28.3 ± 0.3 b | 2.57 ± 0.12 c | 3.04 ± 0.2 bc | 115 ± 4 b | 96 ± 2 b |
| PB46 | 75.0 ± 1.1 bc | 31.1 ± 0.1 b | 0.84 ± 0.03 ab | 266 ± 4 c | 2.84 ± 0.02 c | 28.2 ± 0.4 b | 2.79 ± 0.05 b | 3.29 ± 0.2 b | 105 ± 8 c | 86 ± 3 c |
| PB50 | 76.8 ± 0.8 ab | 33.1 ± 0.1 a | 0.89 ± 0.01 a | 324 ± 4 a | 3.18 ± 0.13 a | 34.1 ± 0.5 a | 3.13 ± 0.21 a | 2.78 ± 0.1 cd | 136 ± 6 a | 127 ± 3 a |
| Cws | 69.1 ± 0.8 d | 26.1 ± 0.1 d | 0.75 ± 0.04 bc | 256 ± 5 d | 2.85 ± 0.06 bc | 27.3 ± 0.5 c | 2.49 ± 0.04 c | 4.20 ± 0.1 a | 96 ± 10 d | 78 ± 3 d |
| Ci | 78.8 ± 0.7 a | 24.1 ± 0.1 g | 0.71 ± 0.02 c | 231 ± 4 e | 2.69 ± 0.08 d | 25.8 ± 0.5 d | 2.27 ± 0.03 d | 2.52 ± 0.1 d | 86 ± 9 e | 67 ± 3 e |
| Treatments | Enzyme Activities (mmole min–1 mg protein–1) | ||
|---|---|---|---|
| APX | CAT | GPX | |
| PB3 | 3.46 ± 0.08 b | 66.61 ± 1.9 bc | 0.210 ± 0.04 b |
| PB46 | 2.83 ± 0.06 c | 70.28 ± 3.7 b | 0.224 ± 0.04 b |
| PB50 | 2.71 ± 0.10 c | 62.94 ± 3.4 c | 0.196 ± 0.02 b |
| Cws | 4.09 ± 0.07 a | 80.36 ± 4.3 a | 0.279 ± 0.03 a |
| Ci | 2.20 ± 0.08 d | 55.92 ± 5.4 d | 0.145 ± 0.03 c |
| Genes | NCBI Accession No. | Primer Sequence | Melting Temp. (°C) | Amplicon Length (bp) |
|---|---|---|---|---|
| LEA | XM_015782086 | F—5′ GGATCACTAGACGCCGTGAA 3′ R—5′ CAGAAATCCTCCCCTGCGAC 3′ | 59.55 60.46 | 151 |
| HSP70 | XM_015776732 | F—5′ GAATCGTGACGGTCTCAGCA 3′ R—5′ CGATGAGGGCTTTCCGTTCT 3′ | 60.11 60.11 | 156 |
| RAB16B | XM_015762125 | F—5′ ATCGATCGACGGCTTTGACA 3′ R—5′ GCCCCTGGTAGTTGTCCATC 3′ | 59.83 60.11 | 154 |
| bZIP23 | XM_015770367 | F—5′ AGATCACGCTGGAGGAGTTC 3′ R—5′ CGGAGGGAACACATTGCTCT 3′ | 59.18 60.04 | 167 |
| AP2/ERF | XM_015785947 | F—5′ GTGACAGCACAGTCACAACG 3′ F—5′ GATGACGAGGCTACCTTCACC 3′ | 59.70 60.20 | 144 |
| SNAC1 | XM_015775072 | F—5′ TGGACCTGAGCTACGACGAT 3′ R—5′ TCACCTCAGAACGGGACCAT 3′ | 60.39 60.54 | 157 |
| Actin | XM_015761709 | F—5′ GGACTCTGGTGATGGTGTCA 3′ R—5′ TTTCCCGTTCAGCAGTGGTA 3′ | 59.02 59.24 | 164 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devarajan, A.K.; Muthukrishanan, G.; Truu, J.; Truu, M.; Ostonen, I.; Kizhaeral S., S.; Panneerselvam, P.; Kuttalingam Gopalasubramanian, S. The Foliar Application of Rice Phyllosphere Bacteria induces Drought-Stress Tolerance in Oryza sativa (L.). Plants 2021, 10, 387. https://doi.org/10.3390/plants10020387
Devarajan AK, Muthukrishanan G, Truu J, Truu M, Ostonen I, Kizhaeral S. S, Panneerselvam P, Kuttalingam Gopalasubramanian S. The Foliar Application of Rice Phyllosphere Bacteria induces Drought-Stress Tolerance in Oryza sativa (L.). Plants. 2021; 10(2):387. https://doi.org/10.3390/plants10020387
Chicago/Turabian StyleDevarajan, Arun Kumar, Gomathy Muthukrishanan, Jaak Truu, Marika Truu, Ivika Ostonen, Subramanian Kizhaeral S., Periyasamy Panneerselvam, and Sabarinathan Kuttalingam Gopalasubramanian. 2021. "The Foliar Application of Rice Phyllosphere Bacteria induces Drought-Stress Tolerance in Oryza sativa (L.)" Plants 10, no. 2: 387. https://doi.org/10.3390/plants10020387