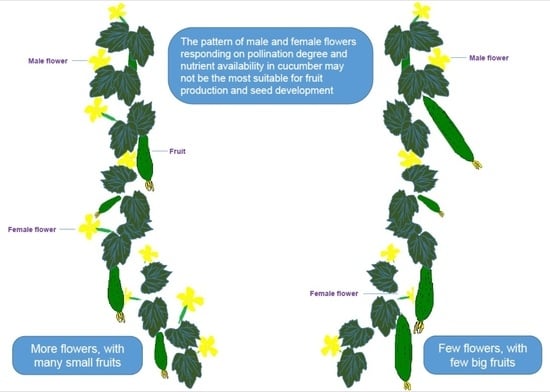
The Patterns of Male and Female Flowers in Flowering Stage May Not Be Optimal Resource Allocation for Fruit and Seed Growth
Abstract
:1. Introduction
2. Results
2.1. Total Number of Male and Female Flowers
2.2. Dynamic Changes in the Number of Male and Female Flowers
2.3. Reproduction Set
2.4. Seed Production
2.5. Correlation between Flowers and Fruits and Seeds
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cultivation
4.3. Experimental Design
4.4. Male and Female Flower Production
4.5. Pollination Settings
4.6. Fruit Production
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, D.Y. Reproductive Ecology of Plants. In Plant Life-History Evolution and Reproductive Ecology; Zhang, D.Y., Ed.; Science Press: Beijing, China, 2003; pp. 101–189. [Google Scholar]
- Zhang, T.; Tan, D.Y. Adaptive significances of sexual system in andromonoecious Capparis spinosa (Capparaceae). J. Syst. Evol. 2008, 46, 861–873. [Google Scholar]
- Buonaiuto, D.M.; Wolkovich, E.M. Differences between flower and leaf phenological responses to environmental variation drive shifts in spring phenological sequences of temperate woody plants. J. Ecol. 2021, 109, 2922–2933. [Google Scholar] [CrossRef]
- Creux, N.M.; Brown, E.A.; Garner, A.G.; Saeed, S.; Scher, C.L.; Holalu, S.V.; Yang, D.I.; Maloof, J.N.; Blackman, B.K.; Harmer, S.L. Flower orientation influences floral temperature, pollinator visits and plant fitness. New Phytol. 2021, 232, 868–879. [Google Scholar] [CrossRef] [PubMed]
- Strauss, S.Y. Floral characters link herbivores, pollinators, and plant fitness. Ecology 1997, 78, 1640–1645. [Google Scholar] [CrossRef]
- Rusman, Q.; Lucas-Barbosa, D.; Hassan, K.; Poelman, E.H. Plant ontogeny determines strength and associated plant fitness consequences of plant-mediated interactions between herbivores and flower visitors. J. Ecol. 2020, 108, 1046–1060. [Google Scholar] [CrossRef] [Green Version]
- Ansaldi, B.H.; Weber, J.J.; Franks, S.J. The role of phenotypic plasticity and pollination environment in the cleistogamous, mixed mating breeding system of Triodanis perfoliata. Plant Biol. 2018, 20, 1068–1074. [Google Scholar] [CrossRef]
- Li, X.M.; She, D.Y.; Zhang, D.Y.; Liao, W.J. Life history trait differentiation and local adaptation in invasive populations of Ambrosia artemisiifolia in China. Oecologia 2015, 177, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Bell, G. On the function of flowers. Proc. R. Soc. Lond. B. 1985, B22, 223–265. [Google Scholar]
- Caruso, C.M. The quantitative genetics of floral trait variation in Lobelia: Potential constraints on adaptive evolution. Evolution 2004, 58, 732–740. [Google Scholar] [CrossRef]
- Worley, A.C.; Barrett, S.C.H. Evolution of floral display in Eichhornia paniculata (Pontederiaceae): Direct and correlated responses to selection on flower size and number. Evolution 2000, 54, 1533–1545. [Google Scholar] [CrossRef]
- Sargent, R.D.; Goodwillie, C.; Kalisz, S.; Rees, R.H. Phylogenetic evidence for a flower size and number trade-off. Am. J. Bot. 2007, 94, 2059–2062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atlan, A.; Hornoy, B.; Delerue, F.; Gonzalez, M.; Pierre, J.S.; Tarayre, M. Phenotypic plasticity in reproductive traits of the perennial shrub ulex europaeus in response to shading: A multi-year monitoring of cultivated clones. PLoS ONE 2015, 10, e0137500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.; Zhang, D.Y.; Ives, A.R.; Li, Q.J. Why do stigmas move in a flexistylous plant? J. Evol. Biol. 2011, 24, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.E.; Dorken, M.E. The Evolution of Males: Support for Predictions from Sex Allocation Theory Using Mating Arrays of Sagittaria latifolia (Alismataceae). Evolution 2011, 65, 2782–2791. [Google Scholar] [CrossRef] [PubMed]
- Torices, R.; Mendez, M. Influence of inflorescence size on sexual expression and female reproductive success in a monoecious species. Plant Biol. 2011, 13, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Carper, A.L.; Adler, L.S.; Irwin, R.E. Effects of florivory on plant-pollinator interactions: Implications for male and female components of plant reproduction. Am. J. Bot. 2016, 103, 1061–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peruzzi, L.; Mancuso, E.; Gargano, D. Males are cheaper, or the extreme consequence of size/age-dependent sex allocation: Sexist gender diphasy in Fritillaria montana (Liliaceae). Bot. J. Linn. Soc. 2012, 168, 323–333. [Google Scholar] [CrossRef] [Green Version]
- Burd, M. Bateman Principle and Plant Reproduction—The Role of Pollen Limitation in Fruit and Seed Set. Bot. Rev. 1994, 60, 83–139. [Google Scholar] [CrossRef]
- Christopher, D.A.; Mitchell, R.J.; Karron, J.D. Pollination intensity and paternity in flowering plants. Ann. Bot. 2020, 125, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.H.; Wolf, S.; Kafkafi, U. Interactive effect of nutrient concentration and container volume on flowering, fruiting, and nutrient uptake of sweet pepper. J. Plant Nutr. 2001, 24, 479–501. [Google Scholar] [CrossRef]
- Garcia, M.B. Sex allocation in a long-lived monocarpic plant. Plant Biol. 2003, 5, 203–209. [Google Scholar] [CrossRef]
- Wright, J.W.; Meagher, T.R. Selection on floral characters in natural Spanish populations of Silene latifolia. J. Evol. Biol. 2004, 17, 382–395. [Google Scholar] [CrossRef] [Green Version]
- Ganeshaiah, K.N.; Shaanker, R.U. Seed abortion in wind-dispersed pods of dalbergia-sissoo—Maternal regulation or sibling rivalry. Oecologia 1988, 77, 135–139. [Google Scholar] [CrossRef]
- Marcelis, L.F.M.; Heuvelink, E.; Hofman-Eijer, L.R.B.; Den Bakker, J.; Xue, L.B. Flower and fruit abortion in sweet pepper in relation to source and sink strength. J. Exp. Bot. 2004, 55, 2261–2268. [Google Scholar] [CrossRef] [Green Version]
- Rosenheim, J.A.; Alon, U.; Shinar, G. Evolutionary balancing of fitness-limiting factors. Am. Nat. 2010, 175, 662–674. [Google Scholar] [CrossRef]
- Schreiber, S.J.; Rosenheim, J.A.; Williams, N.W.; Harder, L.D. Evolutionary and ecological consequences of multiscale variation in pollen receipt for seed production. Am. Nat. 2015, 185, E14–E29. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Garcia, E.; Olano, J.M.; Leroux, O.; Mezquida, E.T. Deciphering the role of reproductive investment, pollination success and predispersal seed predation on reproductive output in Juniperus thurifera. Plant Ecol. Divers 2019, 12, 37–49. [Google Scholar] [CrossRef]
- Song, Y.P.; Ma, K.F.; Ci, D.; Chen, Q.Q.; Tian, J.X.; Zhang, D.Q. Sexual dimorphic floral development in dioecious plants revealed by transcriptome, phytohormone, and DNA methylation analysis in Populus tomentosa. Plant Mol. Biol. 2013, 83, 559–576. [Google Scholar] [CrossRef]
- Barber, N.A.; Adler, L.S.; Bernardo, H.L. Effects of above- and belowground herbivory on growth, pollination, and reproduction in cucumber. Oecologia 2011, 165, 377–386. [Google Scholar] [CrossRef]
- Bai, S.N.; Xu, Z.H. Unisexual cucumber flowers, sex and sex differentiation. Int. Rev. Cel. Mol. Bio. 2013, 304, 1–55. [Google Scholar]
- Boualem, A.; Troadec, C.; Camps, C.; Lemhemdi, A.; Morin, H.; Sari, M.A.; Fraenkel-Zagouri, R.; Kovalski, I.; Dogimont, C.; Perl-Treves, R.; et al. A cucurbit androecy gene reveals how unisexual flowers develop and dioecy emerges. Science 2015, 350, 688–691. [Google Scholar] [CrossRef]
- Liu, W.F.; Qin, Z.W.; Xin, M.; Zhou, X.Y.; Yang, J.; Wang, C.H. Analysis of CsPAP-fib regulation of cucumber female differentiation in response to low night temperature conditions. Sci. Hortic. 2018, 240, 81–88. [Google Scholar] [CrossRef]
- Nicodemo, D.; Malheiros, E.B.; De Jong, D.; Couto, R.H.N. Enhanced production of parthenocarpic cucumbers pollinated with stingless bees and Africanized honey bees in greenhouses. Semin. Cienc. Agrar. 2013, 34, 3625–3633. [Google Scholar] [CrossRef]
- Zhou, Y.; Ahammed, G.J.; Wang, Q.; Wu, C.Q.; Wan, C.P.; Yang, Y.X. Transcriptomic insights into the blue light-induced female floral sex expression in cucumber (Cucumis sativus L.). Sci. Rep. 2018, 8, 14261. [Google Scholar] [CrossRef] [Green Version]
- Diola, V.; Orth, A.I.; Guerra, M.P. Reproductive biology in monoecious and gynoecious cucumber cultivars as a result of IBA application. Hortic. Bras. 2008, 26, 30–34. [Google Scholar] [CrossRef] [Green Version]
- Dorken, M.E.; Barrett, S.C.H. Gender plasticity in Sagittaria sagittifolia (Alismataceae), a monoecious aquatic species. Plant Syst. Evol. 2003, 237, 99–106. [Google Scholar] [CrossRef]
- Golenberg, E.M.; West, N.W. Hormonal interactions and gene regulation can link monoecy and environmental plasticity to the evolution of dioecy in plants. Am. J. Bot. 2013, 100, 1022–1037. [Google Scholar] [CrossRef] [Green Version]
- Paquin, V.; Aarssen, L.W. Allometric gender allocation in Ambrosia artemisiifolia (Asteraceae) has adaptive plasticity. Am. J. Bot. 2004, 91, 430–438. [Google Scholar] [CrossRef]
- Van Drunen, W.E.; Dorken, M.E. Trade-offs between clonal and sexual reproduction in Sagittaria latifolia (Alismataceae) scale up to affect the fitness of entire clones. New Phytol 2012, 196, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Halpern, S.L.; Adler, L.S.; Wink, M. Leaf herbivory and drought stress affect floral attractive and defensive traits in Nicotiana quadrivalvis. Oecologia 2010, 163, 961–971. [Google Scholar] [CrossRef]
- Groeneveld, J.H.; Tscharntke, T.; Moser, G.; Clough, Y. Experimental evidence for stronger cacao yield limitation by pollination than by plant resources. Perspect. Plant Ecol. 2010, 12, 183–191. [Google Scholar] [CrossRef]
- Motzke, I.; Tscharntke, T.; Wanger, T.C.; Klein, A.M. Pollination mitigates cucumber yield gaps more than pesticide and fertilizer use in tropical smallholder gardens. J. Appl. Ecol. 2015, 52, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Zuo, X.A. Pollen limitation and resource limitation affect the reproductive success of Medicago sativa L. BMC Ecol. 2018, 18, 28–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacome-Flores, M.E.; Delibes, M.; Wiegand, T.; Fedriani, J.M. Spatio-temporal arrangement of Chamaerops humilis inflorescences and occupancy patterns by its nursery pollinator, Derelomus chamaeropsis. Ann. Bot. 2018, 121, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Vallejo-Marin, M.; Rausher, M.D. The role of male flowers in andromonoecious species: Energetic costs and siring success in Solanum carolinense L. Evolution 2007, 61, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, M.; Vaughton, G. Sex expression and sexual dimorphism in subdioecious Wurmbea dioica (Colchicaceae). Int. J. Plant Sci. 2001, 162, 589–597. [Google Scholar] [CrossRef]
- Rocheta, M.; Sobral, R.; Magalhaes, J.; Amorim, M.I.; Ribeiro, T.; Pinheiro, M.; Egas, C.; Morais-Cecilio, L.; Costa, M.M.R. Comparative transcriptonnic analysis of male and female flowers of monoecious Quercus suber. Front Plant Sci. 2014, 5, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Teixido, A.L.; Valladares, F. Pollinator-mediated phenotypic selection does not always modulate flower size and number in the large-flowered Mediterranean shrub Cistus ladanifer (Cistaceae). Bot. J. Linn. Soc. 2014, 176, 540–555. [Google Scholar] [CrossRef] [Green Version]
- Dittmar, P.J.; Monks, D.W.; Schultheis, J.R. Maximum potential vegetative and floral production and fruit characteristics of watermelon pollenizers. Hortscience 2009, 44, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Low, J.E.; Aslund, M.L.W.; Rutter, A.; Zeeb, B.A. The effects of pruning and nodal adventitious roots on polychlorinated biphenyl uptake by Cucurbita pepo grown in field conditions. Environ. Pollut. 2011, 159, 769–775. [Google Scholar] [CrossRef]
- Choi, E.Y.; Cho, I.H.; Moon, J.H.; Woo, Y.H. Impact of secondary-lateral branch removal during watermelon production. Hortic Environ. Biotechnol. 2012, 53, 24–31. [Google Scholar] [CrossRef]
- Nabizadeh, E.; Taherifard, E.; Gerami, F. Effect of pruning lateral branches on four varieties of medicinal castor bean plant (Ricinuscommunis L.) yield, growth and development. J. Med. Plants Res. 2011, 5, 5828–5834. [Google Scholar]
- Lord, J.M.; Westoby, M. Accessory costs of seed production and the evolution of angiosperms. Evolution 2012, 66, 200–210. [Google Scholar] [CrossRef]
- Marsal, J.; Basile, B.; Solari, L.; DeJong, T.M. Influence of branch autonomy on fruit, scaffold, trunk and root growth during Stage III of peach fruit development. Tree Physiol. 2003, 23, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.C.; Song, Z.W.; Lu, W.L.; Poschenrieder, C.; Schmidhalter, U. Current soil nutrient status of intensively managed greenhouses. Pedosphere 2012, 22, 825–833. [Google Scholar] [CrossRef]
- Moreno, D.A.; Pulgar, G.; Ruiz, J.M.; Villora, G.; Romero, L. Optimum nutrient range in cucumber (Cucumis sativus L. cv. Brunex F-1 plants): I. Nitrogen and phosphorus parameters. Phyton Int. J. Exp. Bot. 1998, 63, 191–195. [Google Scholar]
- Baker, D.N.; Kanekal, S.G.; Li, X.; Monk, S.P.; Goldstein, J.; Burch, J.L. An extreme distortion of the Van Allen belt arising from the ‘Hallowe’en’ solar storm in 2003. Nature 2004, 432, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, M.R.; Rekha, A.; Ravishankar, K.; Praveen, K.S.; Santosh, L.C. Breaking the intergeneric crossing barrier in papaya using sucrose treatment. Sci. Hortic. 2007, 114, 33–36. [Google Scholar] [CrossRef]
- Owuor, B.O.; Owino, F. Control pollination and pollen management in Sebania sesban (L). Merr. Euphytica 1993, 70, 161–165. [Google Scholar] [CrossRef]
- Dalkilic, Z.; Mestav, H.O. In vitro pollen quantity, viability and germination tests in quince. Afr. J. Biotechnol. 2011, 10, 16516–16520. [Google Scholar]
- Godini, A. Counting pollen grains of some almond cultivars by means of haemocytometer. Rivista della Ortoflorofrutticoltura Italiana 1981, 65, 173–178. [Google Scholar]
- Burd, M. Offspring quality in relation to excess flowers in Pultenaea gunnii (Fabaceae). Evolution 2004, 58, 2371–2376. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, S. Patterns of fruit-set—What controls fruit-flower ratios in plants. Evolution 1986, 40, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Gepts, P. Domestication as a long-term selection experiment. Plant Breed. Rev. 2004, 24, 1–44. [Google Scholar]






| Factors | Dependent v | Df * | F * | p-Value |
|---|---|---|---|---|
| Nutrient | Total number of male flowers | 1 | 4.80 | 0.031 |
| Total number of female flowers | 1 | 21.47 | 0.000 | |
| Number of male flowers per day | 32 | 1.48 | 0.084 | |
| Number of female flowers per day | 32 | 1.68 | 0.033 | |
| Number of male flowers on the 36th day | 1 | 2.50 | 0.117 | |
| Number of male flowers on the 43rd day | 1 | 10.60 | 0.002 | |
| Number of female flowers on the 36th day | 1 | 10.52 | 0.002 | |
| Number of female flowers on the 43rd day | 1 | 5.05 | 0.027 | |
| Ratio of male/female flowers per day | 32 | 0.943 | 0.562 | |
| Ratio of male/female flowers on the 36th day | 1 | 1.01 | 0.317 | |
| Ratio of male/female flowers on the 43rd day | 1 | 0.35 | 0.554 | |
| Pollination | Total number of male flowers | 5 | 0.57 | 0.72 |
| Total number of female flowers | 5 | 0.15 | 0.98 | |
| Number of male flowers per day | 32 | 2.30 | 0.001 | |
| Number of female flowers per day | 32 | 1.61 | 0.045 | |
| Number of male flowers on the 36th day | 32 | 0.97 | 0.437 | |
| Number of male flowers on the 43rd day | 5 | 1.82 | 0.115 | |
| Number of female flowers on the 36th day | 5 | 0.88 | 0.501 | |
| Number of female flowers on the 43rd day | 5 | 2.25 | 0.037 | |
| Ratio of male/female flowers per day | 32 | 1.60 | 0.047 * | |
| Ratio of male/female flowers on the 36th day | 5 | 0.59 | 0.708 | |
| Ratio of male/female flowers on the 43rd day | 5 | 0.29 | 0.919 | |
| Nutrients and Pollination | Total number of male flowers | 5 | 0.80 | 0.554 |
| Total number of female flowers | 5 | 0.15 | 0.98 | |
| Number of male flowers per day | 32 | 1.42 | 0.104 | |
| Number of female flowers per day | 32 | 2.15 | 0.003 | |
| Number of male flowers on the 36th day | 5 | 0.20 | 0.962 | |
| Number of male flowers on the 43rd day | 5 | 1.27 | 0.284 | |
| Number of female flowers on the 36th day | 5 | 0.62 | 0.688 | |
| Number of female flowers on the 43rd day | 5 | 1.07 | 0.382 | |
| Ratio of male/female flowers per day | 32 | 2.38 | 0.001 | |
| Ratio of male/female flowers on the 36th day | 5 | 0.44 | 0.819 | |
| Ratio of male/female flowers on the 43rd day | 5 | 1.06 | 0.384 |
| Factors | Dependent Variables | Df * | F * | p-Value |
|---|---|---|---|---|
| Nutrient | Fruit yield per plant (pollinated fruits) | 1 | 22.7 | 0.000 |
| Fruit yield per plant (non-pollinated fruits) | 1 | 1.20 | 0.278 | |
| Weight of fruit | 1 | 0.06 | 0.810 | |
| Number of fruit | 1 | 18.11 | 0.000 | |
| Percentage fruit set | 1 | 7.03 | 0.009 | |
| Days of fruit growing | 1 | 0.15 | 0.702 | |
| Diameter of fruit | 1 | 0.23 | 0.635 | |
| Length of fruit | 1 | 0.04 | 0.839 | |
| Total no. seeds per plant | 1 | 15.06 | 0.000 | |
| Number of seeds per fruit | 1 | 0.09 | 0.766 | |
| Pollination | Fruit yield per plant (Pollinated fruits) | 5 | 2.39 | 0.043 |
| Fruit yield per plant (Non-pollinated fruits) | 5 | 0.63 | 0.681 | |
| Weight of fruit | 5 | 1.64 | 0.157 | |
| Number of fruit | 5 | 2.49 | 0.036 | |
| Percentage fruit set | 5 | 2.62 | 0.029 | |
| Days of fruit growing | 5 | 1.59 | 0.173 | |
| Diameter of fruit | 5 | 0.76 | 0.580 | |
| Length of fruit | 5 | 1.15 | 0.339 | |
| Total number of seeds per plant | 5 | 6.78 | 0.000 | |
| Number of seeds per fruit | 5 | 6.53 | 0.000 | |
| Nutrients and Pollination | Fruit yield per plant (Pollinated fruits) | 5 | 4.09 | 0.002 |
| Fruit yield per plant (Non-pollinated fruits) | 5 | 0.30 | 0.915 | |
| Weight of fruit | 5 | 1.40 | 0.232 | |
| Number of fruit | 5 | 1.32 | 0.262 | |
| Percentage fruit set | 5 | 0.91 | 0.476 | |
| Days of fruit growing | 5 | 1.40 | 0.235 | |
| Diameter of fruit | 5 | 2.99 | 0.016 | |
| Length of fruit | 5 | 2.12 | 0.072 | |
| Total number of seeds per plant | 5 | 1.30 | 0.564 | |
| Number of seeds per fruit | 5 | 0.78 | 0.270 |
| Female Flowers Per Plant | Number Fruits Per Plant | Non-Pollinated Female Flowers | Male Flowers Per Plant | Non-Pollinated Male Flowers | Fruit Yield Per Plant | Total Seeds Per plant | ||
|---|---|---|---|---|---|---|---|---|
| Number fruits per plant | r | 0.337 | ||||||
| p | 0.000 | |||||||
| n | 117 | |||||||
| Non-pollinated female flowers | r | 0.349 | 0.049 | |||||
| p | 0.000 | 0.601 | ||||||
| n | 117 | 117 | ||||||
| Male flowers per plant | r | 0.451 | 0.088 | 0.246 | ||||
| p | 0.000 | 0.345 | 0.007 | |||||
| n | 117 | 117 | 117 | |||||
| Non-pollinated male flowers | r | 0.259 | −0.173 | 0.147 | 0.444 | |||
| p | 0.005 | 0.063 | 0.113 | 0.000 | ||||
| n | 117 | 117 | 117 | 117 | ||||
| Fruit yield per plant | r | 0.337 | .892 | −0.053 | 0.135 | −0.152 | ||
| p | 0.000 | 0.000 | 0.569 | 0.145 | 0.101 | |||
| n | 117 | 117 | 117 | 117 | 117 | |||
| Total seeds per plant | r | 0.236 | 0.721 | −0.033 | 0.139 | −0.146 | 0.849 | |
| p | 0.01 | 0.000 | 0.725 | 0.136 | 0.116 | 0.000 | ||
| n | 117 | 117 | 117 | 117 | 117 | 117 | ||
| Shoot mass | r | 0.387 | −0.039 | 0.380 | 0.293 | 0.523 | −0.065 | −0.067 |
| p | 0.000 | 0.681 | 0.000 | 0.001 | 0.000 | 0.488 | 0.473 | |
| n | 117 | 117 | 117 | 117 | 117 | 117 | 117 | |
| Seeds per fruit | r | 0.051 | −0.034 | 0.135 | 0.124 | −0.007 | −0.057 | −0.042 |
| p | 0.593 | 0.714 | 0.146 | 0.182 | 0.942 | 0.539 | 0.656 | |
| n | 117 | 117 | 117 | 117 | 117 | 117 | 117 | |
| Fruit weight | r | 0.019 | 0.077 | 0.093 | 0.101 | −0.051 | 0.053 | 0.012 |
| p | 0.861 | 0.465 | 0.379 | 0.336 | 0.629 | 0.614 | 0.911 | |
| n | 92 | 92 | 92 | 92 | 92 | 92 | 92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Yu, G.; Hu, F.; Li, Z.; Li, W.; Peng, C. The Patterns of Male and Female Flowers in Flowering Stage May Not Be Optimal Resource Allocation for Fruit and Seed Growth. Plants 2021, 10, 2819. https://doi.org/10.3390/plants10122819
Gao L, Yu G, Hu F, Li Z, Li W, Peng C. The Patterns of Male and Female Flowers in Flowering Stage May Not Be Optimal Resource Allocation for Fruit and Seed Growth. Plants. 2021; 10(12):2819. https://doi.org/10.3390/plants10122819
Chicago/Turabian StyleGao, Lei, Guozhu Yu, Fangyu Hu, Zhiqi Li, Weihua Li, and Changlian Peng. 2021. "The Patterns of Male and Female Flowers in Flowering Stage May Not Be Optimal Resource Allocation for Fruit and Seed Growth" Plants 10, no. 12: 2819. https://doi.org/10.3390/plants10122819