The Influence of Organic Fertilizers on the Abundance of Soil Microorganism Communities, Agrochemical Indicators, and Yield in East Lithuanian Light Soils
Abstract
:1. Introduction
2. Results and Discussion
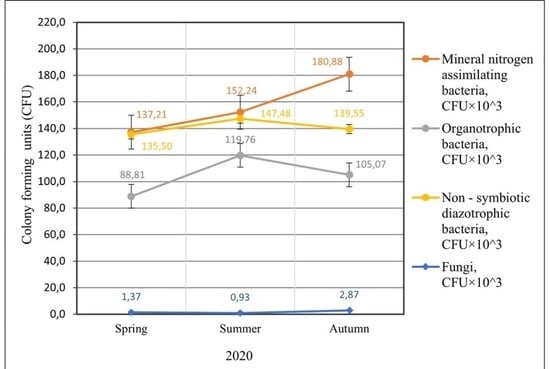
2.1. Abundance of Different Physiological Groups of Soil Microorganisms
2.2. Investigations of Mineral Nitrogen in Soil
2.3. Spring Barley Yield
3. Materials and Methods
3.1. Experimental Treatments and Design
3.2. Soil Sampling and Microbial Count
3.3. Soil Agrochemical Analysis
3.4. Plant Yield Evaluation
3.5. Meteorological Conditions
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Merrill, S.D.; Liebig, A.; Tanaka, D.L.; Krupinsky, J.M.; Hanson, J.D. Comparison of soil quality and productivity at two sites differing in profile structure and topsoil properties. Agric. Ecosyst. Environ. 2013, 179, 53–61. [Google Scholar] [CrossRef]
- Prosser, J.I. Dispersing misconceptions and identifying opportunities for the use of ‘omics’ in soil microbial ecology. Nat. Rev. Microbiol. 2015, 13, 439–446. [Google Scholar] [CrossRef]
- Carini, P.; Marsden, P.J.; Leff, J.W.; Morgan, E.E.; Strickland, M.S.; Fierer, N. Relic DNA is abundant in soil and obscures estimates of soil microbial diversity. Nat. Microbiol. 2016, 19, 16242. [Google Scholar] [CrossRef]
- Adetutu, E.M.; Ball, A.S.; Weber, J.; Aleer, S.; Dandie, C.E.; Juhasz, A.L. Impact of bacterial and fungal processes on 14C-hexadecane mineralization in weathered hydrocarbon con-taminated soil. Sci. Total Environ. 2012, 414, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Savci, S. Investigation of Effect of Chemical Fertilizers on Environment. APCBEE Procedia 2012, 1, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Piotrowska-Długosz, A.; Wilczewski, E. Soil Phosphatase Activity and Phosphorus Content as Influenced by Catch Crops Cultivated as Green Manure. Pol. J. Environ. Stud. 2014, 23, 157–165. [Google Scholar]
- Cao, H.; Chen, R.; Wang, L.; Jiang, L.; Yang, F.; Zheng, S.; Wang, G.; Lin, X. Soil pH, total phosphorus, climate and distance are the major factors influencing microbial activity at a regional spatial scale. Sci. Rep. 2016, 6, 25815. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition—Current Knowledge and Future Directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.; Tang, J.; Li, Z.; Zhou, Z.; Wang, J.; Wang, S.; Cao, Y. Soil Enzyme Activity and Microbial Metabolic Function Diversity in Soda Saline–Alkali Rice Paddy Fields of Northeast China. Sustainability 2020, 12, 10095. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Q.; Zhou, J.; Wei, Q. Pyrosequencing technology reveals the impact of different manure doses on the bacterial community in apple rhizosphere soil. Appl. Soil Ecol. 2014, 78, 28–36. [Google Scholar] [CrossRef]
- Daquiado, A.R.; Kappusamy, S.; Kim, A.Y.; Kim, J.H.; Yoon, Y.; Kim, P.J.; Oh, S.; Kwak, Y.; Lee, Y.B. Pyrosequencing analysis of bacterial community diversity in long-term fertilized paddy field soil. Appl. Soil Ecol. 2016, 108, 84–91. [Google Scholar] [CrossRef]
- He, Z.; Pagiliari, P.H.; Waldrip, H.M. Applied and Environmental Chemistry of Animal Manure: A Review. Pedosphere 2016, 26, 779–816. [Google Scholar] [CrossRef]
- Triberti, L.; Nastri, A.; Balcony, G. Long-term effects of crop rotation, manure and mineral fertilization on carbon sequestration and soil fertility. Eur. J. Agron. 2016, 74, 47–55. [Google Scholar] [CrossRef]
- Zebarth, B.J.; Chabot, R.; Coulombe, J.; Simard, R.R.; Doheret, J.; Tremblay, N. Pelletized organo-mineral fertilizer product as a nitrogen source for potato production. Can. J. Soil Sci. 2005, 85, 387–395. [Google Scholar] [CrossRef] [Green Version]
- Saidu, N.E.B.; Valente, S.; Bana, E.; Kirsch, G.; Bagrel, D.; Montenarh, M. Coumarin polysulfides inhibit cell growth and induce apoptosis in HCT116 colon cancer cells. Bioorg. Med. Chem. 2012, 20, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Mažeika, R.; Staugaitis, G.; Baltrušaitis, J. Engineered Pelletized Organo-Mineral Fertilizers (OMF) from Poultry Manure, Diammonium Phosphate and Potassium Chloride. ACS Sustain. Chem. Eng. 2016, 4, 2279–2285. [Google Scholar] [CrossRef]
- Crusciol, C.A.C.; de Campos, M.; Martello, J.M.; Alves, C.S.; Nascimento, C.A.C.; dos Reis Pereira, J.C.; Cantarella, H. Organomineral Fertilizer as Source of P and K for Sugarcane. Sci. Rep. 2020, 10, 5398. [Google Scholar] [CrossRef]
- Ye, C.; Huang, S.; Sha, C.; Wu, J.; Cui, C.; Su, J.; Ruan, J.; Tan, J.; Tang, H.; Xue, J. Changes of bacterial community in arable soil after short-term application of fresh manures and organic fertilizer. Environ. Techol. 2020, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Šarauskas, E.; Naujokiene, V.; Lekavičienė, K.; Kriaučiūnienė, Z.; Jotautienė, E.; Jasinskas, A.; Zinkevičienė, R. Application of Granular and Non-Granular Organic Fertilizers in Terms of Energy, Environmental and Economic Efficiency. Sustainability 2021, 13, 9740. [Google Scholar] [CrossRef]
- Kominko, H.; Gorazda, K.; Wzorek, Z. The Possibility of Organo-Mineral Fertilizer Production from Sewage Sludge. Waste Biomass Valoriz. 2017, 8, 1781–1791. [Google Scholar] [CrossRef] [Green Version]
- Piaulokaitė-Motuzienė, L.; Končius, D.; Lapinskas, E. The occurrence of microorganisms as affected by different soil agrochemical properties. Agric. Sci. Art. 2005, 89, 154–162. (In Lithuanian) [Google Scholar]
- Kazlauskaitė-Jadzevičė, A.; Marcinkonis, S. Assessment of plant biomass carbon stock in differently renaturalized arable land. Žem. Moksl. 2015, 22, 121–132. (In Lithuanian) [Google Scholar]
- Castaneda, L.E.; Barbosa, O. Metagenomic analysis exploring taxonomic and functional diversity of soil microbial communities in Chilean vineyards and surrounding native forests. PeerJ 2017, 5, e3098. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, L.; Norman, R.; Slaton, N.; Daniels, M. The nitrogen and phosphorous cycle in soils. Agric. Nat. Res. 2013, 2148, 1–4. [Google Scholar]
- Žičkienė, L.; Staugaitis, G.; Mažvila, J.; Masevičienė, A.; Narutytė, I. Mineral nitrogen content fluxes in different texture soils on a rolling to hilly landscape. Žem. Moksl. 2015, 22, 198–208. (In Lithuanian) [Google Scholar]
- Goloran, J.B.; Chen, C.R.; Phillips, I.R. Forms of nitrogen alter plant phosphorus uptake and pathways in rehabilitated highly alkaline bauxite processing residue sand. Land Degrad. Develop. 2017, 28, 628–637. [Google Scholar] [CrossRef]
- Smalstienė, V.; Pranckietienė, I.; Dromantienė, R.; Šidlauskas, G. The influence of different nitrogen forms and application time on winter wheat. Žem. Mok. 2017, 24, 81–90. (In Lithuanian) [Google Scholar] [CrossRef] [Green Version]
- Smith, W.N.; Grant, B.B.; Desjardins, R.L.; Kroebel, R.; Li, C.; Qian, B.; Worth, D.E.; McConkey, B.G.; Drury, C.F. Assessing the effects of climate change on crop production and GHG emissions in Canada. Agric. Ecosyst. Environ. 2013, 179, 139–150. [Google Scholar] [CrossRef]
- Wang, J.; Wang, D.; Zhang, G.; Wang, Y.; Wang, C.; Teng, Y.; Christie, P. Nitrogen and phosphorus leaching losses from intensively managed paddy fields with straw retention. Agric. Water Manag. 2014, 141, 66–73. [Google Scholar] [CrossRef]
- Adomaitis, T.; Mažvila, J.; Vaišvila, Z.; Arbačiauskas, J.; Antanaitis, A.; Lubytė, J.; Šumskis, D. Ilgalaikio tręšimo įtaka anijonų išplovimui. Agriculture 2010, 97, 71–82. (In Lithuanian) [Google Scholar]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gau Chon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EUR-Lex. The Nitrates Directive (91/676/EEC). 1991. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31991L0676&from=EN (accessed on 24 November 2021).
- Kirk, J.L.; Beaudette, L.A.; Hart, M.; Moutoglis, P.; Klironomos, J.N.; Lee, H.; Trevors, J.T. Methods of studying soil microbial diversity. J. Microbiol. Methods 2004, 58, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Jannson, J.K.; Hofmockel, K.S. The soil microbiome—From metagenomics to metaphenomics. Curr. Opin. Microbiol. 2018, 43, 162–168. [Google Scholar] [CrossRef]
- Bučiene, A. On the sustainability of conventional, organic and integrated farming systems. Ecosyst. Health Sustain. Agric. 2012, 1, 42–50. [Google Scholar]
- Grzyb, A.; Wolna-Maruwka, A.; Niewiadomska, A. Environmental factors affecting the mineralization of crop residues. Agronomy 2020, 10, 1951. [Google Scholar] [CrossRef]
- Grzyb, A.; Wolna-Maruwka, A.; Niewiadomska, A. The Significance of Microbial Transformation of Nitrogen Compounds in the Light of Integrated Crop Management. Agronomy 2021, 11, 1415. [Google Scholar] [CrossRef]
- Gautam, A.; Sekaran, U.; Guzman, J.; Kovács, P.; Hernandez, J.L.G.; Kumar, S. Responses of soil microbial community structure and enzymatic activities to long-term application of mineral fertilizer and beef manure. Environ. Sustain. Indic. 2020, 8, 100073. [Google Scholar] [CrossRef]
- Gans, J.; Wolinsky, M.; Dunbar, J.M. Computational improvements reveal great bacterial diversity and high metal toxicity in soil. Science 2005, 309, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Furtak, K.; Gajda, A.M. Activity and Variety of Soil Microorganisms Depending on the Diversity of the Soil Tillage System. In Sustainability of Agroecosystems; Intech Open: London, UK, 2018; pp. 45–61. [Google Scholar]
- Bakšienė, E.; Ražukas, A.; Repečkienė, J.; Titova, J. Influence of different farming systems on the stability of low productivity soil in Southeast Lithuania. Zemdirb.-Agric. 2014, 101, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Piaulokaitė-Motuzienė, L.; Kočius, D. Nitrogen compounds transforming microorganisms succession assessment. Žem. Moksl. 2006, 4, 38–45. (In Lithuanian) [Google Scholar]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. Organic Amendments: Microbial Community Structure, Activity and Abundance of Agriculturally Relevant Microbes Are Driven by Long-Term Fertilization Strategies. Front. Microbiol. 2016, 7, 1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Sha, C.; Wang, M.; Ye, C.; Li, P.; Huang, S. Effect of Organic Fertilizer on Soil Bacteria in Maize Fields. Land 2021, 10, 328. [Google Scholar] [CrossRef]
- Chen, Y.; Xin, L.; Liu, J.; Yuan, M.; Liu, S.; Jiang, W.; Chen, J. Changes in bacterial community of soil induced by long-term straw returning. Sci. Agric. 2016, 74, 349–356. [Google Scholar] [CrossRef]
- Kyllmar, K.; Carlsson, C.; Gustafson, A.; Ulen, B.; Jahnsson, H. Nutrient discharge from small agricultural catchments in Sweden: Characteristics and trends. Agric. Ecosyst. Environ. 2006, 115, 15–26. [Google Scholar] [CrossRef]
- Rutkowska, A.; Fotyma, M. Mineral Nitrogen as a Universal Soil Test to Predict Plant N Requirements and Ground Watter Pollution—Case Study for Poland; Intech Open: London, UK, 2011; pp. 333–350. [Google Scholar]
- Žičkienė, L. Mineral Nitrogen Fluxes in Different Soils. Ph.D. Thesis, Lithuanian Research Centre for Agriculture and Forestry, Dotnuva, Lithuania, 2016; p. 138. [Google Scholar]
- Plošek, L.; Elbl, J.; Lošák, T.; Kužel, S.; Kintl, A.; Juřička, D.; Kynický, J.; Martensson, A.; Brtnický, M. Leaching of mineral nitrogen in the soil influenced by addition of compost and N-mineral fertilizer. Acta Agric. Scand. 2017, 67, 607–614. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Liu, R.; Zhang, A.; Yang, Z. Effect of Nitrate Leaching Caused by Swine Manure Application in Fields of the Yellow River Irrigation Zone of Ningxia, China. Sci. Rep. 2017, 7, 13693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbl, J.; Šimečkova, J.; Škarpa, P.; Kintl, A.; Brtnicky, M.; Vaverkova, M.D. Comparison of the Agricultural Use of Products from OrganicWaste Processing with Conventional Mineral Fertilizer: Potential Effects on Mineral Nitrogen Leaching and Soil Quality. Agronomy 2020, 10, 226. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; He, T.; Cao, T.; Yang, T.; Meng, J.; Chen, W. Effects of biochar application on nitrogen leaching, ammonia volatilization and nitrogen use efficiency in two distinct soils. J. Soil Sci. Plant Nutr. 2017, 17, 515–528. [Google Scholar] [CrossRef] [Green Version]
- Schlegel, A.J.; Assefa, Y.; Bond, H.D.; Haag, L.A.; Stone, L.R. Changes in soil nutrients after 10 years of cattle manure and swine effluent application. Soil Tillage Res. 2017, 172, 48–58. [Google Scholar] [CrossRef]
- Mažeika, R.; Arbačiauskas, J.; Masevičienė, A.; Narutytė, I.; Šumskis, D.; Žičkienė, L.; Rainys, K.; Drapanauskaite, D.; Staugaitis, G.; Baltrusaitis, J. Nutrient Dynamics and Plant Response in Soil to Organic Chicken Manure Based Fertilizers. Waste Biomass Valoriz. 2021, 12, 371–382. [Google Scholar] [CrossRef]
- Ojo, J.A.; Olowoake, A.A.; Obembe, A. Efficacy of organomineral fertilizer and un-amended compost on the growth and yield of watermelon (Citrullus lanatus Thumb) in Ilorin Southern Guinea Savanna zone of Nigeria. Int. J. Recycl. Org. Waste Agric. 2014, 3, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Corrêa, J.C.; Grohskopf, M.A.; Nicoloso, R.D.S.; Lourenço, K.S.; Martini, R. Organic, organomineral, and mineral fertilizers with urease and nitrification inhibitors for wheat and corn under notillage. Pesqui. Agropecu. Bras. 2016, 51, 916–924. [Google Scholar] [CrossRef] [Green Version]
- Davet, P.; Rouxel, F. Detection and Isolation of Soil Fungi; Science Publisher: Hauppauge, NY, USA, 2000. [Google Scholar]
- Kuster, E. Outline of a comparative study of criteria used in characterization of the actinomycetes. J. Syst. Evol. Microbiol. 1959, 9, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Aquilanti, L.; Favilli, F.; Clemeti, F. Comparison of different strategies for isolation and preliminary identification of Azotobacter from soil samples. Soil Biol. Biochem. 2004, 36, 1475–1483. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis; CRC Press: Boca Raton, FL, USA, 2007; p. 1224. [Google Scholar]
- Nelson, P.E.; Toussoun, T.A.; Marasas, W.F.O. Fusarium Species: An Illustrated Manual for Identification; Penn State University Press: University Park, PA, USA, 1990; p. 206. [Google Scholar]
- Watanabe, T. Pictorial Atlas of Soil and Seed Fungi/Morphologies of Cultured Fungi and Key to Species; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Domsch, K.H.; Gams, W.; Anderson, T.H. Compendium of Soil Fungi; IHW-Verlag: Eching, Germany, 2007; p. 672. [Google Scholar]
- Samson, R.A.; Visagie, C.M.; Houbraken, J.; Hong, S.B.; Hubka, V.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Susca, A.; Tanney, J.B.; et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 2014, 78, 141–173. [Google Scholar] [CrossRef] [Green Version]
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Hong, S.B.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Varga, J.; Yaguchi, T.; Samsom, R.A. Identification and nomenclature of the genus Penicillium. Stud. Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef] [Green Version]
- Salina, O. Micromycetes of Trichoderma sect. Longibrachiatum in Lithuania. Bot. Lith. 2007, 13, 261–269. [Google Scholar]
- Egnér, H.; Riehm, H.; Domingo, W.R. Investigations on soil chemical analysis as a basis of the evaluation of plant nutrient status of soils II. Chemical extraction methods for phosphorus and potassium determination. Lantbrukshögsk. Ann. 1960, 26, 199–215. [Google Scholar]
- Association of Analytical Chemists International (AOAC). AOAC Official Method 973.57 Sulfate in Water. Turbidimetric Method; Scientific Research: Atlanta, GA, USA, 2000; pp. 23–24. [Google Scholar]




| Fertilization Variants | Mineral Nitrogen (Nmin) Concentration 0–60 cm Layer mg kg−1 ± Standard Deviation (SD) | |
|---|---|---|
| 2019 Spring | 2020 Spring | |
| C | 6.92 ± 0.41 | 1.29 ± 0.33 |
| MF | 6.71 ± 1.26 | 1.60 ± 0.38 |
| PLM170 | 13.13 ± 1.13 | 1.78 ± 0.40 |
| GPM85 | 12.50 ± 0.46 | 1.64 ± 0.27 |
| GPM170 | 13.73 ± 1.69 | 2.09 ± 0.47 |
| GPM170 + A | 15.68 ± 0.56 | 2.31 ± 0.40 |
| GPM170 + T | 13.96 ± 1.57 | 1.80 ± 0.26 |
| CLM170 | 7.98 ± 1.29 | 1.90 ± 0.30 |
| GCM85 | 7.66 ± 0.11 | 1.61 ± 0.39 |
| GCM170 | 9.13 ± 1.53 | 1.88 ± 0.42 |
| GCM170 + A | 11.57 ± 2.74 | 1.99 ± 0.42 |
| GCM170 + T | 9.95 ± 1.71 | 1.77 ± 0.26 |
| GPM85 + MF | 13.42 ± 0.78 | 2.02 ± 0.29 |
| GCM85 + MF | 9.51 ± 0.37 | 1.84 ± 0.25 |
| Fertilization Variants | Clean, 15% Humidity Grain Yield t/ha ± Standard Deviation (SD) | Grain-Straw Ratio | Straw Yield t/ha ± Standard Deviation (SD) | 1000 Grain Weight g ± Standard Deviation (SD) |
|---|---|---|---|---|
| C | 1.20 ± 0.04 | 1:1.01 | 1.21 ± 0.13 | 38.2 ± 1.68 |
| MF | 2.72 ± 0.20 | 1:0.98 | 2.67 ± 0.47 | 46.4 ± 1.34 |
| PLM170 | 1.49 ± 0.30 | 1:0.90 | 1.33 ± 0.19 | 41.0 ± 0.38 |
| GPM85 | 1.30 ± 0.35 | 1:0.99 | 1.30 ± 0.40 | 38.1 ± 1.98 |
| GPM170 | 1.26 ± 0.11 | 1:0.98 | 1.23 ± 0.12 | 39.7 ± 1.61 |
| GPM170 + A | 1.22 ± 0.13 | 1:0.95 | 1.15 ± 0.09 | 40.5 ± 1.41 |
| GPM170 + T | 1.31 ± 0.14 | 1:0.95 | 1.24 ± 0.12 | 39.3 ± 0.52 |
| CLM170 | 1.48 ± 0.08 | 1:0.89 | 1.31 ± 0.11 | 39.7 ± 2.06 |
| GCM85 | 1.10 ± 0.04 | 1:1.03 | 1.13 ± 0.06 | 38.5 ± 0.86 |
| GCM170 | 1.21 ± 0.04 | 1:1.06 | 1.28 ± 0.16 | 37.7 ± 2.43 |
| GCM170 + A | 1.14 ± 0.02 | 1:1.04 | 1.18 ± 0.07 | 37.9 ± 2.12 |
| GCM170 + T | 1.08 ± 0.03 | 1:1.03 | 1.12 ± 0.05 | 37.7 ± 1.48 |
| GPM85 + MF | 3.15 ± 0.02 | 1:0.97 | 3.04 ± 0.32 | 46.7 ± 0.94 |
| GCM85 + MF | 3.00 ± 0.02 | 1:0.99 | 2.96 ± 0.41 | 45.9 ± 0.58 |
| Abbreviation | Explanation |
|---|---|
| C | control (without fertilizer—N0P0P0) |
| MF | mineral fertilizers (N90/60 *) |
| PLM170 | poultry litter manure (N170) |
| GPM85 | granulated poultry manure (N85) |
| GPM170 | granulated poultry manure (N170) |
| GPM170 + A | granulated poultry manure (N170) + biological substance No 1 (Azotobacter spp.) |
| GPM170 + T | granulated poultry manure (N170) + biological substance No 2 (Trichoderma spp.) |
| CLM170 | peat cattle litter (N170) |
| GCM85 | granulated cattle manure (N85) |
| GCM170 | granulated cattle manure (N170) |
| GCM170 + A | granulated cattle manure (N170) + biological substance No. 1 (Azotobacter spp.) |
| GCM170 + T | granulated cattle manure (N170) + biological substance No. 2 (Trichoderma spp.) |
| GPM85 + MF | granulated poultry manure (N85) + mineral fertilizers (N90/60 *) |
| GCM85 + MF | granulated cattle manure (N85) + mineral fertilizers (N90/60 *) |
| Indicators | Investigation Method | Sampling Time and Frequency, Depth cm | Sources |
|---|---|---|---|
| pH | 1 M KCl extraction by potentiometric method | Samples (pH, mobile P2O5, mobile K2O) are taken in the fall before the experiment is set up (2018) and after the experiment is completed (2023). Sampling depth—0–20 cm. | ISO 10390:2005 |
| Mobile phosphorus (P2O5) | By Egner–Riehm–Domingo (A–L) method in a buffer solution (pH 3.7) extraction (spectrophotometer) | [67] | |
| Mobile potassium (K2O) | By Egner–Riehm–Domingo (A–L) method in a buffer solution (pH 3.7) extraction (lame emission spectrometer) | [67] | |
| Mineral nitrogen (Nmin) (N–NO3 + N–NH4) | Determined in air-dry samples, 1 M KCl extraction, by flow analysis | Samples (Nmin and Smin) are taken every spring after the regeneration of plant vegetation before fertilization with mineral fertilizers. Sampling depth 0–30 and 30–60 cm. | ISO 14256-2:2005 |
| Mineral sulphur (Smin) (S-SO4) | Determined in 1 M KCl extraction by turbidimetric method AOAC-OM-973.57 | [68] |
| 2019 | ||||||
| April | May | June | July | August | September | |
| Precipitation, mm | 1 | 29 | 28 | 50 | 100 | 47 |
| Daily air temperature average, °C | 9.0 | 13.3 | 21.1 | 17.1 | 17.5 | 12.6 |
| 2020 | ||||||
| April | May | June | July | August | September | |
| Precipitation, mm | 6 | 78 | 68 | 67 | 78 | 14 |
| Daily air temperature average, °C | 6.6 | 10.3 | 19.4 | 17.6 | 18.4 | 14.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivojiene, D.; Kacergius, A.; Baksiene, E.; Maseviciene, A.; Zickiene, L. The Influence of Organic Fertilizers on the Abundance of Soil Microorganism Communities, Agrochemical Indicators, and Yield in East Lithuanian Light Soils. Plants 2021, 10, 2648. https://doi.org/10.3390/plants10122648
Sivojiene D, Kacergius A, Baksiene E, Maseviciene A, Zickiene L. The Influence of Organic Fertilizers on the Abundance of Soil Microorganism Communities, Agrochemical Indicators, and Yield in East Lithuanian Light Soils. Plants. 2021; 10(12):2648. https://doi.org/10.3390/plants10122648
Chicago/Turabian StyleSivojiene, Diana, Audrius Kacergius, Eugenija Baksiene, Aiste Maseviciene, and Lina Zickiene. 2021. "The Influence of Organic Fertilizers on the Abundance of Soil Microorganism Communities, Agrochemical Indicators, and Yield in East Lithuanian Light Soils" Plants 10, no. 12: 2648. https://doi.org/10.3390/plants10122648