Vitrification Solutions for Plant Cryopreservation: Modification and Properties
Abstract
:1. Introduction
2. Cryoprotective Substances
2.1. Substances That Can Penetrate through the Cell Wall and into the Protoplast
2.1.1. Glycerol
| Substances | Abr. | Mr | Tm | Tg | Density | LD50 | |
|---|---|---|---|---|---|---|---|
| g mol−1 | °C | °C | g cm−3 | ||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Sulfoxides | |||||||
| Dimethyl sulfoxide [48] | DMSO | 78.13 | 18.45 | −132.15 | 1.10 | ** | |
| Diols | |||||||
| Ethylene glycol [49] | EG | 62.07 | −13 | −113.15 | 1.11 | * | |
| Propylene glycol [50] | PG | 76.06 | −59 | −100.65 | 1.4 | ** | |
| PEG 8000 [51] | PEG | 8000 | 63 | 54.82 | 1.21 | *** | |
| Triols | |||||||
| Glycerol [52] | Gly | 92.09 | 19 | −83.15 | 1.26 | * | |
| Polyalcohols | |||||||
| Sorbitol [53] | Sor | 182.17 | 111.5 | −6 | 1.49 | ** | |
| Monosaccharides | |||||||
| Glucose [54] | Glu | 164.16 | 147 | 22.85 | 1.4 | * | |
| Disaccharides | |||||||
| Sucrose [54] | Suc | 342.3 | 186 | 59.85 | 1.587 | *** | |
| Proteins | |||||||
| Bovine serum albumin [55] | BSA | 66.5 kDa | 69.8 | § | - | ||
| Amide | |||||||
| Formamide [56] | 45.04 | 2.55 | - | 1.13 | * | ||
| Plant Vitrification Solutions | |||||||
| Plant vitrification solution 1 [46] | PVS1 | 42.48 | −41 | −122 | 1.15 | - | |
| Plant vitrification solution 1 [57] | PVS1 | −155 | |||||
| Plant vitrification solution 2 [46] | PVS2 | 37.51 | −44 | −119 | 1.14 | - | |
| Plant vitrification solution 2 [58] | PVS2 | −115 | |||||
| Plant vitrification solution 3 [47] | PVS3 | 56.29 | −35.4 | −93.9 | 1.29 | - | |
| Plant vitrification solution 4 [59] | PVS4 | 73.53 | −33.9 | −112 | 1.31 | - | |
| Plant vitrification solution N [58] | PVSN | - | −50 | −110 | - | - | |
| Vitrification solution L [46] | VSL | 32.97 | −41 | −125 | 1.9 | - | |
| Vitrification solution L [46] | VSL+ | - | −47 | −121 | - | - | |
2.1.2. Dimethyl Sulfoxide
2.1.3. Ethylene Glycol
2.1.4. Propylene Glycol
2.1.5. Polyethylene Glycol
2.2. Substances That Can Penetrate through the Cell Wall
2.2.1. Glucose
2.2.2. Sorbitol
2.2.3. Sucrose
2.2.4. Amides
2.2.5. Bovine Serum Albumin (BSA)
3. Substances That Do Not Penetrate through the Cell Wall
4. Vitrification Solutions and Modifications
| PVS2 | DMSO (%) | Suc (%) | Gly (%) | EG (%) | PG (%) | PEG (%) | Sor (%) | Total (%) | Plant |
|---|---|---|---|---|---|---|---|---|---|
| Sakai § | 15 | 13.7 | 30 | 15 | 73.7 | Citrus sinensis [13] | |||
| PVS2-M1 | 13.7 | 30 | 15 | 15 | 73.7 | Porphyra yezoensis [94] | |||
| PVS2-M2 | 7.5 | 13.7 | 30 | 15 | 7.5 | 73.7 | Porphyra yezoensis [94] | ||
| PVS2-M3 | 12.5 | 13.7 | 25 | 15 | 3 * | 69.2 | Prunus salicina Lindley cv. Methley x Prunus spinosa L. [115] | ||
| PVS2-M4 | 15 | 30 | 15 | 60 | Malus [96] | ||||
| PVS2-M5 | 15 | 30 | 15 | 15 | 75 | Tetraclinis articulata (Vahl.) [116] | |||
| PVS2-M6 | 13 | 15 | 25 | 15 | 2 | 70 | Guazuma crinita Mart. [117] | ||
| PVS2-M7 | 15 | 34.2 | 30 | 15 | 94.2 | Poncirus trifoliata (L.) Raf. × Citrus sinensis (L.) Osbeck. [118] | |||
| PVS2-M8 §§ | 15 | 22.5 | 37.5 | 15 | 52.5 | Allium sativum L. and Dendranthema grandiflora [61], Rubus fruticosus L. and Prunus cerasifera Ehrh [119] | |||
| PVS2-M9 §§§ | 15 | 15 | 30 | 15 | 75 | Guazuma crinita Mart. [13] | |||
| PVS2-M10 | 15 | 13.7 | 30 | 15 | 3 ** | 76.7 | Populus alba L. [120] |
| PVS3 | DMSO (%) | Suc (%) | Gly (%) | EG (%) | Total (%) | Plant |
|---|---|---|---|---|---|---|
| Nishizawa | 50 | 50 | 100 | Asparagus officinalis L. [47] | ||
| PVS3-M1 | 5 | 50 | 50 | 100 | Porphyra yezoensis [94] | |
| PVS3-M2 | 50 | 30 | 80 | Malus domestica Borkh. [69] | ||
| PVS3-M3 | 45 | 45 | 90 | Kalopanax septemlobus [121] | ||
| PVS3-M4 | 40 | 40 | 80 | Gentian, Wasabi, Malus [22,122] Fragaria ananassa [123] | ||
| PVS3-M5 | 60 | 35 | 20 | 105 | Asparagus officinalis L. [47] |
5. Comparison of Vitrification Solutions on Regeneration
6. Vitrification Solution and Cryopreservation Methods
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Engelmann, F. Use of biotechnologies for the conservation of plant biodiversity. Vitr. Cell. Dev. Biol. Anim. 2011, 47, 5–16. [Google Scholar] [CrossRef]
- Wang, M.-R.; Bi, W.; Shukla, M.R.; Ren, L.; Hamborg, Z.; Blystad, D.-R.; Saxena, P.K.; Wang, Q.-C. Epigenetic and Genetic Integrity, Metabolic Stability, and Field Performance of Cryopreserved Plants. Plants 2021, 10, 1889. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, F. In vitro conservation methods. In Biotechnology and Plant Genetic Resources; Callow, J.A., Ford Lloyd, B.V., Newbury, H.J., Eds.; CAB International: Oxford, UK, 1997; pp. 119–162. [Google Scholar]
- Wang, M.-R.; Chen, L.; Da Silva, J.A.T.; Volk, G.M.; Wang, Q.-C. Cryobiotechnology of apple (Malus spp.): Development, progress and future prospects. Plant Cell Rep. 2018, 37, 689–709. [Google Scholar] [CrossRef]
- Panis, B. Sixty years of plant cryopreservation: From freezing hardy mulberry twigs to establishing reference crop collections for future generations. Acta Hortic. 2019, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zámečník, J.; Šesták, J. Constrained States Occurring in Plants Cryo-Processing and the Role of Biological Glasses. In Hot Topics in Thermal Analysis and Calorimetry; Springer: Singapore, 2010; Volume 8, pp. 291–310. [Google Scholar]
- Benson, E.E. Cryopreservation of Phytodiversity: A Critical Appraisal of Theory & Practice. Crit. Rev. Plant Sci. 2008, 27, 141–219. [Google Scholar] [CrossRef]
- Hirsh, A.G. Vitrification in plants as a natural form of cryoprotection. Cryobiology 1987, 24, 214–228. [Google Scholar] [CrossRef]
- Volk, G.M.; Walters, C. Plant vitrification solution 2 lowers water content and alters freezing behavior in shoot tips during cryoprotection. Cryobiology 2006, 52, 48–61. [Google Scholar] [CrossRef]
- Grout, B.W.W. Introduction to the in Vitro Preservation of Plant Cells, Tissues and Organs. In Genetic Preservation of Plant Cells in Vitro; Springer: Singapore, 1995; pp. 1–20. [Google Scholar]
- Benson, E.E. Cryopreservation theory. In Plant Cryopreservation: A Practical Guide; Springer: New York, NY, USA, 2008; pp. 15–32. [Google Scholar]
- Benson, E.E. Cryopreservation. In Plant Conservation Biotechnology; CRC Press: Boca Raton, FL, USA, 1999; pp. 109–122. [Google Scholar]
- Sakai, A.; Kobayashi, S.; Oiyama, I. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep. 1990, 9, 30–33. [Google Scholar] [CrossRef]
- Uragami, A.; Sakai, A.; Nagai, M.; Takahashi, T. Survival of cultured cells and somatic embryos of Asparagus officinalis cryopreserved by vitrification. Plant Cell Rep. 1989, 8, 418–421. [Google Scholar] [CrossRef]
- Leunufna, S.; Keller, E.R.J. Investigating a new cryopreservation protocol for yams (Dioscorea spp.). Plant Cell Rep. 2003, 21, 1159–1166. [Google Scholar] [CrossRef]
- Jiroutová, P.; Sedlák, J. Cryobiotechnology of Plants: A Hot Topic not Only for Gene Banks. Appl. Sci. 2020, 10, 4677. [Google Scholar] [CrossRef]
- Roque-Borda, C.; Kulus, D.; de Souza, A.V.; Kaviani, B.; Vicente, E. Cryopreservation of Agronomic Plant Germplasm Using Vitrification-Based Methods: An Overview of Selected Case Studies. Int. J. Mol. Sci. 2021, 22, 6157. [Google Scholar] [CrossRef]
- Agrawal, A.; Singh, S.; Malhotra, E.V.; Meena, D.P.S.; Tyagi, R.K. In Vitro Conservation and Cryopreservation of Clonally Propagated Horticultural Species. In Conservation and Utilization of Horticultural Genetic Resources; Rajasekharan, P., Rao, V., Eds.; Springer: New York, NY, USA, 2019; pp. 529–578. [Google Scholar]
- Bettoni, J.C.; Bonnart, R.; Volk, G.M. Challenges in implementing plant shoot tip cryopreservation technologies. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 144, 21–34. [Google Scholar] [CrossRef]
- Malik, S.K.; Chaudhury, R. Cryopreservation Techniques for Conservation of Tropical Horticultural Species Using Various Explants. In Conservation and Utilization of Horticultural Genetic Resources; Springer: Singapore, 2019; pp. 579–594. [Google Scholar]
- Panis, B.; Lambardi, M. Status of cryopreservation technologies in plants (crops and forest trees). Role Biotechnol. 2005, 5, 43–54. [Google Scholar]
- Sakai, P.A.; Hirai, D.; Niino, T. Development of PVS-Based Vitrification and Encapsulation–Vitrification Protocols. In Plant Cryopreservation: A Practical Guide; Springer: Singapore, 2008; pp. 33–57. [Google Scholar]
- Höfer, M.; Hanke, M.-V. Cryopreservation of fruit germplasm. Vitr. Cell. Dev. Biol. Anim. 2017, 53, 372–381. [Google Scholar] [CrossRef]
- Kulus, D.; Zalewska, M. Cryopreservation as a tool used in long-term storage of ornamental species—A review. Sci. Hortic. 2014, 168, 88–107. [Google Scholar] [CrossRef]
- Bi, W.-L.; Pan, C.; Hao, X.-Y.; Cui, Z.-H.; Kher, M.M.; Marković, Z.; Wang, Q.-C.; da Silva, J.A.T. Cryopreservation of grapevine (Vitis spp.)—A review. In Vitro Cell. Dev. Biol. Plant 2017, 53, 449–460. [Google Scholar] [CrossRef]
- Yamamoto, S.; Rafique, T.; Fukui, K.; Sekizawa, K.; Niino, T. V-cryo-plate procedure as an effective protocol for cryobanks: Case study of mint cryopreservation. Cryo Lett. 2012, 33, 12–23. [Google Scholar]
- Yamamoto, S.-I.; Rafique, T.; Priyantha, W.S.; Fukui, K.; Matsumoto, T.; Niino, T. Development of a cryopreservation procedure using aluminium cryo-plates. Cryo Lett. 2011, 32, 256–265. [Google Scholar]
- Kim, H.H.; Yoon, J.W.; Park, Y.E.; Cho, E.G.; Sohn, J.K.; Kim, T.K.; Engelmann, F. Cryopreservation of potato cultivated varieties and wild species: Critical factors in droplet vitrification. Cryo Lett. 2006, 27, 223–234. [Google Scholar]
- Panis, B.; Nguyẽn, T.n.T. Cryopreservation of Musa Germplasm; Bioversity International: Rome, Italy, 2001; Volume 5. [Google Scholar]
- Carra, A.; Carimi, F.; Bettoni, J.C.; Pathirana, R. Progress and Challenges in the Application of Synthetic Seed Technology for Ex Situ Germplasm Conservation in Grapevine (Vitis spp.). In Synthetic Seeds; Springer: Singapore, 2019; pp. 439–467. [Google Scholar]
- Fahy, G.M.; Wowk, B.; Wu, J.; Paynter, S. Improved vitrification solutions based on the predictability of vitrification solution toxicity. Cryobiology 2004, 48, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.S.; González-Benito, M.E.; Molina-García, A.D. Glassy State and Cryopreservation of Mint Shoot Tips. Biotechnol. Prog. 2013, 29, 707–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zámečník, J.; Faltus, M.; Bilavčík, A.; Kotková, R. Comparison of cryopreservation methods of vegetatively propagated crops based on thermal analysis. In Current Frontiers Cryopreservation; IntechOpen: London, UK, 2012; pp. 333–358. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Santarius, K.A. Freezing of Isolated Thylakoid Membranes in Complex Media. VII. The Effect of Bovine Serum Albumin. Biochem. Physiol. Pflanz. 1991, 187, 149–162. [Google Scholar] [CrossRef]
- Elmoazzen, H.; Elliott, J.; McGann, L. Cryoprotectant equilibration in tissues. Cryobiology 2005, 51, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Kanaze, F.I.; Kokkalou, E.; Niopas, I.; Georgarakis, M.; Stergiou, A.; Bikiaris, D. Thermal analysis study of flavonoid solid dispersions having enhanced solubility. J. Therm. Anal. Calorim. 2006, 83, 283–290. [Google Scholar] [CrossRef]
- Tao, D.; Li, P.H. Classification of plant cell cryoprotectants. J. Theor. Biol. 1986, 123, 305–310. [Google Scholar] [CrossRef]
- Rall, W.F.; Fahy, G.M. Ice-free cryopreservation of mouse embryos at −196 °C by vitrification. Nat. Cell Biol. 1985, 313, 573–575. [Google Scholar] [CrossRef]
- Gao, D.; Liu, J.; Liu, C.; McGann, L.; Watson, P.; Kleinhans, F.; Mazur, P.; Critser, E.; Critser, J. Andrology: Prevention of osmotic injury to human spermatozoa during addition and removal of glycerol. Hum. Reprod. 1995, 10, 1109–1122. [Google Scholar] [CrossRef]
- Hubálek, Z. Protectants used in the cryopreservation of microorganisms. Cryobiology 2003, 46, 205–229. [Google Scholar] [CrossRef]
- Golan, M.; Jelinkova, S.; Kratochvilova, I.; Skládal, P.; Pešl, M.; Rotrekl, V.; Pribyl, J. AFM Monitoring the Influence of Selected Cryoprotectants on Regeneration of Cryopreserved Cells Mechanical Properties. Front. Physiol. 2018, 9, 804. [Google Scholar] [CrossRef]
- Gerber, D.W.; Byerrum, R.U.; Gee, R.W.; Tolbert, N. Glycerol concentrations in crop plants. Plant Sci. 1988, 56, 31–38. [Google Scholar] [CrossRef]
- Sillanpää, M.; Ncibi, C. Biochemicals. In A Sustainable Bioeconomy; Springer: Berlin/Heidelberg, Germany, 2017; pp. 141–183. [Google Scholar]
- Warner, R.M.; Ampo, E.; Nelson, D.; Benson, J.D.; Eroglu, A.; Higgins, A.Z. Rapid quantification of multi-cryoprotectant toxicity using an automated liquid handling method. Cryobiology 2021, 98, 219–232. [Google Scholar] [CrossRef]
- Suzuki, M.; Tandon, P.; Ishikawa, M.; Toyomasu, T. Development of a new vitrification solution, VSL, and its application to the cryopreservation of gentian axillary buds. Plant Biotechnol. Rep. 2008, 2, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Nishizawa, S.; Sakai, A.; Amano, Y.; Matsuzawa, T. Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci. 1993, 91, 67–73. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, C.T. A new approach to understanding and measuring glass formation in bulk amorphous materials. Intermetallics 2004, 12, 1035–1043. [Google Scholar] [CrossRef]
- Kuleshova, L.; Mac Farlaneb, D.R.; Trounson, A.; Shaw, J. Sugars Exert a Major Influence on the Vitrification Properties of Ethylene Glycol-Based Solutions and Have Low Toxicity to Embryos and Oocytes. Cryobiology 1999, 38, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.; Singh, G. Examination of the concentration dependence of Tg of binary aqueous solutions. Thermochim. Acta 2008, 469, 116–119. [Google Scholar] [CrossRef]
- Jonnalagadda, S.; Robinson, D.H. Effect of the inclusion of PEG on the solid-state properties and drug release from polylactic acid films and microcapsules. J. Appl. Polym. Sci. 2004, 93, 2025–2030. [Google Scholar] [CrossRef]
- Zondervan, R.; Kulzer, F.; Berkhout, G.C.G.; Orrit, M. Local viscosity of supercooled glycerol near Tg probed by rotational diffusion of ensembles and single dye molecules. Proc. Natl. Acad. Sci. USA 2007, 104, 12628–12633. [Google Scholar] [CrossRef] [Green Version]
- Talja, R.A.; Roos, Y.H. Phase and state transition effects on dielectric, mechanical, and thermal properties of polyols. Thermochim. Acta 2001, 380, 109–121. [Google Scholar] [CrossRef]
- Simperler, A.; Kornherr, A.; Chopra, R.; Bonnet, P.A.; Jones, W.; Motherwell, A.W.D.S.; Zifferer, G. Glass Transition Temperature of Glucose, Sucrose, and Trehalose: An Experimental and in Silico Study. J. Phys. Chem. B 2006, 110, 19678–19684. [Google Scholar] [CrossRef]
- Roberts, A.; Finnigan, W.; Kelly, P.; Faulkner, M.; Breitling, R.; Takano, E.; Scrutton, N.; Blaker, J.; Hay, S. Non-covalent protein-based adhesives for transparent substrates—Bovine serum albumin vs. recombinant spider silk. Mater. Today Bio 2020, 7, 100068. [Google Scholar] [CrossRef]
- Anonym. Data Safety Sheet—Formamide. 2015. Available online: https://www.carlroth.com/medias/SDB-4095-IE-EN.pdf?context=bWFzdGVyfHNlY3VyaXR5RGF0YXNoZWV0c3wyNjc1NzV8YXBwbGljYXRpb24vcGRmfHNlY3VyaXR5RGF0YXNoZWV0cy9oNzIvaDc2LzkwNDYwODU3MzAzMzQucGRmfDUyOGVmNzI4NzM3MmU1NDQwYmIzZDYwODI5OTYxNDU2NmZhOWJlNTVkMzVlOWRlZTk2NjQyNjNkYzliMzI0OTk (accessed on 27 November 2021).
- Matsumoto, T. Cryopreservation of Plant Genetic Resources: Conventional and New Methods. Rev. Agric. Sci. 2017, 5, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Vozovyk, K.; Bobrova, O.; Prystalov, A.; Shevchenko, N.; Kuleshova, L. Amorphous state stability of plant vitrification solutions. Biologija 2020, 66, 66. [Google Scholar] [CrossRef]
- Matsumoto, T.; Sakai, A.; Yamada, K. Cryopreservation of in vitro-grown apical meristems of wasabi (Wasabia japonica) by vitrification and subsequent high plant regeneration. Plant Cell Rep. 1994, 13, 442–446. [Google Scholar] [CrossRef]
- Kawai, K.; Suzuki, T.; Oguni, M. Low-Temperature Glass Transitions of Quenched and Annealed Bovine Serum Albumin Aqueous Solutions. Biophys. J. 2006, 90, 3732–3738. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-H.; Lee, Y.-G.; Shin, D.-J.; Ko, H.-C.; Gwag, J.-G.; Cho, E.-G.; Engelmann, F. Development of alternative plant vitrification solutions in droplet-vitrification procedures. Cryo Lett. 2009, 30, 320–334. [Google Scholar] [CrossRef]
- Notman, R.; Noro, M.; O’Malley, B.; Anwar, J. Molecular Basis for Dimethylsulfoxide (DMSO) Action on Lipid Membranes. J. Am. Chem. Soc. 2006, 128, 13982–13983. [Google Scholar] [CrossRef]
- Kim, H.-H.; Kim, J.-B.; Baek, H.-J.; Cho, E.-G.; Chae, Y.-A.; Engelmann, F. Evolution of DMSO concentration in garlic shoot tips during a vitrification procedure. Cryo Lett. 2004, 25, 91–100. [Google Scholar]
- Kim, J.-B.; Kim, H.-H.; Baek, H.-J.; Cho, E.-G.; Kim, Y.-H.; Engelmann, F. Changes in sucrose and glycerol content in garlic shoot tips during freezing using PVS3 solution. Cryo Lett. 2005, 26, 103–112. [Google Scholar]
- Volk, G.M.; Harris, J.L.; Rotindo, K.E. Survival of mint shoot tips after exposure to cryoprotectant solution components. Cryobiology 2006, 52, 305–308. [Google Scholar] [CrossRef]
- Hakura, A.; Mochida, H.; Yamatsu, K. Dimethyl sulfoxide (DMSO) is mutagenic for bacterial mutagenicity tester strains. Mutat. Res. Lett. 1993, 303, 127–133. [Google Scholar] [CrossRef]
- Kapp, R., Jr.; Eventoff, B. Mutagenicity of dimethylsulfoxide (DMSO): In vivo cytogenetics study in the rat. Teratog. Carcinog. Mutagenesis 1981, 1, 141–145. [Google Scholar] [CrossRef]
- Vogin, E.E.; Carson, S.; Cannon, G.; Linegar, C.R.; Rubin, L.F. Chronic toxicity of DMSO in primates. Toxicol. Appl. Pharmacol. 1970, 16, 606–612. [Google Scholar] [CrossRef]
- Halmagyi, A.; Valimareanu, S.; Coste, A.; Deliu, C.; Isac, V. Cryopreservation of Malus shoot tips and subsequent plant regeneration. Rom. Biotechnol. Lett. 2010, 15, 80. [Google Scholar]
- Volk, G. Application of Functional Genomics and Proteomics to Plant Cryopreservation. Curr. Genom. 2010, 11, 24–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, V.R.; Horner, H.T. Calcium oxalate crystals in plants. Bot. Rev. 1980, 46, 361–427. [Google Scholar] [CrossRef]
- Prychid, C.J.; Jabaily, R.S.; Rudall, P. Cellular Ultrastructure and Crystal Development in Amorphophallus (Araceae). Ann. Bot. 2008, 101, 983–995. [Google Scholar] [CrossRef]
- Ranjbar, H.; Ahmadi, H.; Sheshdeh, R.K.; Ranjbar, H. Application of relative sensitivity function in parametric optimization of a tri-ethylene glycol dehydration plant. J. Nat. Gas Sci. Eng. 2015, 25, 39–45. [Google Scholar] [CrossRef]
- Bhattacharya, S. Cryoprotectants and their usage in cryopreservation process. In Cryopreservation Biotechnology in Biomedical and Biological Sciences; IntechOpen: London, UK, 2018; p. 7. [Google Scholar]
- Steuter, A.A.; Mozafar, A.; Goodin, J.R. Water Potential of Aqueous Polyethylene Glycol. Plant Physiol. 1981, 67, 64–67. [Google Scholar] [CrossRef] [Green Version]
- Popova, E.; Bukhov, N.; Popov, A.; Kim, H.-H. Cryopreservation of protocorm-like bodies of the hybrid orchid Bratonia (Miltonia flavescens × Brassia longissima). Cryo Lett. 2010, 31, 426–437. [Google Scholar]
- Fuller, B.J. Cryoprotectants: The essential antifreezes to protect life in the frozen state. Cryo Lett. 2004, 25, 375–388. [Google Scholar]
- Sipen, P.; Anthony, P.; Davey, M.R. Cryopreservation of scalps of Malaysian bananas using a pre-growth method. Cryo Lett. 2011, 32, 197–205. [Google Scholar]
- Acker, J.P.; McGann, L.E. Protective effect of intracellular ice during freezing? Cryobiology 2003, 46, 197–202. [Google Scholar] [CrossRef]
- Bryant, G.; Koster, K.L.; Wolfe, J. Membrane behaviour in seeds and other systems at low water content: The various effects of solutes. Seed Sci. Res. 2001, 11, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Shendurse, A.; Khedkar, C. Glucose: Properties and analysis. Encycl. Food Health 2016, 3, 239–247. [Google Scholar]
- Bhandari, B.R.; Roos, Y.H. Dissolution of sucrose crystals in the anhydrous sorbitol melt. Carbohydr. Res. 2003, 338, 361–367. [Google Scholar] [CrossRef]
- Göldner, E.M.; Seitz, U.; Reinhard, E. Cryopreservation of Digitalis lanata Ehrh. cell cultures: Preculture and freeze tolerance. Plant Cell Tissue Organ Cult. 1991, 24, 19–24. [Google Scholar] [CrossRef]
- Salaj, T.; Matusikova, I.; Panis, B.; Swennen, R.; Salaj, J. Recovery and characterisation of hybrid firs (Abies alba × A. cephalonica, Abies alba × A. numidica) embryogenic tissues after cryopreservation. Cryo Lett. 2010, 31, 206–217. [Google Scholar]
- Subramanian, S.; Raj, A.; Kumar, R.; Rana, S.K.; Jha, A.K.; Gautam, S. Isolation, Culturing and cryopreservation of putative granulosa stem cells from buffalo ovaries. Int. J. Cell Sci. Biotechnol. 2014, 4, 20–25. [Google Scholar]
- Carpenter, J.F.; Crowe, J.H. The mechanism of cryoprotection of proteins by solutes. Cryobiology 1988, 25, 244–255. [Google Scholar] [CrossRef]
- Santarius, K.A.; Giersch, C. Cryopreservation of spinach chloroplast membranes by low-molecular-weight carbohydrates: II. Discrimination between colligative and noncolligative protection. Cryobiology 1983, 20, 90–99. [Google Scholar] [CrossRef]
- Sikora, A.; Dupanov, V.O.; Kratochvíl, J.; Zamecnik, J. Transitions in Aqueous Solutions of Sucrose at Subzero Temperatures. J. Macromol. Sci. Part B 2007, 46, 71–85. [Google Scholar] [CrossRef]
- Sakai, A.; Kobayashi, S.; Oiyama, I. Survival by Vitrification of Nucellar Cells of Navel Orange (Citrus sinensis var. brasiliensis Tanaka) Cooled to −196 °C. J. Plant Physiol. 1991, 137, 465–470. [Google Scholar] [CrossRef]
- Sopalun, K.; Kanchit, K.; Ishikawa, K. Vitrification-based cryopreservation of Grammatophyllum speciosum protocorm. Cryo Lett. 2010, 31, 347–357. [Google Scholar]
- Horvath, A.; Wayman, W.R.; Urbányi, B.; Ware, K.M.; Dean, J.C.; Tiersch, T.R. The relationship of the cryoprotectants methanol and dimethyl sulfoxide and hyperosmotic extenders on sperm cryopreservation of two North-American sturgeon species. Aquaculture 2005, 247, 243–251. [Google Scholar] [CrossRef]
- Bronshteyn, V.L.; Steponkus, P.L. Nucleation and Growth of Ice Crystals in Concentrated Solutions of Ethylene Glycol. Cryobiology 1995, 32, 1–22. [Google Scholar] [CrossRef]
- Rall, W. Factors affecting the survival of mouse embryos cryopreserved by vitrification. Cryobiology 1987, 24, 387–402. [Google Scholar] [CrossRef]
- Turner, S.; Senaratna, T.; Touchell, D.; Bunn, E.; Dixon, K.; Tan, B. Stereochemical arrangement of hydroxyl groups in sugar and polyalcohol molecules as an important factor in effective cryopreservation. Plant Sci. 2001, 160, 489–497. [Google Scholar] [CrossRef]
- Kim, H.-H.; Yoon, J.-W.; Kim, J.-B.; Engelmann, F.; Cho, E.-G. Thermal analysis of garlic shoot tips during a vitrification procedure. Cryo Lett. 2005, 26, 33–44. [Google Scholar]
- Wu, Y.; Zhao, Y.; Zhou, M.; Engelmann, F. Cryopreservation of temperate fruit tree germplasm. In Plant Genetic Resources Network in East Asia. Proceedings of the Meeting for the Regional Network for Conservation and Use of Plant Genetic Resources in East Asia, Ulaanbaatar, Mongolia, 13–16 August 2001; International Plant Genetic Resources Institute (IPGRI): Rome, Italy, 2002; pp. 77–88. [Google Scholar]
- Kim, H.-H.; Popova, E.V.; Yi, J.-Y.; Cho, G.-T.; Park, S.-U.; Lee, S.-C.; Engelmann, F. Cryopreservation of hairy roots of Rubia akane (Nakai) using a droplet-vitrification procedure. Cryo Lett. 2011, 31, 473–484. [Google Scholar]
- Hong, S.; Yin, M.; Shao, X.; Wang, A.; Xu, W. Cryopreservation of embryogenic callus of Dioscorea bulbifera by vitrification. Cryo Lett. 2009, 30, 64–75. [Google Scholar]
- Cho, E.G.; Hor, Y.L.; Kim, H.H.; Rao, V.R.; Engelmann, F. Cryopreservation of Citrus madurensis zygotic embryonic axes by vitrification: Importance of pregrowth and preculture conditions. Cryo Lett. 2002, 22, 391–396. [Google Scholar]
- Ivchenko, T.V.; Vitsenya, T.I.; Shevchenko, N.A.; Bashtan, N.O.; Kornienko, S.I. Hypothermic and Low-Temperature Storage of Garlic (Allium sativum L.) for in Vitro Collections. Probl. Cryobiol. Cryomedicine 2017, 27, 110–120. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, K.; Harata, K.; Mii, M.; Sakai, A.; Yoshimatsu, K.; Shimomura, K. Cryopreservation of zygotic embryos of a Japanese terrestrial orchid (Bletilla striata) by vitrification. Plant Cell Rep. 1997, 16, 754–757. [Google Scholar] [CrossRef]
- Ray, A.; Bhattacharya, S. Cryopreservation of in vitro grown nodal segments of Rauvolfia serpentina by PVS2 vitrification. Cryo Lett. 2009, 29, 321–328. [Google Scholar]
- Sajini, K.K.; Karun, A.; Amamath, C.H.; Engelmann, F. Cryopreservation of coconut (Cocos nucifera L.) zygotic embryos by vitrification. Cryo Lett. 2011, 32, 317–328. [Google Scholar]
- Panis, B.; Swennen, R. Plant cryopreservation: Applications, constraints and prospects. In Society for Low Temperature Biology. Annual Scientific Meeting, AGM and Symposium. Validation, Safety and Ethical Issues Impacting the Low Temperature Storage of Biological Resources; Cryo Letters: Lewes, UK, 2007; pp. 1–29. [Google Scholar]
- Tokatli, Y.O.; Akdemir, H. Cryopreservation of Fraser photinia (Photinia × fraseri Dress.) via vitrification-based one-step freezing techniques. Cryo Lett. 2010, 31, 40–49. [Google Scholar]
- Volk, G.M.; Maness, N.; Rotindo, K. Cryopreservation of garlic (Allium sativum L.) using plant vitrification solution 2. Cryo Lett. 2004, 25, 219–226. [Google Scholar]
- March, G.G.-D.; De Boucaud, M.-T.; Chmielarz, P. Cryopreservation of Prunus avium L. embryogenic tissues. Cryo Lett. 2006, 26, 341–348. [Google Scholar]
- Engelmann-Sylvestre, I.; Engelmann, F. Cryopreservation of in vitro-grown shoot tips of Clinopodium odorum using aluminium cryo-plates. Vitr. Cell. Dev. Biol. Anim. 2015, 51, 185–191. [Google Scholar] [CrossRef]
- Li, B.-Q.; Feng, C.-H.; Wang, M.-R.; Hu, L.-Y.; Volk, G.; Wang, Q.-C. Recovery patterns, histological observations and genetic integrity in Malus shoot tips cryopreserved using droplet-vitrification and encapsulation-dehydration procedures. J. Biotechnol. 2015, 214, 182–191. [Google Scholar] [CrossRef]
- Vollmer, R.; Villagaray, R.; Castro, M.; Anglin, N.; Ellis, D. Cryopreserved potato shoot tips showed genotype-specific response to sucrose concentration in rewarming solution (RS). Plant Cell Tissue Organ Cult. (PCTOC) 2018, 136, 353–363. [Google Scholar] [CrossRef]
- Wang, M.-R.; Zhang, Z.; Zámečník, J.; Bilavčík, A.; Blystad, D.-R.; Haugslien, S.; Wang, Q.-C. Droplet-vitrification for shoot tip cryopreservation of shallot (Allium cepa var. aggregatum): Effects of PVS3 and PVS2 on shoot regrowth. Plant Cell Tissue Organ Cult. 2020, 140, 185–195. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Kretzschmar, A.A.; Bonnart, R.; Shepherd, A.; Volk, G.M. Cryopreservation of 12 Vitis Species Using Apical Shoot Tips Derived from Plants Grown In Vitro. HortScience 2019, 54, 976–981. [Google Scholar] [CrossRef] [Green Version]
- Hammond, S.D.H.; Viehmannova, I.; Zamecnik, J.; Panis, B.; Faltus, M. Droplet-vitrification methods for apical bud cryopreservation of yacon [Smallanthus sonchifolius (Poepp. and Endl.) H. Rob.]. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 147, 197–208. [Google Scholar] [CrossRef]
- Niedermeyer, W.; Parish, G.R.; Moor, H. Reactions of yeast cells to glycerol treatment alterations to membrane structure and glycerol uptake. Protoplasma 1977, 92, 177–193. [Google Scholar] [CrossRef]
- Brison, M.; de Boucaud, M.-T.; Dosba, F. Cryopreservation of in vitro grown shoot tips of two interspecific Prunus rootstocks. Plant Sci. 1995, 105, 235–242. [Google Scholar] [CrossRef]
- Serrano-Martinez, F.; Casas, J.L. Cryopreservation of Tetraclinis articulata (vahl.) Masters. Cryo Lett. 2011, 32, 248–255. [Google Scholar]
- Maruyama, E.; Kinoshita, I.; Ishii, K.; Ohba, K.; Sakai, A. Germplasm conservation of Guazuma crinita, a useful tree in the Peru-Amazon, by the cryopreservation of in vitro-cultured multiple bud clusters. Plant Cell Tissue Organ Cult. (PCTOC) 1997, 48, 161–165. [Google Scholar] [CrossRef]
- Wang, Q.; Batuman, Ö.; Li, P.; Bar-Joseph, M.; Gafny, R. A simple and efficient cryopreservation of in vitro-grown shoot tips of Troyer’citrange [Poncirus trifoliata (L.) Raf. × Citrus sinensis (L.) Osbeck.] by encapsulation-vitrification. Euphytica 2002, 128, 135–142. [Google Scholar] [CrossRef]
- Vujović, T.; Jevremović, D.; Marjanović, T.; Ružić, Đ. Cryopreservation of Serbian autochthonous plum ‘Crvena Ranka’ using aluminium cryo-plates. Genetika 2021, 53, 283–294. [Google Scholar] [CrossRef]
- Lambardi, M.; Fabbri, A.; Caccavale, A. Cryopreservation of white poplar (Populus alba L.) by vitrification of in vitro-grown shoot tips. Plant Cell Rep. 2000, 19, 213–218. [Google Scholar] [CrossRef]
- Shin, D.J.; Kong, H.; Popova, E.V.; Moon, H.K.; Park, S.Y.; Park, S.-U.; Lee, S.C.; Kim, H.H. Cryopreservation of Kalopanax septemlobus embryogenic callus using vitrification and droplet-vitrification. Cryo Lett. 2012, 33, 402–410. [Google Scholar]
- Barraco, G.; Sylvestre, I.; Iapichino, G.; Engelmann, F. Investigating the cryopreservation of nodal explants of Lithodora rosmarinifolia (Ten.) Johnst., a rare, endemic Mediterranean species. Plant Biotechnol. Rep. 2012, 7, 141–146. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Balaraju, K.; Song, J.-Y.; Yi, J.-Y.; Lee, S.-Y.; Lee, J.-R.; Yoon, M.; Kim, H.-H. Cryopreservation of in vitro grown shoot tips of strawberry (Fragaria × ananassa Duch.) genetic resources by droplet-vitrification. Korean J. Plant Resour. 2019, 32, 689–697. [Google Scholar]
- Sakai, A. Development of cryopreservation techniques. In Cryopreservation of Tropical Plant Germplasm: Current Research Progress and Application; CGIAR: Montpellier, France, 2000; pp. 1–7. [Google Scholar]
- Langis, R.; Schnabel, B.; Earle, E.; Steponkus, P. Cryopreservation of Brassica campestris L. cell suspensions by vitrification. Cryo Lett. 1989, 10, 421–428. [Google Scholar]
- Kim, H.-H.; Cho, E.-G.; Baek, H.-J.; Kim, C.-Y.; Keller, E.R.J.; Engelmann, F. Cryopreservation of garlic shoot tips by vitrification: Effects of dehydration, rewarming, unloading and regrowth conditions. Cryo Lett. 2004, 25, 59–70. [Google Scholar]
- Towill, L. Cryopreservation of isolated mint shoot tips by vitrification. Plant Cell Rep. 1990, 9, 178–180. [Google Scholar] [CrossRef]
- Grospietsch, M.; Stodulkova, E.; Zamecnik, J. Effect of osmotic stress on the dehydration tolerance and cryopreservation of Solanum tuberosum shoot tips. Cryo Lett. 1999, 20, 339–346. [Google Scholar]
- Suranthran, P.; Gantait, S.; Sinniah, U.R.; Subramaniam, S.; Alwee, S.S.R.S.; Roowi, S.H. Effect of loading and vitrification solutions on survival of cryopreserved oil palm polyembryoids. Plant Growth Regul. 2012, 66, 101–109. [Google Scholar] [CrossRef]
- Mallon, R.; Bunn, E.; Turner, S.R.; Gonzalez, M.L. Cryopreservation of Centaurea ultreiae (Compositae) a critically endangered species from Galicia (Spain). Cryo Lett. 2008, 29, 363–370. [Google Scholar]
- Turner, S.R.; Senaratna, T.; Bunn, E.; Tan, B.; Dixon, K.; Touchell, D.H. Cryopreservation of Shoot Tips from Six Endangered Australian Species using a Modified Vitrification Protocol. Ann. Bot. 2001, 87, 371–378. [Google Scholar] [CrossRef] [Green Version]
- Halmagyi, A.; Deliu, C.; Coste, A.; Keul, M.; Cheregi, O.; Cristea, V. Vitrification of potato shoot tips for germplasm cryopreservation. Contrib. Bot. 2004, 39, 187–193. [Google Scholar]
- Shevchenko, N.; Mozgovska, A.; Bobrova, O.; Bashtan, N.; Kovalenko, G.; Ivchenko, T. Post-Thaw Survival of Meristems from In Vitro Sweet Potato (Ipomoea batatas (L.) Lam.) Plants. Biol. Life Sci. Forum 2020, 4, 43. [Google Scholar] [CrossRef]
- Watanabe, K.; Steponkus, P.L. Vitrification of Oryza sativa L. cell suspensions. Cryo Lett. 1995, 16, 255–262. [Google Scholar]
- Folgado, R.; Panis, B.; Sergeant, K.; Renaut, J.; Swennen, R.; Hausman, J.-F. Unravelling the effect of sucrose and cold pretreatment on cryopreservation of potato through sugar analysis and proteomics. Cryobiology 2015, 71, 432–441. [Google Scholar] [CrossRef]
- Dumet, D.; Grapin, A.; Bailly, C.; Dorion, N. Revisiting crucial steps of an encapsulation/desiccation based cryopreservation process: Importance of thawing method in the case of Pelargonium meristems. Plant Sci. 2002, 163, 1121–1127. [Google Scholar] [CrossRef]
- Matsumoto, T. An approach to enhance dehydration tolerance of alginate-coated dried meristems cooled to −196 °C. Cryo Lett. 1995, 16, 299–306. [Google Scholar]
- Reed, B.M. Cryopreservation—Practical considerations. In Plant Cryopreservation: A Practical Guide; Springer: Berlin/Heidelberg, Germany, 2008; pp. 3–13. [Google Scholar]
- Sakai, A.; Engelmann, F. Vitrification, encapsulation-vitrification and droplet-vitrification: A review. Cryo Lett. 2007, 28, 151–172. [Google Scholar]
- Volk, G.M.; Shepherd, A.N.; Bonnart, R. Successful Cryopreservation of Vitis Shoot Tips: Novel Pre-treatment Combinations Applied to Nine Species. Cryo Lett. 2019, 39, 322–330. [Google Scholar]
- Benelli, C.; Carvalho, L.; EL Merzougui, S.; Petruccelli, R. Two Advanced Cryogenic Procedures for Improving Stevia rebaudiana (Bertoni) Cryopreservation. Plants 2021, 10, 277. [Google Scholar] [CrossRef]
- O’Brien, C.; Hiti-Bandaralage, J.C.A.; Folgado, R.; Lahmeyer, S.; Hayward, A.; Folsom, J.; Mitter, N. First report on cryopreservation of mature shoot tips of two avocado (Persea americana Mill.) rootstocks. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 144, 103–113. [Google Scholar] [CrossRef]
- Sharma, S.; Parasher, K.; Mukherjee, P.; Sharma, Y.P. Cryopreservation of a Threatened Medicinal Plant, Valeriana Jatamansi Jones, Using Vitrification and Assessment of Biosynthetic Stability of Regenerants. Cryo Lett. 2021, 42, 300–308. [Google Scholar]
- Fahy, G.M.; Macfarlane, D.R.; Angell, C.A.; Meryman, H.T. Vitrification as an approach to cryopreservation. Cryobiology 1984, 21, 407–426. [Google Scholar] [CrossRef]
- Kim, H.-H.; Lee, J.-K.; Yoon, J.-W.; Ji, J.-J.; Nam, S.-S.; Hwang, H.-S.; Cho, E.-G.; Engelmann, F. Cryopreservation of garlic bulbil primordia by the droplet-vitrification procedure. Cryo Lett. 2006, 27, 143–153. [Google Scholar]
- Kartha, K.; Leung, N.; Mroginski, L. In vitro Growth Responses and Plant Regeneration from Cryopreserved Meristems of Cassava (Manihot esculenta Crantz). Zeitschrift für Pflanzenphysiologie 1982, 107, 133–140. [Google Scholar] [CrossRef]
- Ellis, D.; Skogerboe, D.; Andre, C.; Hellier, B.; Volk, G. Implementation of garlic cryopreservation techniques in the national plant germplasm system. Cryo Lett. 2006, 27, 99–106. [Google Scholar]
- Tanaka, D.; Niino, T.; Isuzugawa, K.; Hikage, T.; Uemura, M. Cryopreservation of shoot apices of in-vitro grown gentian plants: Comparison of vitrification and encapsulation-vitrification protocols. Cryo Lett. 2004, 25, 167–176. [Google Scholar]
- Kaczmarczyk, A.; Shvachko, N.; Lupysheva, Y.; Hajirezaei, M.-R.; Keller, E.R.J. Influence of alternating temperature preculture on cryopreservation results for potato shoot tips. Plant Cell Rep. 2008, 27, 1551–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaefer-Menuhr, A.; Schumacher, H.-M.; Mix-Wagner, G. Long-term storage of old potato varieties by cryopreservation of meristems in liquid nitrogen. Landbauforsch. Voelkenrode 1994, 44, 301–313. [Google Scholar]
- Martinez-Montero, M.E.; Martinez, J.; Engelmann, F. Cryopreservation of sugarcane somatic embryos. Cryo Lett. 2008, 29, 229–242. [Google Scholar]
- Senula, A.D.; Keller, E.R.J.; Sanduijav, T.; Yohannes, T. Cryopreservation of cold-acclimated mint (Mentha spp.) shoot tips using a simple vitrification protocol. Cryo Lett. 2007, 28, 1–12. [Google Scholar]
- Bettoni, J.C.; Marković, Z.; Bi, W.; Volk, G.M.; Matsumoto, T.; Wang, Q.-C. Grapevine Shoot Tip Cryopreservation and Cryotherapy: Secure Storage of Disease-Free Plants. Plants 2021, 10, 2190. [Google Scholar] [CrossRef]
- Panis, B.; Nagel, M.; Houwe, I.V.D. Challenges and Prospects for the Conservation of Crop Genetic Resources in Field Genebanks, in In Vitro Collections and/or in Liquid Nitrogen. Plants 2020, 9, 1634. [Google Scholar] [CrossRef] [PubMed]
- Gámez-Pastrana, R.; González-Arnao, M.T.; Martínez-Ocampo, Y.; Engelmann, F. Thermal events in calcium alginate beads during encapsulation dehydration and encapsulation-vitrification protocols. Acta Hortic. 2011, 908, 47–54. [Google Scholar] [CrossRef]
- Funnekotter, B.; Mancera, R.L.; Bunn, E. Advances in understanding the fundamental aspects required for successful cryopreservation of Australian flora. Vitr. Cell. Dev. Biol. Anim. 2017, 53, 289–298. [Google Scholar] [CrossRef]
- Funnekotter, B.; Bunn, E.; Mancera, R.L. Cryo-mesh: A simple alternative cryopreservation protocol. Cryo Lett. 2017, 38, 155–159. [Google Scholar]
- Nadarajan, J.; Pritchard, H.W. Biophysical Characteristics of Successful Oilseed Embryo Cryoprotection and Cryopreservation Using Vacuum Infiltration Vitrification: An Innovation in Plant Cell Preservation. PLoS ONE 2014, 9, e96169. [Google Scholar] [CrossRef]
- Bruňáková, K.; Zámečník, J.; Urbanová, M.; Čellárová, E. Dehydration status of ABA-treated and cold-acclimated Hypericum perforatum L. shoot tips subjected to cryopreservation. Thermochim. Acta 2011, 525, 62–70. [Google Scholar] [CrossRef]
- Šesták, J.; Zamecnik, J. Can clustering of liquid water and thermal analysis be of assistance for better understanding of biological germplasm exposed to ultra-low temperatures. J. Therm. Anal. Calorim. 2007, 88, 411–416. [Google Scholar] [CrossRef]
| PVS1 | DMSO (%) | Suc (%) | Gly (%) | EG (%) | PG (%) | PEG (%) | Sor (%) | Total (%) | Plant |
|---|---|---|---|---|---|---|---|---|---|
| Uragami | 7 | 22 | 15 | 15 | 9.1 | 68.1 | Asparagus officinalis L. [14] | ||
| PVS1-M1 | 6 | 22 | 13 | 13 | 54 | Malus sp. [96] | |||
| PVS1-M2 | 6 | 19 | 13 | 13 | 9.1 | 60.1 | Porphyra yezoensis [94] | ||
| PVS1-M3 | 6 | 13.7 | 22 | 13 | 13 | 67.7 | Allium sativum L. [97] | ||
| PVS1-M4 | 10 | 22 | 13 | 13 | 58 | Dioscorea opposite [98] | |||
| PVS1-M5 | 7 | 22 | 15 | 15 | 59 | Citrus madurensis [99] | |||
| PVS1-M6 | 31.1 | 18.4 | 15 | 64.5 | Allium sativum L. [100] | ||||
| PVS1-M7 | 7 | 15 | 22 | 30 | 74 | Bletila strata [101] | |||
| PVS1-M8 | 5 | 13.7 | 13.7 | 32.4 | Citrus madurensis [99] |
| DMSO (%) | Suc (%) | Gly (%) | EG (%) | PEG * (%) | Sor (%) | BSA (%) | CaCl2 (mM) | Total (%) | Plant | |
|---|---|---|---|---|---|---|---|---|---|---|
| PVS4 | 20.5 | 35 | 20 | 75.5 | Various plants [124] | |||||
| PVS4-M1 | 5 | 5 | 10 | Malus [96] | ||||||
| PVS4-M2 ** | 10 | 15 | 20 | 30 | 10 | 75 | Citrus madurensis [99] Bromus inermis Leyss [46] | |||
| PVS4-M3 *** | 10 | 5 | 20 | 30 | 10 | 65 | Bromus inermis Leyss [46] | |||
| Steponkus | 43.5 | 16 | 6 | 65.5 | Secale cereale L. [125] | |||||
| Steponkus-M1 | 13.7 | 50 | 15 | 6 | 84.7 | Allium sativum L. [126] | ||||
| Towill | 7.8 | 35 | 10 | 52.8 | Mentha aquatica× M. spicata [127] | |||||
| Towill-M1 | 10 | 35 | 5 | 50 | Guazuma crinita Mart. [117] | |||||
| Towill-M2 | 6.8 | 13.7 | 35 | 10 | 65.5 | Allium sativum L. [126] |
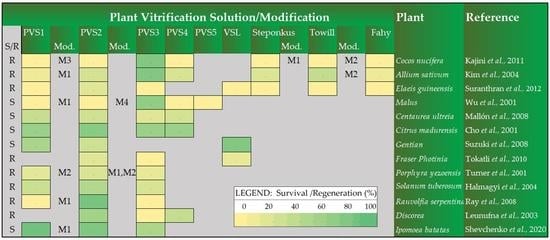
| PVS1 | PVS2 | PVS3 | PVS4 | PVS5 | VSL | Steponkus | Towill | Fahy | Plant | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mod. | Mod. | Mod. | Mod. | R/S ** | ||||||||||
| 0 | M3 | 0 | 80 | 0 | 0 | M1 | 0 | M2 | 0 | R | Cocos nucifera L. [103] | |||
| 11 | M1 | 27 | 80 | 25 | 23 | 39 | M2 | 11 | R | Allium sativum L. [126] | ||||
| 0 | 20 | 0 | 0 | 0 § | 0 | 0 | R | Elaeis guineensis [129] | ||||||
| 0 | M1 | 0 | M4 | 70 | 0 | 0 | S | Malus [96] | ||||||
| 36 | 30 | 20 | 28 | S | Centaurea ultreia [130] | |||||||||
| 65 | 75 | 65 | 65 | S | Citrus madurensis [99] | |||||||||
| 55 | 32 | 80 | S | Gentian [46] | ||||||||||
| 80 | 20 | 0 | 14 | R | Fraser Photinia [105] | |||||||||
| 18 | M2 | 24 | M1,M2 | 15 | R | Porphyra yezoensis [131] | ||||||||
| 34 | 49 | 25 | R | Solanum tuberosum L. [132] | ||||||||||
| 0 | M1 | 87 | 0 | R | Rauvolfia serpentina L. [102] | |||||||||
| 59 | 0 | 38 | R | Discorea [15] | ||||||||||
| 92 | M1 | 82 | 52 §§§§ | 83 * | S | Ipomoea batatas [133] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamecnik, J.; Faltus, M.; Bilavcik, A. Vitrification Solutions for Plant Cryopreservation: Modification and Properties. Plants 2021, 10, 2623. https://doi.org/10.3390/plants10122623
Zamecnik J, Faltus M, Bilavcik A. Vitrification Solutions for Plant Cryopreservation: Modification and Properties. Plants. 2021; 10(12):2623. https://doi.org/10.3390/plants10122623
Chicago/Turabian StyleZamecnik, Jiri, Milos Faltus, and Alois Bilavcik. 2021. "Vitrification Solutions for Plant Cryopreservation: Modification and Properties" Plants 10, no. 12: 2623. https://doi.org/10.3390/plants10122623