Aberrant Mannosylated and Highly Fucosylated Glycoepitopes of Prostatic Acid Phosphatase as Potential Ligands for Dendritic-Cell Specific ICAM-Grabbing Nonintegrin (DC-SIGN) in Human Seminal Plasma—A Step towards Explaining Idiopathic Infertility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seminal Plasma Samples
2.2. Quantification of Prostatic Acid Phosphatase
2.3. Mild Oxidation of the Glycans on Capture Antibodies
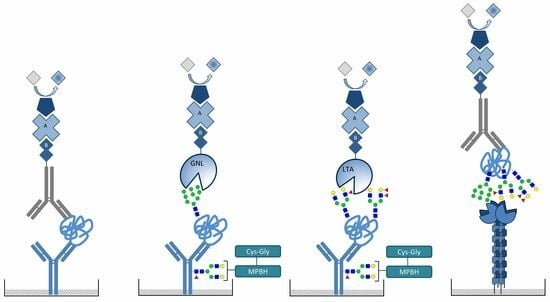
2.4. Detection of Mannosylated and Fucosylated Glycoepitopes of Prostatic acid Phosphatase
2.5. DC-SIGN Interaction with PAP
2.6. Statistical Analysis
3. Results
3.1. Concentration of Prostatic Acid Phosphatase
3.2. Detection of Mannose and High-Fucose Type Glycoepitopes in Prostatic Acid Phosphatase
3.3. DC-SIGN Reactivity with Seminal Plasma Prostatic Acid Phosphatase
3.4. Correlation Scatter Plots between DC-SIGN Reactivity and Galanthus Nivalis or Lotus Tetragonolobus Lectin Reactivity and Spearman Rank Correlation Test
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kong, H.Y.; Byun, J. Emerging roles of human prostatic Acid phosphatase. Biomol. Ther. 2013, 21, 10–20. [Google Scholar] [CrossRef]
- Sharma, M.; Gupta, S.; Dhole, B.; Kumar, A. The Prostate Gland. In Basics of Human Andrology; Kumar, A., Sharma, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 17–36. [Google Scholar] [CrossRef]
- Ziyyat, A.; Barraud-Lange, V.; Sifer, C.; Ducot, B.; Wolf, J.P.; Soufir, J.C. Paradoxical increase of sperm motility and seminal carnitine associated with moderate leukocytospermia in infertile patients. Fertil. Steril. 2008, 90, 2257–2263. [Google Scholar] [CrossRef]
- Collins, K.A.; Bennett, A.T. Persistence of spermatozoa and prostatic acid phosphatase in specimens from deceased individuals during varied postmortem intervals. Am. J. Forensic Med. Pathol. 2001, 22, 228–232. [Google Scholar] [CrossRef]
- Curi, S.M.; Ariagno, J.I.; Chenlo, P.H.; Mendeluk, G.R.; Pugliese, M.N.; Sardi Segovia, L.M.; Repetto, H.E.; Blanco, A.M. Asthenozoospermia: Analysis of a large population. Arch. Androl. 2003, 49, 343–349. [Google Scholar] [CrossRef]
- Veeramani, S.; Yuan, T.C.; Chen, S.J.; Lin, F.F.; Petersen, J.E.; Shaheduzzaman, S.; Srivastava, S.; MacDonald, R.G.; Lin, M.F. Cellular prostatic acid phosphatase: A protein tyrosine phosphatase involved in androgen-independent proliferation of prostate cancer. Endocr. Relat. Cancer. 2005, 12, 805–822. [Google Scholar] [CrossRef]
- Hassan, M.I.; Aijaz, A.; Ahmad, F. Structural and functional analysis of human prostatic acid phosphatase. Expert Rev. Anticancer Ther. 2010, 10, 1055–1068. [Google Scholar] [CrossRef]
- Muniyan, S.; Chaturvedi, N.K.; Dwyer, J.G.; Lagrange, C.A.; Chaney, W.G.; Lin, M.F. Human prostatic acid phosphatase: Structure, function and regulation. Int. J. Mol. Sci. 2013, 14, 10438–10464. [Google Scholar] [CrossRef]
- White, K.Y.; Rodemich, L.; Nyalwidhe, J.O.; Comunale, M.A.; Clements, M.A.; Lance, R.S.; Schellhammer, P.F.; Mehta, A.S.; Semmes, O.J.; Drake, R.R. Glycomic characterization of prostate-specific antigen and prostatic acid phosphatase in prostate cancer and benign disease seminal plasma fluids. J. Proteome Res. 2009, 8, 620–630. [Google Scholar] [CrossRef]
- Clark, G.F.; Grassi, P.; Pang, P.C.; Panico, M.; Lafrenz, D.; Drobnis, E.Z.; Baldwin, M.R.; Morris, H.R.; Haslam, S.M.; Schedin-Weiss, S.; et al. Tumor biomarker glycoproteins in the seminal plasma of healthy human males are endogenous ligands for DC-SIGN. Mol. Cell Proteom. 2012, 11, M111.008730. [Google Scholar] [CrossRef]
- Pang, P.C.; Tissot, B.; Drobnis, E.Z.; Morris, H.R.; Dell, A.; Clark, G.F. Analysis of the human seminal plasma glycome reveals the presence of immunomodulatory carbohydrate functional groups. J. Proteome Res. 2009, 8, 4906–4915. [Google Scholar] [CrossRef]
- Kałuża, A.; Jarząb, A.; Gamian, A.; Kratz, E.M.; Zimmer, M.; Ferens-Sieczkowska, M. Preliminary MALDI-TOF-MS analysis of seminal plasma N-glycome of infertile men. Carbohydr. Res. 2016, 435, 19–25. [Google Scholar] [CrossRef]
- Lan, R.; Xin, M.; Hao, Z.; You, S.; Xu, Y.; Wu, J.; Dang, L.; Zhang, X.; Sun, S. Biological Functions and Large-Scale Profiling of Protein Glycosylation in Human Semen. J. Proteome Res. 2020, 19, 3877–3889. [Google Scholar] [CrossRef]
- Xin, M.; You, S.; Xu, Y.; Shi, W.; Zhu, B.; Shen, J.; Wu, J.; Li, C.; Chen, Z.; Su, Y.; et al. Precision Glycoproteomics Reveals Distinctive N-Glycosylation in Human Spermatozoa. Mol. Cell Proteom. 2022, 21, 100214. [Google Scholar] [CrossRef]
- Merlotti, A.; Dantas, E.; Remes Lenicov, F.; Ceballos, A.; Jancic, C.; Varese, A.; Rubione, J.; Stover, S.; Geffner, J.; Sabatté, J. Fucosylated clusterin in semen promotes the uptake of stress-damaged proteins by dendritic cells via DC-SIGN. Hum. Reprod. 2015, 7, 1545–1556. [Google Scholar] [CrossRef]
- van Liempt, E.; Bank, C.M.; Mehta, P.; Garciá-Vallejo, J.J.; Kawar, Z.S.; Geyer, R.; Alvarez, R.A.; Cummings, R.D.; van Kooyk, Y.; van Die, I. Specificity of DC-SIGN for mannose- and fucose-containing glycans. FEBS Lett. 2006, 580, 6123–6131. [Google Scholar] [CrossRef]
- Chen, S.; Haab, B.B. Analysis of glycans on serum proteins using antibody microarrays. Methods Mol. Biol. 2009, 520, 39–58. [Google Scholar] [CrossRef]
- Lu, C.; Wonsidler, J.L.; Li, J.; Du, Y.; Block, T.; Haab, B.; Chen, S. Chemically-blocked antibody microarray for multiplexed high-throughput profiling of specific protein glycosylation in complex samples. J. Vis. Exp. 2012, 63, e3791. [Google Scholar] [CrossRef]
- Sabatte, J.; Faigle, W.; Ceballos, A.; Morelle, W.; Rodríguez Rodrígues, C.; Remes Lenicov, F.; Thépaut, M.; Fieschi, F.; Malchiodi, E.; Fernández, M.; et al. Semen clusterin is a novel DC-SIGN ligand. J. Immunol. 2011, 187, 5299–5309. [Google Scholar] [CrossRef]
- Politch, J.A.; Tucker, L.; Bowman, F.P.; Anderson, D.J. Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum. Reprod. 2007, 22, 2928–2935. [Google Scholar] [CrossRef]
- Badia, R.; Iborra, A.; Palacio, J.R.; Antich, M.; Martínez, P. The effect of oxidative environment on immunosuppressive properties of human seminal plasma. Am. J. Reprod. Immunol. 2008, 60, 354–360. [Google Scholar] [CrossRef]
- Guerin, L.R.; Prins, J.R.; Robertson, S.A. Regulatory T-cells and immune tolerance in pregnancy: A new target for infertility treatment? Hum. Reprod. Update 2009, 15, 517–535. [Google Scholar] [CrossRef]
- Pang, P.C.; Tissot, B.; Drobnis, E.Z.; Sutovsky, P.; Morris, H.R.; Clark, G.F.; Dell, A. Expression of bisecting type and Lewisx/Lewisy terminated N-glycans on human sperm. J. Biol. Chem. 2007, 282, 36593–36602. [Google Scholar] [CrossRef] [PubMed]
- Crespo, H.J.; Lau, J.T.; Videira, P.A. Dendritic cells: A spot on sialic Acid. Front. Immunol. 2013, 4, 491. [Google Scholar] [CrossRef] [PubMed]
- van Kooyk, Y.; Geijtenbeek, T.B. DC-SIGN: Escape mechanism for pathogens. Nat. Rev. Immunol. 2003, 3, 697–709. [Google Scholar] [CrossRef]
- Guo, Y.; Feinberg, H.; Conroy, E.; Mitchell, D.A.; Alvarez, R.; Blixt, O.; Taylor, M.E.; Weis, W.I.; Drickamer, K. Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nat. Struct. Mol. Biol. 2004, 11, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vallejo, J.J.; van Kooyk, Y. The physiological role of DC-SIGN: A tale of mice and men. Trends Immunol. 2013, 34, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Gringhuis, S.I.; den Dunnen, J.; Litjens, M.; van der Vlist, M.; Geijtenbeek, T.B. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat. Immunol. 2009, 10, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- García-Vallejo, J.J.; van Kooyk, Y. Endogenous ligands for C-type lectin receptors: The true regulators of immune homeostasis. Immunol. Rev. 2009, 230, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, S.J.; García-Vallejo, J.J.; van Kooyk, Y. Dendritic cells and C-type lectin receptors: Coupling innate to adaptive immune responses. Immunol. Cell Biol. 2008, 86, 580–587. [Google Scholar] [CrossRef]
- Clark, G.F. The role of glycans in immune evasion: The human fetoembryonic defence system hypothesis revisited. Mol. Hum. Reprod. 2014, 20, 185–199. [Google Scholar] [CrossRef]
- Clark, G.F.; Schust, D.J. Manifestations of immune tolerance in the human female reproductive tract. Front. Immunol. 2013, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Guerin, L.R.; Moldenhauer, L.M.; Hayball, J.D. Activating T regulatory cells for tolerance in early pregnancy—The contribution of seminal fluid. J. Reprod. Immunol. 2009, 83, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res. 2005, 322, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, B.; Jarząb, A.; Kratz, E.M.; Zimmer, M.; Gamian, A.; Ferens-Sieczkowska, M. Terminal Mannose Residues in Seminal Plasma Glycoproteins of Infertile Men Compared to Fertile Donors. Int. J. Mol. Sci. 2015, 16, 14933–14950. [Google Scholar] [CrossRef]
- Olejnik, B.; Kratz, E.M.; Zimmer, M.; Ferens-Sieczkowska, M. Glycoprotein fucosylation is increased in seminal plasma of subfertile men. Asian J. Androl. 2015, 17, 274–280. [Google Scholar] [CrossRef]
- Figdor, C.G.; van Kooyk, Y.; Adema, G.J. C-type lectin receptors on dendritic cells and Langerhans cells. Nat. Rev. Immunol. 2002, 2, 77–84. [Google Scholar] [CrossRef]
- Geijtenbeek, T.B.; van Duijnhoven, G.C.; van Vliet, S.J.; Krieger, E.; Vriend, G.; Figdor, C.G.; van Kooyk, Y. Identification of different binding sites in the dendritic cell-specific receptor DC-SIGN for intercellular adhesion molecule 3 and HIV-1. J. Biol. Chem. 2002, 277, 11314–11320. [Google Scholar] [CrossRef]





| Group | Subject Age [Years] | Sperm Count [×106/mL] | Sperm Motility [%] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | Range | Mean ± SD | Median | Range | Mean ± SD | Median | Range | |
| C (n = 20) | 31 ± 5 | 32 | 22–39 | 56.3 ± 35.0 | 52.7 | 20.4–99.2 | 55 ± 11 | 56 | 36–71 |
| N (n = 25) | 34 ± 4 | 34 | 28–42 | 61.8 ± 22.0 | 61.5 | 23.0–109.7 | 51 ± 10 | 52 | 33–67 |
| A (n = 28) | 34 ± 5 | 32 | 29–47 | 35.2 ± 18.9 | 28.6 | 19.0–94.7 | 25 ± 7 | 26 | 4–30 |
| O (n = 20) | 32 ± 4 | 32 | 27–40 | 7.9 ± 2.0 | 8.2 | 3.0–11.2 | 46 ± 11 | 41 | 33–67 |
| OA (n = 23) | 33 ± 6 | 33 | 24–49 | 6.1 ± 4.0 | 6.4 | 0.3–10.8 | 18 ± 9 | 18 | 1–29 |
| T (n = 19) | 37 ± 6 | 37 | 33–41 | 62.0 ± 19.0 | 68.0 | 40.4–77.6 | 40 ± 23 | 48 | 14–58 |
| PAP concentration (mg/mL) | Group | ||||||
| C | N | A | O | OA | T | ||
| n = 20 | n = 25 | n = 28 | n = 20 | n = 23 | n = 19 | ||
| Mean ± SD | 0.83 ± 0.57 | 0.71 ± 0.40 | 0.66 ± 0.46 | 0.61 ± 0.35 | 0.61 ± 0.31 | 0.65 ± 0.44 | |
| Median | 0.69 | 0.73 | 0.49 | 0.52 | 0.54 | 0.49 | |
| Range | 0.27–1.29 | 0.46–0.89 | 0.34–0.75 | 0.39–0.83 | 0.38–0.79 | 0.30–0.87 | |
| Lectins Reactivity (AU) | Group | ||||||
|---|---|---|---|---|---|---|---|
| C | N | A | O | OA | T | ||
| n = 20 | n = 25 | n = 28 | n = 20 | n = 23 | n = 19 | ||
| GNL reactivity | Mean ± SD | 0.41 ± 0.27 | 0.78 ± 0.38 | 0.48 ± 0.30 | 0.26 ± 0.23 | 0.21 ± 0.12 | 0.25 ± 0.17 |
| pOA = 0.000001 pO = 0.000002 pT = 0.000013 pC = 0.043308 | pOA = 0.007676 pO = 0.036319 | ||||||
| Median | 0.30 | 0.70 | 0.46 | 0.19 | 0.22 | 0.22 | |
| Range | 0.24–0.51 | 0.45–1.03 | 0.26–0.58 | 0.1–0.36 | 0.1–0.34 | 0.15–0.31 | |
| LTL reactivity | Mean ± SD | 0.19 ± 0.09 | 0.25 ± 0.12 | 0.20 ± 0.15 | 0.19 ± 0.16 | 0.17 ± 0.15 | 0.16 ± 0.1 |
| pOA = 0.046826 | |||||||
| Median | 0.18 | 0.23 | 0.17 | 0.15 | 0.14 | 0.15 | |
| Range | 0.13–0.24 | 0.18–0.28 | 0.10–0.28 | 0.08–0.26 | 0.09–0.19 | 0.08–0.22 | |
| DC-SIGN reactivity | Mean ± SD | 0.32 ± 0.11 | 0.37 ± 0.1 | 0.34 ± 0.12 | 0.26 ± 0.08 | 0.29 ± 0.12 | 0.37 ± 0.12 |
| pO = 0.015318 | |||||||
| Median | 0.30 | 0.36 | 0.32 | 0.26 | 0.30 | 0.33 | |
| Range | 0.24–0.35 | 0.29–0.45 | 0.25–0.42 | 0.20–0.31 | 0.22–0.35 | 0.29–0.47 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kałuża, A.; Trzęsicka, K.; Drzyzga, D.; Ferens-Sieczkowska, M. Aberrant Mannosylated and Highly Fucosylated Glycoepitopes of Prostatic Acid Phosphatase as Potential Ligands for Dendritic-Cell Specific ICAM-Grabbing Nonintegrin (DC-SIGN) in Human Seminal Plasma—A Step towards Explaining Idiopathic Infertility. Biomolecules 2024, 14, 58. https://doi.org/10.3390/biom14010058
Kałuża A, Trzęsicka K, Drzyzga D, Ferens-Sieczkowska M. Aberrant Mannosylated and Highly Fucosylated Glycoepitopes of Prostatic Acid Phosphatase as Potential Ligands for Dendritic-Cell Specific ICAM-Grabbing Nonintegrin (DC-SIGN) in Human Seminal Plasma—A Step towards Explaining Idiopathic Infertility. Biomolecules. 2024; 14(1):58. https://doi.org/10.3390/biom14010058
Chicago/Turabian StyleKałuża, Anna, Katarzyna Trzęsicka, Damian Drzyzga, and Mirosława Ferens-Sieczkowska. 2024. "Aberrant Mannosylated and Highly Fucosylated Glycoepitopes of Prostatic Acid Phosphatase as Potential Ligands for Dendritic-Cell Specific ICAM-Grabbing Nonintegrin (DC-SIGN) in Human Seminal Plasma—A Step towards Explaining Idiopathic Infertility" Biomolecules 14, no. 1: 58. https://doi.org/10.3390/biom14010058