YidC from Escherichia coli Forms an Ion-Conducting Pore upon Activation by Ribosomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. The Cloning of pTrc99a-YidCΔC and Construction of a Single-Cysteine YidC Derivative
2.3. The Purification and Reconstitution of YidC into Vesicles
2.4. The Labeling of YidC
2.5. BN-PAGE Analysis
2.6. In Vivo pBpa Crosslinking
2.7. CyoA Leader Peptide
2.8. Reconstitution of YidC into Planar Bilayers
2.9. Single Ion Channel Measurements
2.10. Ribosome Expression and Purification
2.11. Labeling of Ribosomes after Purification
2.12. Single-Molecule Microscopy Measurements
2.13. Fluorescence Correlation Spectroscopy (FCS)
2.14. Ribosome Nascent Chains
2.15. Calculation of Channel Ion Selectivity
2.16. Estimation of the Pore Diameter
2.17. Modeling of YidC Dimers with AlphaFold
3. Results
3.1. Purified and Reconstituted YidC Displays Ion Channel Activity in the Presence of Its Substrate FoC or Empty Ribosomes
3.2. YidC with C-Terminal Deletion Retains Ribosome Binding Activity
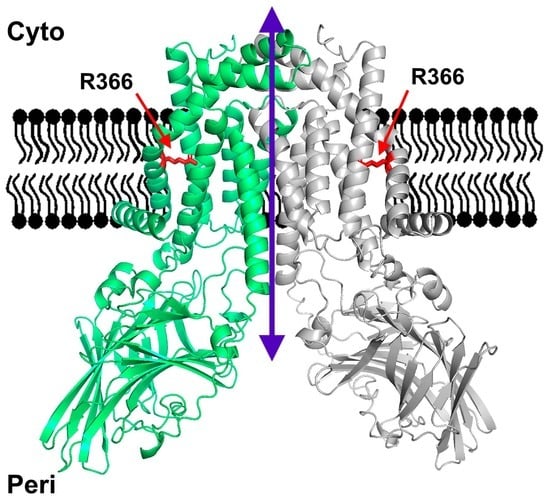
3.3. Structural YidC Models
3.4. Stoichiometry of the Reconstituted YidC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vogtle, F.N.; Koch, H.G.; Meisinger, C. A common evolutionary origin reveals fundamental principles of protein insertases. PLoS Biol. 2022, 20, e3001558. [Google Scholar] [CrossRef]
- Samuelson, J.C.; Jiang, F.; Yi, L.; Chen, M.; de Gier, J.W.; Kuhn, A.; Dalbey, R.E. Function of YidC for the insertion of M13 procoat protein in Escherichia coli: Translocation of mutants that show differences in their membrane potential dependence and Sec requirement. J. Biol. Chem. 2001, 276, 34847–34852. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Jiang, F.; Chen, M.; Cain, B.; Bolhuis, A.; Dalbey, R.E. YidC Is Strictly Required for Membrane Insertion of Subunits a and c of the F1F0ATP Synthase and SecE of the SecYEG Translocase. Biochemistry 2003, 42, 10537–10544. [Google Scholar] [CrossRef]
- Welte, T.; Kudva, R.; Kuhn, P.; Sturm, L.; Braig, D.; Muller, M.; Warscheid, B.; Drepper, F.; Koch, H.G. Promiscuous targeting of polytopic membrane proteins to SecYEG or YidC by the Escherichia coli signal recognition particle. Mol. Biol. Cell 2012, 23, 464–479. [Google Scholar] [CrossRef] [PubMed]
- Scotti, P.A.; Urbanus, M.L.; Brunner, J.; de Gier, J.W.; von Heijne, G.; van der Does, C.; Driessen, A.J.M.; Oudega, B.; Luirink, J. YidC, the Escherichia coli homologue of mitochondrial Oxa1p, is a component of the Sec translocase. EMBO J. 2000, 19, 542–549. [Google Scholar] [CrossRef]
- Beck, K.; Eisner, G.; Trescher, D.; Dalbey, R.E.; Brunner, J.; Muller, M. YidC, an assembly site for polytopic Escherichia coli membrane proteins located in immediate proximity to the SecYE translocon and lipids. EMBO Rep. 2001, 2, 709–714. [Google Scholar] [CrossRef]
- Houben, E.N.; Urbanus, M.L.; Van Der Laan, M.; Ten Hagen-Jongman, C.M.; Driessen, A.J.; Brunner, J.; Oudega, B.; Luirink, J. YidC and SecY mediate membrane insertion of a Type I transmembrane domain. J. Biol. Chem. 2002, 277, 35880–35886. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Kaback, H.R.; Dalbey, R.E. YidC protein, a molecular chaperone for LacY protein folding via the SecYEG protein machinery. J. Biol. Chem. 2013, 288, 28180–28194. [Google Scholar] [CrossRef]
- Kudva, R.; Denks, K.; Kuhn, P.; Vogt, A.; Muller, M.; Koch, H.G. Protein translocation across the inner membrane of Gram-negative bacteria: The Sec and Tat dependent protein transport pathways. Res. Microbiol. 2013, 164, 505–534. [Google Scholar] [CrossRef]
- Sachelaru, I.; Winter, L.; Knyazev, D.G.; Zimmermann, M.; Vogt, A.; Kuttner, R.; Ollinger, N.; Siligan, C.; Pohl, P.; Koch, H.-G. YidC and SecYEG form a heterotetrameric protein translocation channel. Sci. Rep. 2017, 7, 101. [Google Scholar] [CrossRef]
- Sachelaru, I.; Petriman, N.A.; Kudva, R.; Kuhn, P.; Welte, T.; Knapp, B.; Drepper, F.; Warscheid, B.; Koch, H.G. YidC occupies the lateral gate of the SecYEG translocon and is sequentially displaced by a nascent membrane protein. J. Biol. Chem. 2013, 288, 16295–16307. [Google Scholar] [CrossRef] [PubMed]
- Petriman, N.A.; Jauss, B.; Hufnagel, A.; Franz, L.; Sachelaru, I.; Drepper, F.; Warscheid, B.; Koch, H.G. The interaction network of the YidC insertase with the SecYEG translocon, SRP and the SRP receptor FtsY. Sci. Rep. 2018, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Nass, K.J.; Ilie, I.M.; Saller, M.J.; Driessen, A.J.M.; Caflisch, A.; Kammerer, R.A.; Li, X. The role of the N-terminal amphipathic helix in bacterial YidC: Insights from functional studies, the crystal structure and molecular dynamics simulations. Biochim. Biophys. Acta Biomembr. 2022, 1864, 183825. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Pop, O.I.; Haan, G.J.; Baars, L.; Koningstein, G.; Klepsch, M.M.; Genevaux, P.; Luirink, J.; de Gier, J.W. Biogenesis of MalF and the MalFGK(2) maltose transport complex in Escherichia coli requires YidC. J. Biol. Chem. 2008, 283, 17881–17890. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Zhao, Y.; Zheng, J.; Zhou, H.; Zhang, X.C.; Tian, C.; Huang, Y. Structure of YidC from Thermotoga maritima and its implications for YidC-mediated membrane protein insertion. FASEB J. 2018, 32, 2411–2421. [Google Scholar] [CrossRef] [PubMed]
- Kumazaki, K.; Chiba, S.; Takemoto, M.; Furukawa, A.; Nishiyama, K.; Sugano, Y.; Mori, T.; Dohmae, N.; Hirata, K.; Nakada-Nakura, Y.; et al. Structural basis of Sec-independent membrane protein insertion by YidC. Nature 2014, 509, 516–520. [Google Scholar] [CrossRef]
- Kumazaki, K.; Kishimoto, T.; Furukawa, A.; Mori, H.; Tanaka, Y.; Dohmae, N.; Ishitani, R.; Tsukazaki, T.; Nureki, O. Crystal structure of Escherichia coli YidC, a membrane protein chaperone and insertase. Sci. Rep. 2014, 4, 7299. [Google Scholar] [CrossRef]
- Chen, Y.; Capponi, S.; Zhu, L.; Gellenbeck, P.; Freites, J.A.; White, S.H.; Dalbey, R.E. YidC Insertase of Escherichia coli: Water Accessibility and Membrane Shaping. Structure 2017, 25, 1403–1414.e1403. [Google Scholar] [CrossRef]
- Chen, Y.; Sotomayor, M.; Capponi, S.; Hariharan, B.; Sahu, I.D.; Haase, M.; Lorigan, G.A.; Kuhn, A.; White, S.H.; Dalbey, R.E. A hydrophilic microenvironment in the substrate-translocating groove of the YidC membrane insertase is essential for enzyme function. J. Biol. Chem. 2022, 298, 101690. [Google Scholar] [CrossRef]
- Pohl, E.E.; Krylov, A.V.; Block, M.; Pohl, P. Changes of the membrane potential profile induced by verapamil and propranolol. Biochim. Biophys. Acta 1998, 1373, 170–178. [Google Scholar] [CrossRef]
- Ahern, C.A.; Horn, R. Stirring up controversy with a voltage sensor paddle. Trends Neurosci. 2004, 27, 303–307. [Google Scholar] [CrossRef]
- Chanda, B.; Asamoah, O.K.; Blunck, R.; Roux, B.; Bezanilla, F. Gating charge displacement in voltage-gated ion channels involves limited transmembrane movement. Nature 2005, 436, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Roux, B.; MacKinnon, R. The cavity and pore helices in the KcsA K+ channel: Electrostatic stabilization of monovalent cations. Science 1999, 285, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Hannesschlaeger, C.; Horner, A.; Pohl, P. Intrinsic Membrane Permeability to Small Molecules. Chem. Rev. 2019, 119, 5922–5953. [Google Scholar] [CrossRef] [PubMed]
- Smalinskaite, L.; Kim, M.K.; Lewis, A.J.O.; Keenan, R.J.; Hegde, R.S. Mechanism of an intramembrane chaperone for multipass membrane proteins. Nature 2022, 611, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Gumbart, J.; Chipot, C.; Schulten, K. Free energy of nascent-chain folding in the translocon. J. Am. Chem. Soc. 2011, 133, 7602–7607. [Google Scholar] [CrossRef]
- Lewis, A.J.O.; Hegde, R.S. A unified evolutionary origin for the ubiquitous protein transporters SecY and YidC. BMC Biol. 2021, 19, 266. [Google Scholar] [CrossRef]
- Ravaud, S.; Stjepanovic, G.; Wild, K.; Sinning, I. The crystal structure of the periplasmic domain of the Escherichia coli membrane protein insertase YidC contains a substrate binding cleft. J. Biol. Chem. 2008, 283, 9350–9358. [Google Scholar] [CrossRef]
- Oliver, D.C.; Paetzel, M. Crystal structure of the major periplasmic domain of the bacterial membrane protein assembly facilitator YidC. J. Biol. Chem. 2008, 283, 5208–5216. [Google Scholar] [CrossRef]
- Boy, D.; Koch, H.G. Visualization of distinct entities of the SecYEG translocon during translocation and integration of bacterial proteins. Mol. Biol. Cell 2009, 20, 1804–1815. [Google Scholar] [CrossRef]
- Kohler, R.; Boehringer, D.; Greber, B.; Bingel-Erlenmeyer, R.; Collinson, I.; Schaffitzel, C.; Ban, N. YidC and Oxa1 form dimeric insertion pores on the translating ribosome. Mol. Cell 2009, 34, 344–353. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.P.; Phillips, B.P.; Yagita, Y.; Juszkiewicz, S.; Wagner, A.; Malinverni, D.; Keenan, R.J.; Miller, E.A.; Hegde, R.S. The architecture of EMC reveals a path for membrane protein insertion. eLife 2020, 9, e57887. [Google Scholar] [CrossRef] [PubMed]
- Pleiner, T.; Tomaleri, G.P.; Januszyk, K.; Inglis, A.J.; Hazu, M.; Voorhees, R.M. Structural basis for membrane insertion by the human ER membrane protein complex. Science 2020, 369, 433–436. [Google Scholar] [CrossRef] [PubMed]
- McDowell, M.A.; Heimes, M.; Fiorentino, F.; Mehmood, S.; Farkas, A.; Coy-Vergara, J.; Wu, D.; Bolla, J.R.; Schmid, V.; Heinze, R.; et al. Structural Basis of Tail-Anchored Membrane Protein Biogenesis by the GET Insertase Complex. Mol. Cell 2020, 80, 72–86.e77. [Google Scholar] [CrossRef] [PubMed]
- Saparov, S.M.; Erlandson, K.; Cannon, K.; Schaletzky, J.; Schulman, S.; Rapoport, T.A.; Pohl, P. Determining the conductance of the SecY protein translocation channel for small molecules. Mol. Cell 2007, 26, 501–509. [Google Scholar] [CrossRef]
- Young, T.S.; Ahmad, I.; Yin, J.A.; Schultz, P.G. An enhanced system for unnatural amino acid mutagenesis in E. coli. J. Mol. Biol. 2010, 395, 361–374. [Google Scholar] [CrossRef]
- Jauss, B.; Petriman, N.A.; Drepper, F.; Franz, L.; Sachelaru, I.; Welte, T.; Steinberg, R.; Warscheid, B.; Koch, H.G. Non-competitive binding of PpiD and YidC to the SecYEG translocon expands the global view on the SecYEG interactome in E. coli. J. Biol. Chem. 2019, 294, 19167–19183. [Google Scholar] [CrossRef]
- Kedrov, A.; Sustarsic, M.; de Keyzer, J.; Caumanns, J.J.; Wu, Z.C.; Driessen, A.J. Elucidating the native architecture of the YidC: Ribosome complex. J. Mol. Biol. 2013, 425, 4112–4124. [Google Scholar] [CrossRef]
- Hanahan, D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 1983, 166, 557–580. [Google Scholar] [CrossRef]
- Urbanus, M.L.; Fröderberg, L.; Drew, D.; Björk, P.; de Gier, J.-W.L.; Brunner, J.; Oudega, B.; Luirink, J. Targeting, Insertion, and Localization of Escherichia coli YidC. J. Biol. Chem. 2002, 277, 12718–12723. [Google Scholar] [CrossRef]
- Hannesschlaeger, C.; Pohl, P. Membrane Permeabilities of Ascorbic Acid and Ascorbate. Biomolecules 2018, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Pohl, P.; Saparov, S.M.; Borgnia, M.J.; Agre, P. Highly selective water channel activity measured by voltage clamp: Analysis of planar lipid bilayers reconstituted with purified AqpZ. Proc. Natl. Acad. Sci. USA 2001, 98, 9624–9629. [Google Scholar] [CrossRef] [PubMed]
- Woodbury, D.J.; Hall, J.E. Role of channels in the fusion of vesicles with a planar bilayer. Biophys. J. 1988, 54, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Ederth, J.; Mandava, C.S.; Dasgupta, S.; Sanyal, S. A single-step method for purification of active His-tagged ribosomes from a genetically engineered Escherichia coli. Nucleic Acids Res. 2009, 37, e15. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, S.C.; Kim, H.D.; Gonzalez, R.L., Jr.; Puglisi, J.D.; Chu, S. tRNA dynamics on the ribosome during translation. Proc. Natl. Acad. Sci. USA 2004, 101, 12893–12898. [Google Scholar] [CrossRef] [PubMed]
- Magde, D.; Elson, E.L.; Webb, W.W. Fluorescence Correlation Spectroscopy. II. Experimental Realization. Biopolymers 1974, 13, 29–61. [Google Scholar] [CrossRef]
- Hoomann, T.; Jahnke, N.; Horner, A.; Keller, S.; Pohl, P. Filter gate closure inhibits ion but not water transport through potassium channels. Proc. Natl. Acad. Sci. USA 2013, 110, 10842–10847. [Google Scholar] [CrossRef]
- Wickles, S.; Singharoy, A.; Andreani, J.; Seemayer, S.; Bischoff, L.; Berninghausen, O.; Soeding, J.; Schulten, K.; van der Sluis, E.O.; Beckmann, R. A structural model of the active ribosome-bound membrane protein insertase YidC. Elife 2014, 3, e03035. [Google Scholar] [CrossRef]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Pahari, S.; Sun, L.; Alexov, E. PKAD: A database of experimentally measured pKa values of ionizable groups in proteins. Database 2019, 2019, baz024. [Google Scholar] [CrossRef]
- Evans, R.; O’Neill, M.; Pritzel, A.; Antropova, N.; Senior, A.; Green, T.; Žídek, A.; Bates, R.; Blackwell, S.; Yim, J.; et al. Protein complex prediction with AlphaFold-Multimer. bioRxiv 2021. bioRxiv:2021.2010.2004.463034. [Google Scholar] [CrossRef]
- Mirdita, M.; Steinegger, M.; Söding, J. MMseqs2 desktop and local web server app for fast, interactive sequence searches. Bioinformatics 2019, 35, 2856–2858. [Google Scholar] [CrossRef]
- Steinegger, M.; Meier, M.; Mirdita, M.; Vöhringer, H.; Haunsberger, S.J.; Söding, J. HH-suite3 for fast remote homology detection and deep protein annotation. BMC Bioinform. 2019, 20, 473. [Google Scholar] [CrossRef]
- Knyazev, D.G.; Winter, L.; Bauer, B.W.; Siligan, C.; Pohl, P. Ion conductivity of the bacterial translocation channel SecYEG engaged in translocation. J. Biol. Chem. 2014, 289, 24611–24616. [Google Scholar] [CrossRef] [PubMed]
- Kruger, V.; Deckers, M.; Hildenbeutel, M.; van der Laan, M.; Hellmers, M.; Dreker, C.; Preuss, M.; Herrmann, J.M.; Rehling, P.; Wagner, R.; et al. The mitochondrial oxidase assembly protein1 (Oxa1) insertase forms a membrane pore in lipid bilayers. J. Biol. Chem. 2012, 287, 33314–33326. [Google Scholar] [CrossRef]
- Knyazev, D.G.; Lents, A.; Krause, E.; Ollinger, N.; Siligan, C.; Papinski, D.; Winter, L.; Horner, A.; Pohl, P. The bacterial translocon SecYEG opens upon ribosome binding. J. Biol. Chem. 2013, 288, 17941–17946. [Google Scholar] [CrossRef]
- Knyazev, D.G.; Kuttner, R.; Bondar, A.N.; Zimmerman, M.; Siligan, C.; Pohl, P. Voltage Sensing in Bacterial Protein Translocation. Biomolecules 2020, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Schurholz, T.; Schindler, H. Lipid-protein surface films generated from membrane vesicles: Selfassembly, composition, and film structure. Eur. Biophys. J. 1991, 20, 71–78. [Google Scholar] [CrossRef]
- Samuelson, J.C.; Chen, M.; Jiang, F.; Moller, I.; Wiedmann, M.; Kuhn, A.; Phillips, G.J.; Dalbey, R.E. YidC mediates membrane protein insertion in bacteria. Nature 2000, 406, 637–641. [Google Scholar] [CrossRef]
- du Plessis, D.J.; Nouwen, N.; Driessen, A.J. Subunit a of cytochrome o oxidase requires both YidC and SecYEG for membrane insertion. J. Biol. Chem. 2006, 281, 12248–12252. [Google Scholar] [CrossRef]
- van Bloois, E.; Haan, G.J.; de Gier, J.W.; Oudega, B.; Luirink, J. Distinct requirements for translocation of the N-tail and C-tail of the Escherichia coli inner membrane protein CyoA. J. Biol. Chem. 2006, 281, 10002–10009. [Google Scholar] [CrossRef]
- Szyrach, G.; Ott, M.; Bonnefoy, N.; Neupert, W.; Herrmann, J.M. Ribosome binding to the Oxa1 complex facilitates co-translational protein insertion in mitochondria. EMBO J. 2003, 22, 6448–6457. [Google Scholar] [CrossRef]
- Geng, Y.; Kedrov, A.; Caumanns, J.J.; Crevenna, A.H.; Lamb, D.C.; Beckmann, R.; Driessen, A.J.M. Role of the Cytosolic Loop C2 and the C Terminus of YidC in Ribosome Binding and Insertion Activity. J. Biol. Chem. 2015, 290, 17250–17261. [Google Scholar] [CrossRef] [PubMed]
- Seitl, I.; Wickles, S.; Beckmann, R.; Kuhn, A.; Kiefer, D. The C-terminal regions of YidC from Rhodopirellula baltica and Oceanicaulis alexandrii bind to ribosomes and partially substitute for SRP receptor function in Escherichia coli. Mol. Microbiol. 2014, 91, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Palmer, S.R.; Hasona, A.; Nagamori, S.; Kaback, H.R.; Dalbey, R.E.; Brady, L.J. Functional overlap but lack of complete cross-complementation of Streptococcus mutans and Escherichia coli YidC orthologs. J. Bacteriol. 2008, 190, 2458–2469. [Google Scholar] [CrossRef]
- Koch, H.G.; Moser, M.; Schimz, K.L.; Muller, M. The integration of YidC into the cytoplasmic membrane of Escherichia coli requires the signal recognition particle, SecA and SecYEG. J. Biol. Chem. 2002, 277, 5715–5718. [Google Scholar] [CrossRef] [PubMed]
- Serek, J.; Bauer-Manz, G.; Struhalla, G.; van den Berg, L.; Kiefer, D.; Dalbey, R.; Kuhn, A. Escherichia coli YidC is a membrane insertase for Sec-independent proteins. EMBO J. 2004, 23, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Lotz, M.; Haase, W.; Kuhlbrandt, W.; Collinson, I. Projection structure of yidC: A conserved mediator of membrane protein assembly. J. Mol. Biol. 2008, 375, 901–907. [Google Scholar] [CrossRef]
- Klenner, C.; Kuhn, A. Dynamic Disulfide Scanning of the Membrane-inserting Pf3 Coat Protein Reveals Multiple YidC Substrate Contacts. J. Biol. Chem. 2012, 287, 3769–3776. [Google Scholar] [CrossRef]
- Steinberg, R.; Knuepffer, L.; Origi, A.; Asti, R.; Koch, H.-G. Co-translational protein targeting in bacteria. FEMS Microbiol. Lett. 2018, 365, fny095. [Google Scholar] [CrossRef]
- Sarmah, P.; Shang, W.; Origi, A.; Licheva, M.; Kraft, C.; Ulbrich, M.; Lichtenberg, E.; Wilde, A.; Koch, H.G. mRNA targeting eliminates the need for the signal recognition particle during membrane protein insertion in bacteria. Cell Rep. 2023, 42, 112140. [Google Scholar] [CrossRef]
- Neves-Petersen, M.T.; Klitgaard, S.; Pascher, T.; Skovsen, E.; Polivka, T.; Yartsev, A.; Sundstrom, V.; Petersen, S.B. Flash photolysis of cutinase: Identification and decay kinetics of transient intermediates formed upon UV excitation of aromatic residues. Biophys. J. 2009, 97, 211–226. [Google Scholar] [CrossRef]
- Oswald, J.; Njenga, R.; Natriashvili, A.; Sarmah, P.; Koch, H.G. The Dynamic SecYEG Translocon. Front. Mol. Biosci. 2021, 8, 664241. [Google Scholar] [CrossRef] [PubMed]
- Troman, L.; Alvira, S.; Daum, B.; Gold, V.A.M.; Collinson, I. Interaction of the periplasmic chaperone SurA with the inner membrane protein secretion (SEC) machinery. Biochem. J. 2023, 480, 283–296. [Google Scholar] [CrossRef]
- Schulze, R.J.; Komar, J.; Botte, M.; Allen, W.J.; Whitehouse, S.; Gold, V.A.; Lycklama, A.N.J.A.; Huard, K.; Berger, I.; Schaffitzel, C.; et al. Membrane protein insertion and proton-motive-force-dependent secretion through the bacterial holo-translocon SecYEG-SecDF-YajC-YidC. Proc. Natl. Acad. Sci. USA 2014, 111, 4844–4849. [Google Scholar] [CrossRef]
- Zhu, R.; Sinwel, D.; Hasenhuetl, P.S.; Saha, K.; Kumar, V.; Zhang, P.; Rankl, C.; Holy, M.; Sucic, S.; Kudlacek, O.; et al. Nanopharmacological Force Sensing to Reveal Allosteric Coupling in Transporter Binding Sites. Angew. Chem. Int. Ed. 2016, 55, 1719–1722. [Google Scholar] [CrossRef] [PubMed]
- Götzke, H.; Palombo, I.; Muheim, C.; Perrody, E.; Genevaux, P.; Kudva, R.; Müller, M.; Daley, D.O. YfgM Is an Ancillary Subunit of the SecYEG Translocon in Escherichia coli. J. Biol. Chem. 2014, 289, 19089–19097. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.W.; Collinson, I. A bacterial secretosome for regulated envelope biogenesis and quality control. bioRxiv 2022. bioRxiv:2022.2001.2012.476021. [Google Scholar] [CrossRef]
- Simon, S.M.; Blobel, G. Signal peptides open protein-conducting channels in E. coli. Cell 1992, 69, 677–684. [Google Scholar] [CrossRef]
- Nargang, F.E.; Preuss, M.; Neupert, W.; Herrmann, J.M. The Oxa1 protein forms a homooligomeric complex and is an essential part of the mitochondrial export translocase in Neurospora crassa. J. Biol. Chem. 2002, 277, 12846–12853. [Google Scholar] [CrossRef]
- Reif, S.; Randelj, O.; Domanska, G.; Dian, E.A.; Krimmer, T.; Motz, C.; Rassow, J. Conserved mechanism of Oxa1 insertion into the mitochondrial inner membrane. J. Mol. Biol. 2005, 354, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Kulawiak, B.; Hoepker, J.; Gebert, M.; Guiard, B.; Wiedemann, N.; Gebert, N. The mitochondrial protein import machinery has multiple connections to the respiratory chain. Biochim. Biophys. Acta 2013, 1827, 612–626. [Google Scholar] [CrossRef]
- Soman, R.; Yuan, J.; Kuhn, A.; Dalbey, R.E. Polarity and charge of the periplasmic loop determine the YidC and sec translocase requirement for the M13 procoat lep protein. J. Biol. Chem. 2014, 289, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Horner, A.; Zocher, F.; Preiner, J.; Ollinger, N.; Siligan, C.; Akimov, S.A.; Pohl, P. The mobility of single-file water molecules is governed by the number of H-bonds they may form with channel-lining residues. Sci. Adv. 2015, 1, e1400083. [Google Scholar] [CrossRef] [PubMed]
- Spann, D.; Pross, E.; Chen, Y.; Dalbey, R.E.; Kuhn, A. Each protomer of a dimeric YidC functions as a single membrane insertase. Sci. Rep. 2018, 8, 589. [Google Scholar] [CrossRef]
- Klenner, C.; Yuan, J.; Dalbey, R.E.; Kuhn, A. The Pf3 coat protein contacts TM1 and TM3 of YidC during membrane biogenesis. FEBS Lett. 2008, 582, 3967–3972. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knyazev, D.G.; Winter, L.; Vogt, A.; Posch, S.; Öztürk, Y.; Siligan, C.; Goessweiner-Mohr, N.; Hagleitner-Ertugrul, N.; Koch, H.-G.; Pohl, P. YidC from Escherichia coli Forms an Ion-Conducting Pore upon Activation by Ribosomes. Biomolecules 2023, 13, 1774. https://doi.org/10.3390/biom13121774
Knyazev DG, Winter L, Vogt A, Posch S, Öztürk Y, Siligan C, Goessweiner-Mohr N, Hagleitner-Ertugrul N, Koch H-G, Pohl P. YidC from Escherichia coli Forms an Ion-Conducting Pore upon Activation by Ribosomes. Biomolecules. 2023; 13(12):1774. https://doi.org/10.3390/biom13121774
Chicago/Turabian StyleKnyazev, Denis G., Lukas Winter, Andreas Vogt, Sandra Posch, Yavuz Öztürk, Christine Siligan, Nikolaus Goessweiner-Mohr, Nora Hagleitner-Ertugrul, Hans-Georg Koch, and Peter Pohl. 2023. "YidC from Escherichia coli Forms an Ion-Conducting Pore upon Activation by Ribosomes" Biomolecules 13, no. 12: 1774. https://doi.org/10.3390/biom13121774