Effects of Nickel at Environmentally Relevant Concentrations on Human Corneal Epithelial Cells: Oxidative Damage and Cellular Apoptosis
Abstract
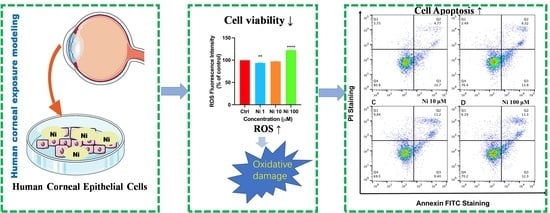
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture and Cell Exposure
2.3. Cell Morphology and Viability Assay
2.4. Measurement of ROS
2.5. Measurement of Cell Apoptosis Rate
2.6. RNA Extraction, Quantitative Real-Time PCR Analysis
2.7. Statistical Analysis
3. Results and Discussions
3.1. Ni exposure Decreased Cell Viability and Altered Cell Morphology
3.2. Ni Exposure Aggravated Oxidative Damage
3.3. Ni Exposure-Induced Cell Apoptosis
3.4. Ni Exposure Up-Regulated mRNA Expression of Apoptosis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeer-Wanklyn, C.J.; Zamble, D.B. Microbial nickel: Cellular uptake and delivery to enzyme centers. Curr. Opin. Chem. Biol. 2017, 37, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Alquezar, C.; Felix, J.B.; McCandlish, E.; Buckley, B.T.; Caparros-Lefebvre, D.; Karch, C.M.; Golbe, L.I.; Kao, A.W. Heavy metals contaminating the environment of a progressive supranuclear palsy cluster induce tau accumulation and cell death in cultured neurons. Sci. Rep. 2020, 10, 569. [Google Scholar] [CrossRef] [PubMed]
- Zambelli, B.; Ciurli, S. Nickel and Human Health. Metal. Ions Life Sci. 2013, 13, 321–357. [Google Scholar]
- Norris, M.R.; Bielory, L. Cosmetics and ocular allergy. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 404–410. [Google Scholar] [CrossRef]
- Xiang, P.; Jia, Y.; Wang, K.; Li, M.-Y.; Qin, Y.-S.; He, R.-W.; Gao, P.; Liu, Y.; Liu, X.; Ma, L.Q. Water extract of indoor dust induces tight junction disruption in normal human corneal epithelial cells. Environ. Pollut. 2018, 243, 301–307. [Google Scholar] [CrossRef]
- Chao, H. Metal Ions in Life Sciences. In Interrelations between Essential Metal Ions and Human Diseases; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer: Dordrecht, The Netherlands, 2013; Volume 13, ISBN 978-94-007-7499-5. [Google Scholar]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Guo, H.; Liu, H.; Jian, Z.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L.; et al. Nickel induces inflammatory activation via NF-κB, MAPKs, IRF3 and NLRP3 inflammasome signaling pathways in macrophages. Aging 2019, 11, 11659–11672. [Google Scholar] [CrossRef]
- Kalagbor, I.A.; Dibofori-Orji, A.N.; Ekpete, O.A. Exposure to Heavy Metals in Soot Samples and Cancer Risk Assessment in Port Harcourt, Nigeria. J. Health Pollut. 2019, 9, 191211. [Google Scholar] [CrossRef]
- Scarselli, A.; Di Marzio, D.; Marinaccio, A.; Iavicoli, S. Nickel compounds in the workplaces: Occupations and activities involving high-risk exposures in Italy. Am. J. Ind. Med. 2018, 61, 968–977. [Google Scholar] [CrossRef]
- Guo, H.; Liu, H.; Jian, Z.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L.; et al. Immunotoxicity of nickel: Pathological and toxicological effects. Ecotoxicol. Environ. Saf. 2020, 203, 111006. [Google Scholar] [CrossRef]
- E Meyers, V.; Garcìa, H.D.; Monds, K.; Cooper, B.L.; James, J.T. Ocular toxicity of authentic lunar dust. BMC Ophthalmol. 2012, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- Miao, Q.; Xu, Y.; Zhang, H.; Xu, P.; Ye, J. Cigarette smoke induces ROS mediated autophagy impairment in human corneal epithelial cells. Environ. Pollut. 2019, 245, 389–397. [Google Scholar] [CrossRef]
- Dreher, K.L.; Jaskot, R.H.; Lehmann, J.R.; Richards, J.H.; Ghio, J.K.M.A.J.; Costa, D.L. Soluble Transition Metals Mediate Residual Oil Fly Ash Induced Acute Lung Injury. J. Toxicol. Environ. Health 1997, 50, 285–305. [Google Scholar]
- Gavett, S.H.; Madison, S.L.; Dreher, K.L.; Winsett, D.W.; McGee, J.K.; Costa, D.L. Metal and Sulfate Composition of Residual Oil Fly Ash Determines Airway Hyperreactivity and Lung Injury in Rats. Environ. Res. 1997, 72, 162–172. [Google Scholar] [CrossRef]
- Getachew, B.; Amde, M.; Danno, B.L. Level of selected heavy metals in surface dust collected from electronic and electrical material maintenance shops in selected Western Oromia towns, Ethiopia. Environ. Sci. Pollut. Res. 2019, 26, 18593–18603. [Google Scholar] [CrossRef]
- Cao, S.; Duan, X.; Zhao, X.; Wang, B.; Ma, J.; Fan, D.; Sun, C.; He, B.; Wei, F.; Jiang, G. Health risk assessment of various metal(loid)s via multiple exposure pathways on children living near a typical lead-acid battery plant, China. Environ. Pollut. 2015, 200, 16–23. [Google Scholar] [CrossRef]
- Xiang, P.; Liu, R.-Y.; Li, C.; Gao, P.; Cui, X.-Y.; Ma, L.Q. Effects of organophosphorus flame retardant TDCPP on normal human corneal epithelial cells: Implications for human health. Environ. Pollut. 2017, 230, 22–30. [Google Scholar] [CrossRef]
- Wolkoff, P. Ocular discomfort by environmental and personal risk factors altering the precorneal tear film. Toxicol. Lett. 2010, 199, 203–212. [Google Scholar] [CrossRef]
- Sunderman, F.W.; Allpass, P.R.; Mitchell, J.M.; Baselt, R.C.; Albert, D.M. Eye malformations in rats: Induction by prenatal exposure to nickel carbonyl. Science 1979, 203, 550–553. [Google Scholar] [CrossRef]
- Hu, W.; Yu, Z.; Gao, X.; Wu, Y.; Tang, M.; Kong, L. Study on the damage of sperm induced by nickel nanoparticle exposure. Environ. Geochem. Health 2020, 42, 1715–1724. [Google Scholar] [CrossRef]
- Siddiqui, M.; Ahamed, M.; Ahmad, J.; Khan, M.M.; Musarrat, J.; Al-Khedhairy, A.; Alrokayan, S. Nickel oxide nanoparticles induce cytotoxicity, oxidative stress and apoptosis in cultured human cells that is abrogated by the dietary antioxidant curcumin. Food Chem. Toxicol. 2012, 50, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.Z.; Kleve, M.G. Nickel nanowires induced and reactive oxygen species mediated apoptosis in human pancreatic adenocarcinoma cells. Int. J. Nanomed. 2011, 6, 1475–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puttaswamy, N.; Liber, K. Influence of inorganic anions on metals release from oil sands coke and on toxicity of nickel and vanadium to Ceriodaphnia dubia. Chemosphere 2012, 86, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Mlhave, L.; Kjrgaard, S.K.; Attermann, J. Effects in the eyes caused by exposure to office dust. Indoor Air 2002, 12, 165–174. [Google Scholar] [CrossRef]
- Lyu, D.; Chen, Z.; Almansoob, S.; Chen, H.; Ye, Y.; Song, F.; Zhang, L.; Qin, Z.; Tang, Q.; Yin, H. Transcriptomic profiling of human corneal epithelial cells exposed to airborne fine particulate matter (PM2.5). Ocul. Surf. 2020, 18, 554–564. [Google Scholar] [CrossRef]
- Lewis, J.B.; Messer, R.L.W.; Pitts, L.; Hsu, S.D.; Hansen, J.M.; Wataha, J.C. Ni(II) ions dysregulate cytokine secretion from human monocytes. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2009, 88, 358–365. [Google Scholar] [CrossRef]
- Freitas, M.; Barcellos-De-Souza, P.; Barja-Fidalgo, C.; Fernandes, E. Nickel induces apoptosis in human neutrophils. BioMetals 2013, 26, 13–21. [Google Scholar] [CrossRef]
- Ahmad, J.; Alhadlaq, H.A.; Siddiqui, M.A.; Saquib, Q.; Al-Khedhairy, A.A.; Musarrat, J.; Ahamed, M. Concentration-dependent induction of reactive oxygen species, cell cycle arrest and apoptosis in human liver cells after nickel nanoparticles exposure. Environ. Toxicol. 2015, 30, 137–148. [Google Scholar] [CrossRef]
- Lemp, M.A. New Strategies in the Treatment of Dry-Eye States. Cornea 1999, 18, 625–632. [Google Scholar] [CrossRef]
- Kim, A.; Park, S.; Sung, J.H. Cell Viability and Immune Response to Low Concentrations of Nickel and Cadmium: An In Vitro Model. Int. J. Environ. Res. Public Health 2020, 17, 9218. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, M.; Pei, L.; Liu, X.; Wei, L.; Li, A.; Mei, Y.; Xu, Q. Effects of heavy metal mixture exposure on hematological and biomedical parameters mediated by oxidative stress. Sci. Total Environ. 2020, 705, 134865. [Google Scholar] [CrossRef]
- Han, A.; Zou, L.; Gan, X.; Li, Y.; Liu, F.; Chang, X.; Zhang, X.; Tian, M.; Li, S.; Su, L.; et al. ROS generation and MAPKs activation contribute to the Ni-induced testosterone synthesis disturbance in rat Leydig cells. Toxicol. Lett. 2018, 290, 36–45. [Google Scholar] [CrossRef]
- Wang, Y.F.; Shyu, H.W.; Chang, Y.C.; Tseng, W.C.; Huang, Y.L.; Lin, K.H.; Chou, M.C.; Liu, H.L.; Chen, C.Y. Nickel (II)-induced cytotoxi city and apoptosis in human proximal tubule cells through a ROS- and mitochondria-mediated pathway. Toxicol. Appl. Pharmacol. 2012, 259, 177–186. [Google Scholar] [CrossRef]
- Sun, J.; Yang, Q.; Wang, D.; Wang, S.; Chen, F.; Zhong, Y.; Yi, K.; Yao, F.; Jiang, C.; Li, S.; et al. Nickel toxicity to the performance and microbial community of enhanced biological phosphorus removal system. Chem. Eng. J. 2017, 313, 415–423. [Google Scholar] [CrossRef]
- Nzengue, Y.; Steiman, R.; Rachidi, W.; Favier, A.; Guiraud, P. Oxidative Stress Induced by Cadmium in the C6 Cell Line: Role of Copper and Zinc. Biol. Trace Elem. Res. 2012, 146, 410–419. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, L.; Liu, M.; Huang, B.; Zhang, N.; Mehmood, R.; Nan, K.; Li, Q.; Chen, W.; Lin, S. In situ scavenging of mitochondrial ROS by anti-oxidative MitoQ/hyaluronic acid nanoparticles for environment-induced dry eye disease therapy. Chem. Eng. J. 2020, 398, 125621. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Ott, M.; Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria, oxidative stress and cell death. Apoptosis 2007, 12, 913–922. [Google Scholar] [CrossRef]
- Kawanishi, S.; Yamamoto, I.K. Active oxygen species in DNA damage induced by carcinogenic metal compounds. Environ. Health Perspect. 1994, 102, 17–20. [Google Scholar]
- Nezhat, F.; Cohen, C.; Rahaman, J.; Gretz, H.; Cole, P.; Kalir, T. Comparative immunohistochemical studies of bcl-2 and p53 proteins in benign and malignant ovarian endometriotic cysts. Cancer 2002, 94, 2935–2940. [Google Scholar] [CrossRef]
- Wang, K.; Ma, J.-Y.; Li, M.-Y.; Qin, Y.-S.; Bao, X.-C.; Wang, C.-C.; Cui, D.-L.; Xiang, P.; Ma, L.Q. Mechanisms of Cd and Cu induced toxicity in human gastric epithelial cells: Oxidative stress, cell cycle arrest and apoptosis. Sci. Total Environ. 2021, 756, 143951. [Google Scholar] [CrossRef]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive Oxygen Species (ROS) Regulates Different Types of Cell Death by Acting as a Rheostat. Oxidative Med. Cell. Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef]
- Häcker, G.; Paschen, S.A. Therapeutic targets in the mitochondrial apoptotic pathway. Expert Opin. Ther. Targets 2007, 11, 515–526. [Google Scholar] [CrossRef]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Su, H.; Bidère, N.; Zheng, L.; Cubre, A.; Sakai, K.; Dale, J.; Salmena, L.; Hakem, R.; Straus, S.; Leonardo, M. Requirement for Caspase-8 in NF-κB Activation by Antigen Receptor. Science 2005, 307, 1465–1468. [Google Scholar] [CrossRef]
- Fujisawa, T.; Chang, M.M.J.; Velichko, S.; Thai, P.; Hung, L.Y.; Huang, F.; Phuong, N.; Chen, Y.; Wu, R. NF-κB Mediates IL-1β– and IL-17A–Induced MUC5B Expression in Airway Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2011, 45, 246–252. [Google Scholar] [CrossRef]
- Zheng, Q.; Tan, Q.; Ren, Y.; Reinach, P.S.; Li, L.; Ge, C.; Qu, J.; Chen, W. Hyperosmotic Stress-Induced TRPM2 Channel Activation Stimulates NLRP3 Inflammasome Activity in Primary Human Corneal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3259–3268. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Gurung, P.; Kanneganti, T.-D. Novel Roles for Caspase-8 in IL-1β and Inflammasome Regulation. Am. J. Pathol. 2015, 185, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S. Apoptosis by death receptor. Cell 1997, 88, 355–365. [Google Scholar] [CrossRef]
- Allan, L.A.; Clarke, P.R. Apoptosis and autophagy: Regulation of caspase-9 by phosphorylation. FEBs J. 2010, 276, 6063–6073. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Forward Primer | Reverse Primer | |
|---|---|---|
| β-actin | GACATCCGCAAAGACCTG | GGAAGGTGGACAGCGAG |
| NF-κB | GAAGAAAATGGTGGAGTCTG | GGTTCACTAGTTTCCAAGTC |
| IL-1β | AGCTACGAATCTCCGACCAC | CGTTATCCCATGTGTCGAAGAA |
| Caspase-3 | CATGGAAGCGAATCAATGGACT | CTGTACCAGACCGAGATGTCA |
| Caspase-8 Caspase-9 | CGGACTCTCCAAGAGAACAGG | TCAAAGGTCGTGGTCAAAGCC |
| CTCAGACCAGAGATTCGCAAAC | GCATTTCCCCTCAAACTCTCAA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.-N.; Liu, H.; Liu, M.-M.; Yang, D.-L.; Bi, J.; Chen, Q.-Q.; Chen, W.; Xiang, P. Effects of Nickel at Environmentally Relevant Concentrations on Human Corneal Epithelial Cells: Oxidative Damage and Cellular Apoptosis. Biomolecules 2022, 12, 1283. https://doi.org/10.3390/biom12091283
Zhang Z-N, Liu H, Liu M-M, Yang D-L, Bi J, Chen Q-Q, Chen W, Xiang P. Effects of Nickel at Environmentally Relevant Concentrations on Human Corneal Epithelial Cells: Oxidative Damage and Cellular Apoptosis. Biomolecules. 2022; 12(9):1283. https://doi.org/10.3390/biom12091283
Chicago/Turabian StyleZhang, Zhen-Ning, Hai Liu, Mi-Mi Liu, Dan-Lei Yang, Jue Bi, Qian-Qian Chen, Wei Chen, and Ping Xiang. 2022. "Effects of Nickel at Environmentally Relevant Concentrations on Human Corneal Epithelial Cells: Oxidative Damage and Cellular Apoptosis" Biomolecules 12, no. 9: 1283. https://doi.org/10.3390/biom12091283