PCSK9 Plasma Levels Are Associated with Mechanical Vascular Impairment in Familial Hypercholesterolemia Subjects without a History of Atherosclerotic Cardiovascular Disease: Results of Six-Month Add-On PCSK9 Inhibitor Therapy
Abstract
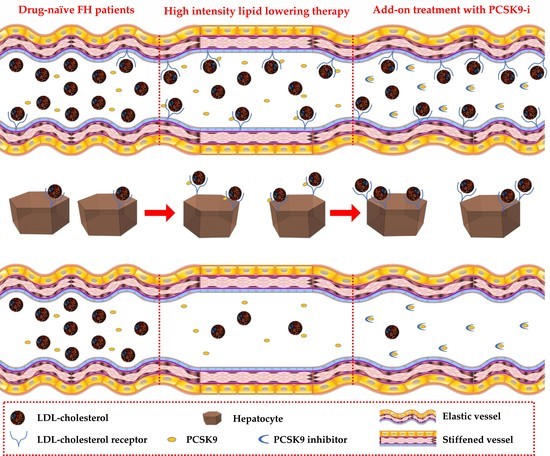
:1. Introduction
2. Materials and Methods
2.1. Pulse Wave Velocity Evaluation
2.2. PCSK9 Plasma Levels
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Writing Group, M.; Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.P.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef]
- Boren, J.; Williams, K.J. The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: A triumph of simplicity. Curr. Opin. Lipidol. 2016, 27, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.; Sjouke, B.; Choque, B.; Kastelein, J.J.; Hovingh, G.K. The PCSK9 decade. J. Lipid Res. 2012, 53, 2515–2524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farnier, M. PCSK9: From discovery to therapeutic applications. Arch. Cardiovasc. Dis. 2014, 107, 58–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidah, N.G.; Awan, Z.; Chretien, M.; Mbikay, M. PCSK9: A key modulator of cardiovascular health. Circ. Res. 2014, 114, 1022–1036. [Google Scholar] [CrossRef]
- Kuzmich, N.; Andresyuk, E.; Porozov, Y.; Tarasov, V.; Samsonov, M.; Preferanskaya, N.; Veselov, V.; Alyautdin, R. PCSK9 as a Target for Development of a New Generation of Hypolipidemic Drugs. Molecules 2022, 27, 434. [Google Scholar] [CrossRef]
- Mayne, J.; Dewpura, T.; Raymond, A.; Cousins, M.; Chaplin, A.; Lahey, K.A.; Lahaye, S.A.; Mbikay, M.; Ooi, T.C.; Chretien, M. Plasma PCSK9 levels are significantly modified by statins and fibrates in humans. Lipids Health Dis. 2008, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Feng, X.; Zhou, Y. PCSK9 Variants in Familial Hypercholesterolemia: A Comprehensive Synopsis. Front. Genet. 2020, 11, 1020. [Google Scholar] [CrossRef]
- Costet, P.; Hoffmann, M.M.; Cariou, B.; Guyomarc’h Delasalle, B.; Konrad, T.; Winkler, K. Plasma PCSK9 is increased by fenofibrate and atorvastatin in a non-additive fashion in diabetic patients. Atherosclerosis 2010, 212, 246–251. [Google Scholar] [CrossRef]
- Guo, Y.L.; Liu, J.; Xu, R.X.; Zhu, C.G.; Wu, N.Q.; Jiang, L.X.; Li, J.J. Short-term impact of low-dose atorvastatin on serum proprotein convertase subtilisin/kexin type 9. Clin. Drug Investig. 2013, 33, 877–883. [Google Scholar] [CrossRef]
- Careskey, H.E.; Davis, R.A.; Alborn, W.E.; Troutt, J.S.; Cao, G.; Konrad, R.J. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J. Lipid. Res. 2008, 49, 394–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welder, G.; Zineh, I.; Pacanowski, M.A.; Troutt, J.S.; Cao, G.; Konrad, R.J. High-dose atorvastatin causes a rapid sustained increase in human serum PCSK9 and disrupts its correlation with LDL cholesterol. J. Lipid. Res. 2010, 51, 2714–2721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khera, A.V.; Qamar, A.; Reilly, M.P.; Dunbar, R.L.; Rader, D.J. Effects of niacin, statin, and fenofibrate on circulating proprotein convertase subtilisin/kexin type 9 levels in patients with dyslipidemia. Am. J. Cardiol. 2015, 115, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Awan, Z.; Seidah, N.G.; MacFadyen, J.G.; Benjannet, S.; Chasman, D.I.; Ridker, P.M.; Genest, J. Rosuvastatin, proprotein convertase subtilisin/kexin type 9 concentrations, and LDL cholesterol response: The JUPITER trial. Clin. Chem. 2012, 58, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Raal, F.; Panz, V.; Immelman, A.; Pilcher, G. Elevated PCSK9 levels in untreated patients with heterozygous or homozygous familial hypercholesterolemia and the response to high-dose statin therapy. J. Am. Heart Assoc. 2013, 2, e000028. [Google Scholar] [CrossRef] [Green Version]
- Berthold, H.K.; Seidah, N.G.; Benjannet, S.; Gouni-Berthold, I. Evidence from a randomized trial that simvastatin, but not ezetimibe, upregulates circulating PCSK9 levels. PLoS ONE 2013, 8, e60095. [Google Scholar] [CrossRef] [Green Version]
- Nozue, T.; Hattori, H.; Ogawa, K.; Kujiraoka, T.; Iwasaki, T.; Michishita, I. Effects of Statin Therapy on Plasma Proprotein Convertase Subtilisin/kexin Type 9 and Sortilin Levels in Statin-Naive Patients with Coronary Artery Disease. J. Atheroscler. Thromb. 2016, 23, 848–856. [Google Scholar] [CrossRef] [Green Version]
- Sahebkar, A.; Simental-Mendia, L.E.; Guerrero-Romero, F.; Golledge, J.; Watts, G.F. Effect of statin therapy on plasma proprotein convertase subtilisin kexin 9 (PCSK9) concentrations: A systematic review and meta-analysis of clinical trials. Diabetes Obes. Metab. 2015, 17, 1042–1055. [Google Scholar] [CrossRef]
- Hentze, H.; Jensen, K.K.; Chia, S.M.; Johns, D.G.; Shaw, R.J.; Davis, H.R., Jr.; Shih, S.J.; Wong, K.K. Inverse relationship between LDL cholesterol and PCSK9 plasma levels in dyslipidemic cynomolgus monkeys: Effects of LDL lowering by ezetimibe in the absence of statins. Atherosclerosis 2013, 231, 84–90. [Google Scholar] [CrossRef]
- Davignon, J.; Dubuc, G. Statins and ezetimibe modulate plasma proprotein convertase subtilisin kexin-9 (PCSK9) levels. Trans. Am. Clin. Climatol. Assoc. 2009, 120, 163–173. [Google Scholar]
- Dubuc, G.; Tremblay, M.; Pare, G.; Jacques, H.; Hamelin, J.; Benjannet, S.; Boulet, L.; Genest, J.; Bernier, L.; Seidah, N.G.; et al. A new method for measurement of total plasma PCSK9: Clinical applications. J. Lipid Res. 2010, 51, 140–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, K.; Iwahashi, N.; Endo, T.; Himeno, H.; Fukui, K.; Kobayashi, S.; Shimizu, M.; Iwasawa, Y.; Morita, Y.; Wada, A.; et al. Long-term effects of ezetimibe-plus-statin therapy on low-density lipoprotein cholesterol levels as compared with double-dose statin therapy in patients with coronary artery disease. Atherosclerosis 2012, 224, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Nozue, T. Lipid Lowering Therapy and Circulating PCSK9 Concentration. J. Atheroscler. Thromb. 2017, 24, 895–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galema-Boers, A.M.H.; Lenzen, M.J.; Sijbrands, E.J.; Roeters van Lennep, J.E. Proprotein convertase subtilisin/kexin 9 inhibition in patients with familial hypercholesterolemia: Initial clinical experience. J. Clin. Lipidol. 2017, 11, 674–681. [Google Scholar] [CrossRef]
- Robinson, J.G.; Farnier, M.; Kastelein, J.J.P.; Roth, E.M.; Taskinen, M.R.; Colhoun, H.M.; Brunet, A.; DiCioccio, A.T.; Lecorps, G.; Pordy, R.; et al. Relationship between alirocumab, PCSK9, and LDL-C levels in four phase 3 ODYSSEY trials using 75 and 150 mg doses. J. Clin. Lipidol. 2019, 13, 979–988.e910. [Google Scholar] [CrossRef] [Green Version]
- Afanasieva, O.; Ezhov, M.V.; Klesareva, E.; Razova, O.; Chubykina, U.; Egiazaryan, M.; Sherstyuk, E.; Afanasieva, M.; Utkina, E.; Pokrovsky, S. Effect of Evolocumab on Lipoprotein(a) and PCSK9 in Healthy Individuals with Elevated Lipoprotein(a) Level. J. Cardiovasc. Dev. Dis 2020, 7, 45. [Google Scholar] [CrossRef]
- Ridker, P.M. Clinician’s Guide to Reducing Inflammation to Reduce Atherothrombotic Risk: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2018, 72, 3320–3331. [Google Scholar] [CrossRef]
- Scicali, R.; Di Pino, A.; Urbano, F.; Ferrara, V.; Marchisello, S.; Di Mauro, S.; Scamporrino, A.; Filippello, A.; Piro, S.; Rabuazzo, A.M.; et al. Analysis of S100A12 plasma levels in hyperlipidemic subjects with or without familial hypercholesterolemia. Acta Diabetol 2019, 56, 899–906. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [Green Version]
- Ruscica, M.; Tokgozoglu, L.; Corsini, A.; Sirtori, C.R. PCSK9 inhibition and inflammation: A narrative review. Atherosclerosis 2019, 288, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Mandraffino, G.; Scicali, R.; Rodriguez-Carrio, J.; Savarino, F.; Mamone, F.; Scuruchi, M.; Cinquegrani, M.; Imbalzano, E.; Di Pino, A.; Piro, S.; et al. Arterial stiffness improvement after adding on PCSK9 inhibitors or ezetimibe to high-intensity statins in patients with familial hypercholesterolemia: A Two-Lipid Center Real-World Experience. J. Clin. Lipidol 2020, 14, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Reference Values for Arterial Stiffness, C. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ’Establishing normal and reference values’. Eur. Heart J. 2010, 31, 2338–2350. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Kim, S.H. Pulse Wave Velocity in Atherosclerosis. Front. Cardiovasc. Med. 2019, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Kubozono, T.; Miyata, M.; Kawasoe, S.; Ojima, S.; Yoshifuku, S.; Miyahara, H.; Maenohara, S.; Ohishi, M. High Pulse Wave Velocity Has a Strong Impact on Early Carotid Atherosclerosis in a Japanese General Male Population. Circ. J. 2017, 81, 310–315. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Fan, F.; Kou, M.; Yang, Y.; Cheng, G.; Jia, J.; Gao, L.; Zhou, Z.; Chen, D.; Zhang, Y.; et al. Brachial-Ankle Pulse Wave Velocity is Associated with the Risk of New Carotid Plaque Formation: Data from a Chinese Community-based Cohort. Sci. Rep. 2018, 8, 7037. [Google Scholar] [CrossRef]
- Zureik, M.; Temmar, M.; Adamopoulos, C.; Bureau, J.M.; Courbon, D.; Thomas, F.; Bean, K.; Touboul, P.J.; Ducimetiere, P.; Benetos, A. Carotid plaques, but not common carotid intima-media thickness, are independently associated with aortic stiffness. J. Hypertens. 2002, 20, 85–93. [Google Scholar] [CrossRef]
- Scicali, R.; Mandraffino, G.; Di Pino, A.; Scuruchi, M.; Ferrara, V.; Squadrito, G.; Purrello, F.; Piro, S. Impact of high neutrophil-to-lymphocyte ratio on the cardiovascular benefit of PCSK9 inhibitors in familial hypercholesterolemia subjects with atherosclerotic cardiovascular disease: Real-world data from two lipid units. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 3401–3406. [Google Scholar] [CrossRef]
- Casula, M.; Olmastroni, E.; Pirillo, A.; Catapano, A.L.; Members of the Lipigen Steering Commettee. Evaluation of the performance of Dutch Lipid Clinic Network score in an Italian FH population: The LIPIGEN study. Atherosclerosis 2018, 277, 413–418. [Google Scholar] [CrossRef] [Green Version]
- Levenson, A.E.; Shah, A.S.; Khoury, P.R.; Kimball, T.R.; Urbina, E.M.; de Ferranti, S.D.; Maahs, D.M.; Dolan, L.M.; Wadwa, R.P.; Biddinger, S.B. Obesity and type 2 diabetes are associated with elevated PCSK9 levels in young women. Pediatr. Diabetes 2017, 18, 755–760. [Google Scholar] [CrossRef]
- Kim, C.H.; An, H.; Kim, S.H.; Shin, D. Pharmacokinetic and pharmacodynamic interaction between ezetimibe and rosuvastatin in healthy male subjects. Drug Des. Devel. Ther. 2017, 11, 3461–3469. [Google Scholar] [CrossRef] [Green Version]
- Filippatos, T.D.; Kei, A.; Rizos, C.V.; Elisaf, M.S. Effects of PCSK9 Inhibitors on Other than Low-Density Lipoprotein Cholesterol Lipid Variables. J. Cardiovasc. Pharmacol. Ther. 2018, 23, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, M.; Plana, N.; Ibarretxe, D.; Cabre, A.; Gonzalez, M.; Ribalta, J.; Masana, L. Circulating PCSK9 levels are positively correlated with NMR-assessed atherogenic dyslipidaemia in patients with high cardiovascular risk. Clin. Sci. 2015, 128, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Ruscica, M.; Camera, M.; Rossetti, L.; Macchi, C.; Colciago, A.; Zanotti, I.; Lupo, M.G.; Adorni, M.P.; Cicero, A.F.G.; et al. PCSK9 induces a pro-inflammatory response in macrophages. Sci. Rep. 2018, 8, 2267. [Google Scholar] [CrossRef] [Green Version]
- Ruscica, M.; Ferri, N.; Fogacci, F.; Rosticci, M.; Botta, M.; Marchiano, S.; Magni, P.; D’Addato, S.; Giovannini, M.; Borghi, C.; et al. Circulating Levels of Proprotein Convertase Subtilisin/Kexin Type 9 and Arterial Stiffness in a Large Population Sample: Data From the Brisighella Heart Study. J. Am. Heart Assoc. 2017, 6, 5764. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.M.; Oemrawsingh, R.M.; Garcia-Garcia, H.M.; Boersma, E.; van Geuns, R.J.; Serruys, P.W.; Kardys, I.; Akkerhuis, K.M. PCSK9 in relation to coronary plaque inflammation: Results of the ATHEROREMO-IVUS study. Atherosclerosis 2016, 248, 117–122. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Xhaard, C.; Lamiral, Z.; Borges-Canha, M.; Neves, J.S.; Dandine-Roulland, C.; LeFloch, E.; Deleuze, J.F.; Bacq-Daian, D.; Bozec, E.; et al. PCSK9 Protein and rs562556 Polymorphism Are Associated With Arterial Plaques in Healthy Middle-Aged Population: The STANISLAS Cohort. J. Am. Heart Assoc. 2020, 9, e014758. [Google Scholar] [CrossRef]
- Toth, S.; Fedacko, J.; Pekarova, T.; Hertelyova, Z.; Katz, M.; Mughees, A.; Kuzma, J.; Stefanic, P.; Kopolovets, I.; Pella, D. Elevated Circulating PCSK9 Concentrations Predict Subclinical Atherosclerotic Changes in Low Risk Obese and Non-Obese Patients. Cardiol. Ther. 2017, 6, 281–289. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, Z.; Yan, B.; Yin, H.; Tai, S.; Peng, J.; Cui, Y.; Gui, Y.; Belke, D.; Zhou, S.; et al. PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) Triggers Vascular Smooth Muscle Cell Senescence and Apoptosis: Implication of Its Direct Role in Degenerative Vascular Disease. Arter. Thromb. Vasc. Biol 2022, 42, 67–86. [Google Scholar] [CrossRef]
- Burggraaf, B.; Pouw, N.M.C.; Arroyo, S.F.; van Vark-van der Zee, L.C.; van de Geijn, G.M.; Birnie, E.; Huisbrink, J.; van der Zwan, E.M.; Mulder, M.T.; Rensen, P.C.N.; et al. A placebo-controlled proof-of-concept study of alirocumab on postprandial lipids and vascular elasticity in insulin-treated patients with type 2 diabetes mellitus. Diabetes Obes. Metab. 2020, 22, 807–816. [Google Scholar] [CrossRef]
- Scicali, R.; Russo, G.I.; Di Mauro, M.; Manuele, F.; Di Marco, G.; Di Pino, A.; Ferrara, V.; Rabuazzo, A.M.; Piro, S.; Morgia, G.; et al. Analysis of Arterial Stiffness and Sexual Function after Adding on PCSK9 Inhibitor Treatment in Male Patients with Familial Hypercholesterolemia: A Single Lipid Center Real-World Experience. J. Clin. Med. 2020, 9, 597. [Google Scholar] [CrossRef] [PubMed]


| Controls n = 26 | HeFH (Baseline) n = 26 | p-Value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age | 43 (9) | 42 (14) | 0.969 |
| Female, n (%) | 9 (34.6) | 9 (34.6) | - |
| BMI, kg/m2 | 23.8 (1.8) | 27.1 (5.2) | 0.05 |
| ASCVD, n (%) | 0 (0) | 0 (0) | - |
| Hypertension | 0 (0) | 0 (0) | - |
| Type 2 Diabetes, n | 0 (0) | 0 (0) | - |
| Carotid plaque | 0 (0) | 0 (0) | - |
| Lipid profile | |||
| TC max (mg/dL) | - | 353 (49) | N/A |
| LDL-C max (mg/dL) | - | 273 (49) | N/A |
| TC (mg/dL) | 202 (15) | 342 (48) | <0.001 |
| HDL-C (mg/dL) | 65 (7) | 52 (11) | <0.001 |
| TG (mg/dL) | 90 (30) | 132 (68) | 0.120 |
| LDL-C (mg/dL) | 119 (11) | 264 (45) | <0.001 |
| PCSK9 (ng/mL) | 161 (4) | 196 (7) | <0.001 |
| CV risk-associated variables | |||
| SBP (mmHg) | 121 (9) | 124 (12) | 0.296 |
| DBP (mmHg) | 70 (6) | 77 (13) | 0.068 |
| PWV (m/s) | 4.9 (0.4) | 9.6 (3.1) | <0.001 |
| Treatment | |||
| Rosuvastatin 20 mg, n (%) | - | 18 (69.2) | - |
| Atorvastatin 40 mg, n (%) | - | 8 (30.8) | - |
| Ezetimibe 10 mg, n (%) | - | 26 (100) | - |
| T0 | T1 | T2 | p-Value (T1 vs. T0) | p-Value (T2 vs. T1) | |
|---|---|---|---|---|---|
| Study variables | |||||
| TC | 342 (48) | 212 (30) | 132 (31) | <0.001 | <0.001 |
| HDL-C | 52 (11) | 53 (12) | 48 (14) | 0.75 | 0.35 |
| TG | 132 (68) | 95 (44) | 94 (29) | 0.02 | 0.92 |
| LDL-C | 264 (45) | 140 (28) | 65 (26) | <0.001 | <0.001 |
| PCSK9 (ng/mL) | 196 (7) | 281 (8) | 189 (10) | <0.001 | <0.001 |
| PCSK9 (n-fold) | 1.22 (0.15) | 1.77 (0.30) | 1.17 (0.24) | <0.001 | <0.001 |
| PWV (m/s) | 9.6 (3.1) | 8.6 (2.5) | 7.9 (2.1) | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toscano, A.; Cinquegrani, M.; Scuruchi, M.; Di Pino, A.; Piro, S.; Ferrara, V.; Morace, C.; Lo Gullo, A.; Imbalzano, E.; Purrello, F.; et al. PCSK9 Plasma Levels Are Associated with Mechanical Vascular Impairment in Familial Hypercholesterolemia Subjects without a History of Atherosclerotic Cardiovascular Disease: Results of Six-Month Add-On PCSK9 Inhibitor Therapy. Biomolecules 2022, 12, 562. https://doi.org/10.3390/biom12040562
Toscano A, Cinquegrani M, Scuruchi M, Di Pino A, Piro S, Ferrara V, Morace C, Lo Gullo A, Imbalzano E, Purrello F, et al. PCSK9 Plasma Levels Are Associated with Mechanical Vascular Impairment in Familial Hypercholesterolemia Subjects without a History of Atherosclerotic Cardiovascular Disease: Results of Six-Month Add-On PCSK9 Inhibitor Therapy. Biomolecules. 2022; 12(4):562. https://doi.org/10.3390/biom12040562
Chicago/Turabian StyleToscano, Arianna, Maria Cinquegrani, Michele Scuruchi, Antonino Di Pino, Salvatore Piro, Viviana Ferrara, Carmela Morace, Alberto Lo Gullo, Egidio Imbalzano, Francesco Purrello, and et al. 2022. "PCSK9 Plasma Levels Are Associated with Mechanical Vascular Impairment in Familial Hypercholesterolemia Subjects without a History of Atherosclerotic Cardiovascular Disease: Results of Six-Month Add-On PCSK9 Inhibitor Therapy" Biomolecules 12, no. 4: 562. https://doi.org/10.3390/biom12040562